James I.N. Introduction to Circulating Atmospheres
Подождите немного. Документ загружается.

9.2 Wave propagation and mean flow interactions
319
90W
- 90E
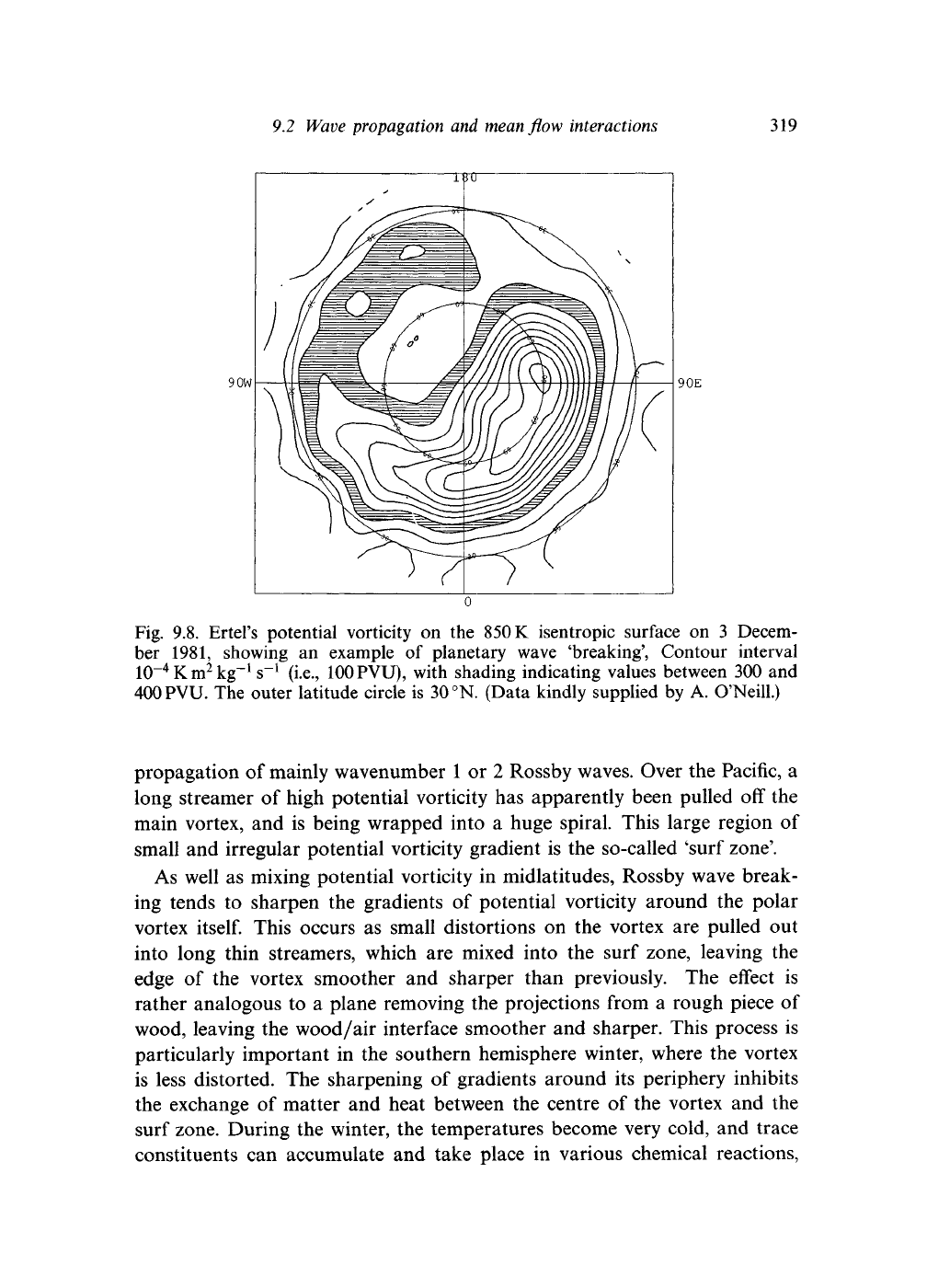
Fig. 9.8. Ertel's potential vorticity on the
850
K isentropic surface on 3 Decem-
ber 1981, showing an example of planetary wave 'breaking', Contour interval
10~
4
Km
2
kg~
1
s"
1
(i.e.,
100
PVU),
with shading indicating values between 300 and
400
PVU.
