Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

362 CHAPTER 9
The isostripper overhead vapor is a propane-enriched isobutane stream and HF which
is condensed and separated into settling drum. The HF phase is pumped back to the
reactor section. The HF saturated hydrocarbon phase is charged to the depropanizer.
The depropanizer and its associated HF stripper remove propane from the isobutane
recycle. The depropanizer bottoms is returned to the reactors as part of the recy-
cle isobutane. The depropanizer overhead containing the propane product and HF
are condensed and separated in the overhead receiver. The acid phase is returned to
the reactor section and the acid-saturated propane is stripped free of acid in the HF
Stripper column. The HF stripper bottoms is an acid-free propane product which is
treated with hot alumina to remove organic fluorides, cooled and treated with KOH
pellets to remove traces of HF and water.
Acid regeneration
A key advantage of HF alkylation over sulfuric is the ability to recover the acid from
the byproduct polymer, water, and other contaminants. In the UOP HF Alkylation
Process, a small stream of circulating acid is stripped with superheated isobutane in
a small monel tower called the Acid Regenerator. The regenerator overhead is HF
and isobutane that are recycled to the reactor; the regenerator bottoms is polymer and
the HF–water azeotrope which are neutralized with aqueous KOH. The neutralized
polymer has good fuel value. The amount of polymer produced is generally only 1 to
2 barrels per 1,000 barrels of alkylate product.
KOH regeneration
The UOP process minimizes chemical costs by regenerating the KOH used to treat
products and all waste and storm water. This KOH regeneration is accomplished using
lime. As the lime is mixed into the KOH, regeneration of the KOH takes place by the
following reaction:
2KF + Ca(OH)
2
→ 2KOH + CaF
2
The calcium fluoride forms a precipitate and can be easily separated from the regen-
erated KOH.
Process variables in HF alkylation
Key variables are reactor temperature, isobutane to olefin molar ratio, acid strength,
and acid to hydrocarbon volume ratio.
Reaction temperature is one of the more important process variables as it has a
significant influence on the octane number of the product. Almost all HF alkylation
reactors are operated below 100
◦
F. At higher temperatures a decrease in alkylate
octane number will occur. Above 120
◦
F polymerization and cracking side reactions
become excessive reducing alkylate quality. In many cases, acid regeneration capacity
of the HF alkylation unit would not be able to maintain proper control of the acid
strength at temperatures above 110
◦
F. Extremely low reaction temperatures may cause
incomplete alkylation. Thus reaction temperatures below 80
◦
F are typically not used.
GASOLINE COMPONENTS 363
Isobutane to olefin molar ratio is generally the most important variable that the refiner
has the most control over within limitations of isostripper fractionation loadings. As
the isobutane to olefin molar ratio is increased, octane increases; thus the flow of
isobutane recycle is usually kept at a practical maximum at all times, up to the capac-
ity of the isostripper. The reasons to reduce the recycle ratio are for the conservation
of fractionation energy or for a reduction in the hydride transfer reaction. The higher
energy consumption, the greater consumption of isobutane (due to the hydride trans-
fer) and the increased production of propane (due to the hydride transfer) must be
justified economically against higher product quality. Another practical limitation of
isobutane circulation is possible entrainment of acid from the reactor section acid
settler to the isostripper because of inadequate settling time.
Acid strength is usually kept between 85 and 95 mass% HF. Maintenance of this
strength level results from a balance between the performance of the unit feed treating
systems for sulfur and water removal and acid regeneration operation. In some cases
oxygenate removal systems or diene removal systems are also used on the feed where
there are known to be high oxygenates (such as downstream of an MTBE unit, or
high diolefins (from severe FCC conditions). The action of the acid on reactions is a
complex phenomenon and is dependent on the type as well as the amount of diluents.
The fresh acid is supplied by acid manufacturers at 99.0
+
wt% HF. This purity is too
high for optimum performance of the HF alkylation process. As the water content
of the circulating acid increases, carbon steel that is not attacked by anhydrous HF,
becomes less resistant to acid attack.
The acid to hydrocarbon volume ratio used in the reactor of the UOP process is
generally around 1:1. At some point below 0.8–1, excess polymerization occurs. In
the most extreme cases, alkylate production could stop.
Two other variable related topics are reaction time and pressure. As the reaction time
decreases, the combined fluorides leaving the reactor section will increase. However,
any reduction in time is limited by the settling capacity and there is generally little
effect within the permissable operating ranges of a particular unit. Excessive velocities
in the settler will cause free (above saturation level) acid to be carried over into the
isostripper. This carryover may result in corrosion of the upper trays of the isostripper
and discolored alkylate as heavier contaminants in the acid that is carried over drop
into the alkylate product. Pressure is not really a process variable as long as it is kept
high enough that all of the hydrocarbon and acid in the reactor section remain in the
liquid state.