The outer latitude circle is
30
°N.
(Data kindly supplied by A. O'Neill.)
propagation of mainly wavenumber
1
or 2 Rossby waves. Over the Pacific, a
long streamer of high potential vorticity has apparently been pulled off the
main vortex, and is being wrapped into a huge spiral. This large region of
small and irregular potential vorticity gradient is the so-called 'surf zone'.
As well as mixing potential vorticity in midlatitudes, Rossby wave break-
ing tends to sharpen the gradients of potential vorticity around the polar
vortex
itself.
This occurs as small distortions on the vortex are pulled out
into long thin streamers, which are mixed into the surf zone, leaving the
edge of the vortex smoother and sharper than previously. The effect is
rather analogous to a plane removing the projections from a rough piece of
wood, leaving the wood/air interface smoother and sharper. This process is
particularly important in the southern hemisphere winter, where the vortex
is less distorted. The sharpening of gradients around its periphery inhibits
the exchange of matter and heat between the centre of the vortex and the
surf
zone.
During the winter, the temperatures become very cold, and trace
constituents can accumulate and take place in various chemical reactions,

320 The stratosphere
without mixing down to lower latitudes where they would be photolysed by
sunlight. The formation of this cold 'containment vessel' is an important
element in generating the infamous ozone hole.
Now let us return to a consideration of the vertical propagation of Rossby
waves in the stratosphere. We can use the work of Section 6.5 which set
up a framework for discussing the propagation of Rossby waves on a zonal
flow U = U(y,z
f
). By making a 'slowly varying' approximation, we can
follow the propagation of wave activity into regions where U is different.
As we saw in Section 6.5, the Eliassen-Palm flux vector is parallel to the
local group velocity. But in frictionless, steady and adiabatic conditions,
the Eliassen-Palm theorem must hold, and so the Eliassen-Palm flux must
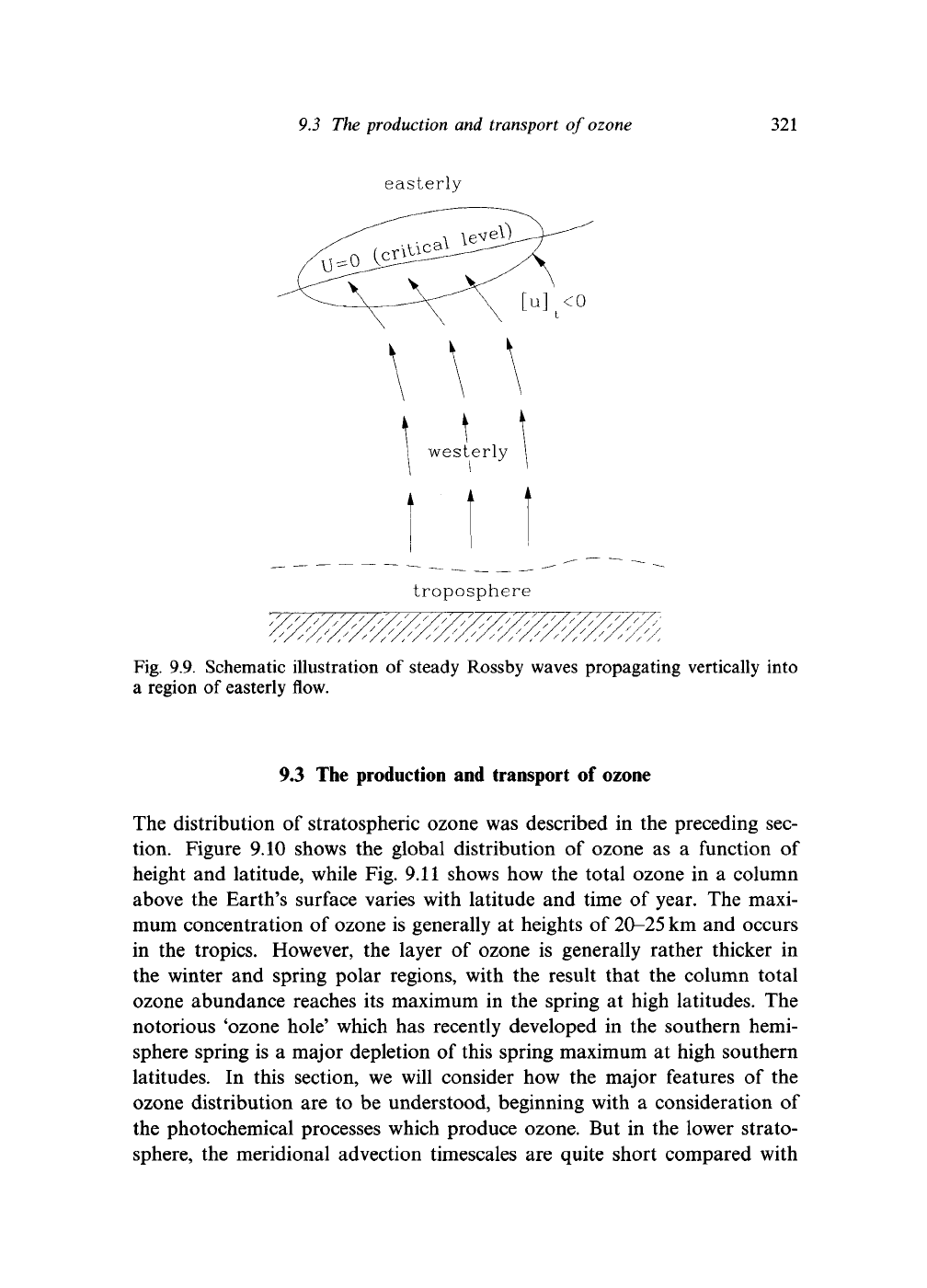
have zero divergence. But now consider the situation depicted in Fig. 9.9, in