HF feed contaminants
As with many refining processes, the control of contaminants coming into the unit with
the normal feedstocks is critical to the long and dependable operation of the HF alky-
lation unit. Above the recommended maximum levels of feed stock contaminants, acid
consumption, acid regeneration requirements, and in some cases unit corrosion and
364 CHAPTER 9
product quality are all measurably affected. Generally all contaminants are kept as
low as possible within the capabilities of the feed treating systems. The major feed
contaminants normally found in alkylation feeds are water or oxygenates, sulfur com-
pounds, nitrogen compounds, non-condensibles, and diolefin.
HF alkylation maintenance
Because HF is highly corrosive to most materials, careful control and maintenance of
equipment metallurgy and condition is required. Carbon steel is the primary material
used for vessels and piping and it can be used only because of a corrosion barrier layer
of iron fluoride that forms on any carbon steel surface exposed to HF. The iron fluoride
layer is tenacious and serves as a barrier against further carbon steel corrosion as
long as the deposit remains undisturbed. Under certain conditions, such as when wet
acid is in the unit, this iron fluoride scale can soften and break off leading to fouling
and corrosion issues. In severe cases this can lead to considerable unscheduled feed
outages as well as clear safety issues. Most refiners aggressively monitor their equip-
ment’s remaining corrosion allowance and use regularly scheduled valve and flange
replacement to head off any problems. For maintenance during an FCC turnaround,
the normal time most alkylation turnarounds are maintained, many refiners choose
to dissolve all the iron fluoride scale by using a chemical cleaning company.
There are other areas within an alkylation unit where conditions are too severe for
carbon steel and monel is the primary metallurgy for such areas as monel has good
resistance even for high water content HF. Temperature is also an important variable
for corrosion rate of both carbon steel and at higher temperatures for monel. Another
use of monel is for moving parts where use of carbon steel would cause cementing of
parts together a iron fluoride scale formed on each steel surface.
Sulfuric acid alkylation
Today there are two processes for H
2
SO
4
alkylation the Cascade process licensed by
Exxon Mobil and MW Kellogg and the Stratco effluent refrigerated process.
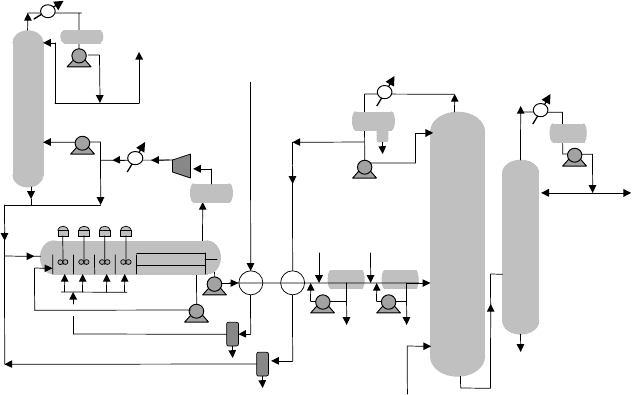
Figure 9.1.3 shows the cascade alkylation process.
Feed pretreatment
It usually consists of deethanizing and Merox-treating of FCC olefins. Some refiners
have added selective hydrogenation units (SHP) to saturate dienes and reduce acid
consumption. Feeds are generally not dried.
Reaction
The FCC olefins are chilled and coalesced to remove water and injected through
sparge rings to 3–6 agitated reaction zones in a large horizontal reactor/settler vessel.
Recycle isobutane from the deisobutanizer and the refrigeration system and recycle
acid from the settler are fed to a pre-flash zone and “cascade” from one zone through
GASOLINE COMPONENTS 365
D
E
P
R
O
P
A
N
I
Z
E
R
D
E
I
S
O
B
U
T
A
N
I
Z
E
R
D
E
B
U
T
A
N
I
Z
E
R
Propane
Refrigerant
~
~~ ~~ ~~ ~~
Settler
FCC
Olefins
Acid to
Reactors
Fresh
Acid
Alkaline
Water
Mixed
Butanes
Alkylate
n-Butane
Coalesers
Acid Recycle
Compressor
Recycle
i-Butane
Reactor
Figure 9.1.3. Cascade auto-refrigerated alkylation process.
specially designed weirs from which the process name derives. Typical isobutane
olefin ratios are 8–12 for the process. The first zone in the cascade reactor has the
lowest operating temperature and the highest isobutane concentration and produces
the highest octane alkylate. As additional olefin is injected in subsequent zones the
temperature increases and isobutane concentration decreases and successively lower
octanes are produced. Because isobutane and H
2
SO
4
are highly immiscible, each
zone requires a mixer with high power inputs to produce a tight emulsion. After
the final reaction zone the emulsion is allowed to settle. The settler acid phase is
pumped back to the lead zone and the hydrocarbon phase effluent is pumped to effluent
treating.
Refrigeration
The heat of reaction is removed by “auto-refrigeration” at reaction temperatures of
35–65
◦
F. While refrigeration is often viewed as costly, in this process it conserves the
heat of reaction to distill 4–5 moles of isobutane recycle per mole of olefin alkylated
and concentrates propane. Isobutane and propane vaporized from the reactor are
compressed, and condensed with cooling water and recycled as “refrigerant” to the
reactors. A fraction of the refrigerant is charged to the depropanizer to remove propane
contained in the feeds from the unit.
Effluent treating
The hydrocarbon effluent containing alkylate and excess isobutane is warmed by
chilling recycle isobutane and feed and treated to remove traces of entrained acid and
366 CHAPTER 9
ester reaction intermediates. Treating systems include washing with fresh acid and
aqueous caustic (as shown). Caustic and water washes, bauxite, and KOH pellets have
also been used.
Fractionation
After effluent treating the balance of reactor isobutane requirement is distilled from
the Alkylate and n-butane deisobutanizer tower (DIB). Most refiners charge saturated
butanes from other units to the DIB for isobutane/n-butane splitting. N-butane is
distilled from the Alkylate for control of product RVP in a debutanizer, and in some
cases an n-butane vapor draw from the DIB. Finally, in a few units, aviation alkylate
is produced by removing heavy ends in a Rerun column.
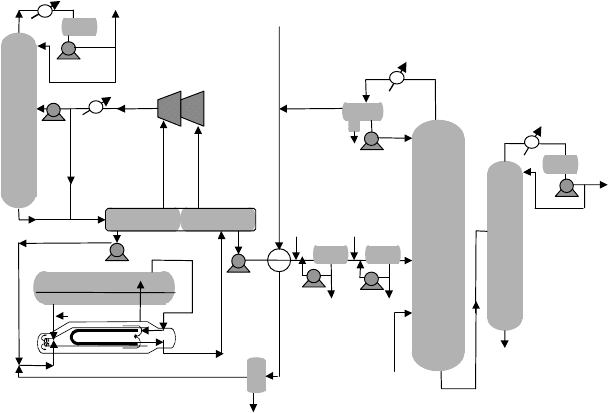
Stratco effluent refrigerated alkylation process
In the Stratco process (Figure 9.1.4.), the principal differences from the Cascade
process are in the reactor and refrigeration design and that the reaction is carried out
without vaporization.
Reaction
Treated feeds and recycle isobutane are first chilled and coalesced to remove water
and charged to several Stratco contactors. Feed and isobutane from the DIB and
n-Butane
Propane
D
E
P
R
O
P
A
N
I
Z
E
R
Refrigerant
Flash
Effluent
Flash
Refrigerant
Settler
Acid Recycle
STRATCO Contactors
Effluent
Compressor
FCC
Olefins
Recycle
i-Butane
D
E
I
S
O
B
U
T
A
N
I
Z
E
R
D
E
B
U
T
A
N
I
Z
E
R
Coalescer
Acid to
Contactors
Acid
Acid
Wash
Alkaline
Water
Wash
Mixed
Butanes
Alkylate
Figure 9.1.4. Stratco effluent refrigerated alkylation process.
GASOLINE COMPONENTS 367
refrigeration and recycle acid from the settler are emulsified together by the high
power impeller of the Stratco Contactor. After reaction and chilling the emulsion
passes to the settler located above the contactors for acid separation. The acid phase
is recycled by gravity to the contactor impeller. And the hydrocarbon phase (effluent)
routed in the tube-side of the contactor heat exchanger.
Refrigeration
The heat of reaction is removed by chilling the emulsion in shell side of the contactor
heat exchanger by partially vaporizing settler effluent on the tube side. Refrigerant
vapor is separated from the effluent liquid in a flash drum, compressed, and condensed.
A portion of the condensed refrigerant is routed to a depropanizer. The balance
of the refrigeration and depropanizer bottoms are flash cooled and returned to the
reactors.
Effluent treating and fractionation
These steps are essentially the same as in the Cascade process.
Process variable in H
2
SO
4
alkylation
Temperature
Maximum octanes for butenes and pentenes are obtained at about 35–40
◦
F and for
propylene at about 50
◦
F. Typically, octane decreases at about 0.04–0.06 octanes per
◦
F at higher temperatures.
Isobutane concentration
Reaction selectivity and octane increase with isobutane concentration. Typical re-
sponses are about 0.1 octane per 1 vol% isobutane in effluent.
Acid strength
Octane is maximized at about 93 wt% acidity. It declines at about 0.2–0.3 RON per
1% acidity decline below that. The economics of acid cost usually require lower final
spending strengths (as low as 88 wt%), so multi-reactor alkylation units typically
feed fresh acid to several reactors in parallel operating at 93% then reuse the 93% as
makeup to other reactors in series.
Space velocity
Octane decreases with the charge rate of olefin. Alkylation reactors are typically
designed at 0.15–0.30. LHSV defined as the volume of olefin charged per hour per
volume of acid. Operations at much higher throughputs are possible but low octanes
and high acid consumption generally result.
368 CHAPTER 9
Acid fraction
Octane increases with acid fraction and peaks at about 60 vol% acid in emulsion.
Power per unit volume
Octane depends upon the mass transfer rate of isobutane from the hydrocarbon phase
into the acid phase. According to Sprow
5
reaction selectivity and octane increase with
interfacial area which depends upon power per unit volume to the 0.25 power.
Sulfuric acid consumption and regeneration
Sulfuric acid alkylation uses about 100 times as much acid as HF alkylation because
H
2
SO
4
cannot be stripped from the conjunct polymer, water, and contaminants. Sul-
furic acid is typically spent at 88–91 wt% H
2
SO
4
, below which it is too weak to carry
out alkylation. The spent acid is not a waste. It is regenerated by burning the organics
which reduces the H
2
SO
4
to SO
2
and water vapor. The SO
2
is then dried and oxidized
to SO
3
and scrubbed with water to produce 98.5–99.5% H
2
SO
4
.
For sulfuric alkylation, aside from the direct hazards of contacting the acid, there are
transportation safety issues around the need to ship and regenerate 20–500 tons of
H
2
SO
4
daily. Several refiners have built dedicated acid plants to regenerate spent acid
from the alkylation unit or pipelines to and from an acid manufacturer.
Acid consumption is generally expressed as the pounds of acid diluted from 99%
to 90% by conjunct polymer, water, and other contaminants per gallon or barrel of
alkylate. Consumption can be as low as 16 lbs per barrel alkylate for diene-free,
MTBE raffinates run at ideal process conditions. Acid consumption can exceed 40 lb/
bbl of alkylate when running high levels of isobutylene, pentenes, and propylene at
high temperatures.
H
2
SO
4
contaminants
Dienes
Butadiene and pentadienes are a major contaminants affecting acid consumption. Di-
enes and can account for 10–30% of the acid consumed. Dienes can dilute 5–20 lbs
of 99% acid down to 90% per lb of diene. Some refiners use diolefin selective hydro-
genation units (such as UOP-Huls SHP™) to eliminate this source of acid consump-
tion.
Water and oxygenates
Water can also account for 10–20% of acid consumption. Wet feeds, wet isobutane
recycle, and high reactor temperatures are the main sources. Oxygenates including
acetone from the FCC, methanol and dimethyl ether from MTBE and TAME units
also consume acid. Water and oxygenates dilute 10 lbs of 99% acid to 90 per lb of
contaminant.
GASOLINE COMPONENTS 369
Sulfur and nitrogen compounds
Dilute about 20 lbs of 99% acid to 90% per lb of sulfur or nitrogen.
C6+
Dilutes 2 lbs of 99% acid to 90% per lb of contaminant. This can be a significant
contributor to acid consumption when alkylating pentenes.
Ethylene
Dilutes 20 lbs of 99% acid to 90% per lb of contaminant by forming stable sulfates
that stay in the acid.
Ethane and lighter
While inert, light ends increase compressor discharge pressure for total condensation.
These indirectly increase acid consumption when limited by horsepower by increasing
reactor temperature.
Table 9.1.1. Alkylation Product Properties/Yields
HF Alkylation
Volume Yields per Butene only with
vol olefin FCC Propene/Butene FCC Butene only Butene Isomerization
Isobutane Consumed 1.28 1.15 1.15
C5 plus Alkylate Produced 1.78 1.77 1.77
C5 plus Alkylate Properties
Specific Gravity 0.693 0.697 0.697
RON-0 93.3 95.5 96.5
MON-0 91.7 93.5 94.5
RVP (PSI) 2.8 2.7 2.7
H
2
SO
4
Alkylation
Volume Yields per
vol olefin FCC Propene/Butene FCC Butene only
Isobutane Consumed 1.2 1.12
C5 plus Alkylate Produced 1.72 1.72
C5 plus Alkylate Properties
Specific Gravity 0.693 0.697
RON-0 92 96
MON-0 90.4 94
RVP (PSI) 2.8 2.7
370 CHAPTER 9
Alkylate properties
Table 9.1.1 illustrates typical product properties for alkylate from HF and H
2
SO
4
alkylation units for propylene-butylene and butylene only units. Alkylate is composed
of primarily C
7
’s and C
8
paraffins with a relatively narrow boiling range. Motor fuel
alkylate has desirable T50 and T90 distillation properties in addition to the long
recognized low endpoint. RON is in the mid to low 90s and MON is 1–2 units
lower. Its high MON is an important property of alkylates. The reid vapor pressure of
debutanized alkylate can be as low as 5–6 PSIA except when alkylating pentenes. Its
low RVP and very low sulfur and the absence of olefins and aromatics make alkylate
a valuable component of reformulated gasolines by reducing Evaporative, NO
x
and
toxics emissions. These properties (or rather lack thereof ) cause alkylate to trade at
a premium over its already high octane value.
Almost all recently designed new alkylation units have been butylenes only or butylene
with some portion of the pentenes. In most cases for butylene only units, the feedstock
is pretreated in a selective hydrogenation unit to both remove the diolefins which
increase acid passivation tar precursors and for HF alkylation units to isomerize most
of the low octane producing 1-butene to the more desirable 2-butene isomers. Such a
pretreating unit can raise the octane of HF alkylate product by one 1 Road ON as can
also be seen in the third column of Table 9.1.1.
Recent developments
Motor fuel alkylation has recently received a boost from the planned phase-out of
MTBE in gasoline blending formulations. Without MTBE, the best conventional
components for today’s reformulated gasolines are high octane paraffinic components
like alkylate.
While environmental legislation has increased demand for alkylate, its catalysts
H
2
SO
4
and HF came under intense environmental scrutiny in recent years. While
both acids are corrosive and toxic, HF is especially hazardous since it has the po-
tential to form toxic aerosols, which can travel significant distances downwind of an
accidental release. This scrutiny challenged the petroleum industry to enhance safety
and even to seek new alkylation catalysts.
r
UOP-Texaco and Mobil-Phillips developed cosolvents called Alkad™ and Re-
vap™, respectively, to reduce HF aerosoling.
r
A consortium of refiners and HF manufacturers lead by Amoco developed HF
dispersion models and water spray mitigation with extensive, large-scale testing to
design of release control systems. Together, cosolvents and water spray mitigation
can reduce downwind HF concentrations by over 95% should a release occur.
GASOLINE COMPONENTS 371
r
Through the American Petroleum Institute, the industry developed a comprehensive
document for safe operation of HF alkylation units entitled ‘Recommended Practice
for Safe Operation of Hydrofluoric Acid Alkylation Units’, API Recommended
Practice 751, first published in June, 1992 and reissued in February, 1999.
r
Finally the efforts to find new catalysts for alkylation have yielded solid cata-
lyst processes such as UOP’s Alkylene™ process that are ready for commerciali-
zation.
Conclusions
Motor fuel alkylation using liquid hydrofluoric or sulfuric acids is one of the oldest
catalytic processes used in refining and petrochemical operations. The liquid acid
processes remain important despite concerns with safety and environmental properties
of the liquid acids. Solid catalyst alkylation using processes such as Alkylene™ (UOP
LLC) are emerging technology.
References
1. V. M. Ipatieff and A. V. Grosse, J Am. Chem. Soc., 1935 37 1616.
2. Pines, Herman, ChemTech, March 1982 150.
3. Hooper, John H. D. Chemistry and Industry, 1986 20, 683.
4. Jernigan, E. C., Gwyn, J. E. and Claridge, E. L. Chem. Eng. Prog. 1965 61(11) 94.
5. Sprow, F. B., Ind. Eng. Chem. Process Des. Dev., 1969 8(2), 254.
6. V. N. Ipatieff and R. E. Schaad, Mixed polymerization of butenes by solid phosphoric acid
catalyst. Ind Eng Chem (May 1938) 30 596–599.
7. A. Chauvel and G. Lefebvre, Petrochemical Processes.Vol1,Synthesis-Gas Derivatives
and Major Hydrocarbons, ed. Technip (1989) 183–187.