which the zonal wind changes sign at some level in the stratosphere. Steady
Rossby waves cannot propagate in easterly conditions. They are evanescent
and, as we saw in Eq. (6.54), their poleward temperature flux is zero. In this
region, both
V •
F and F itself
will
be zero. In the westerly flow,
V •
F must be
zero,
but F itself will generally be nonzero. In the simplest example of the
Charney-Drazin model, F will be vertical and constant with height. In the
vicinity of the 'critical level' where (7 = 0, there must be a large convergence
of F, and, consequently, the Eliassen-Palm theorem must break down there.
Assuming that friction and heating remain small, the simplest solution is
to predict that the flow will become unsteady at this level. Equation (9.6)
suggests that this unsteadiness will be manifested as an easterly acceleration
at the critical level. Thus, if there is a constant flux of wave activity into the
base of the stratosphere, the region of easterly flow will descend until it fills
the stratosphere.
This kind of
process
is the basis of current theories of sudden stratospheric
warmings. Once easterly flow is established at some upper level (where the
assumptions of frictionless, adiabatic flow may become invalid so that the
atmosphere is not constrained by the Eliassen-Palm theorem), the region
of easterlies can descend on a relatively fast dynamical timescale. Once
the easterly regime is established, wave activity will virtually die away, and
the usual westerly regime can only be re-established on a slower radiative
timescale. The associated large temperature rises result from meridional
circulations which maintain thermal wind balance as the zonal wind changes.
It is easy to show that in the stably stratified stratosphere, quite small vertical
displacements of air can result in large temperature changes.
9.3 The production and transport
of
ozone
321
easterly
westerly
troposphere
Fig. 9.9. Schematic illustration
of
steady Rossby waves propagating vertically into
a region of easterly flow.
9.3 The production and transport of ozone
The distribution of stratospheric ozone was described in the preceding sec-
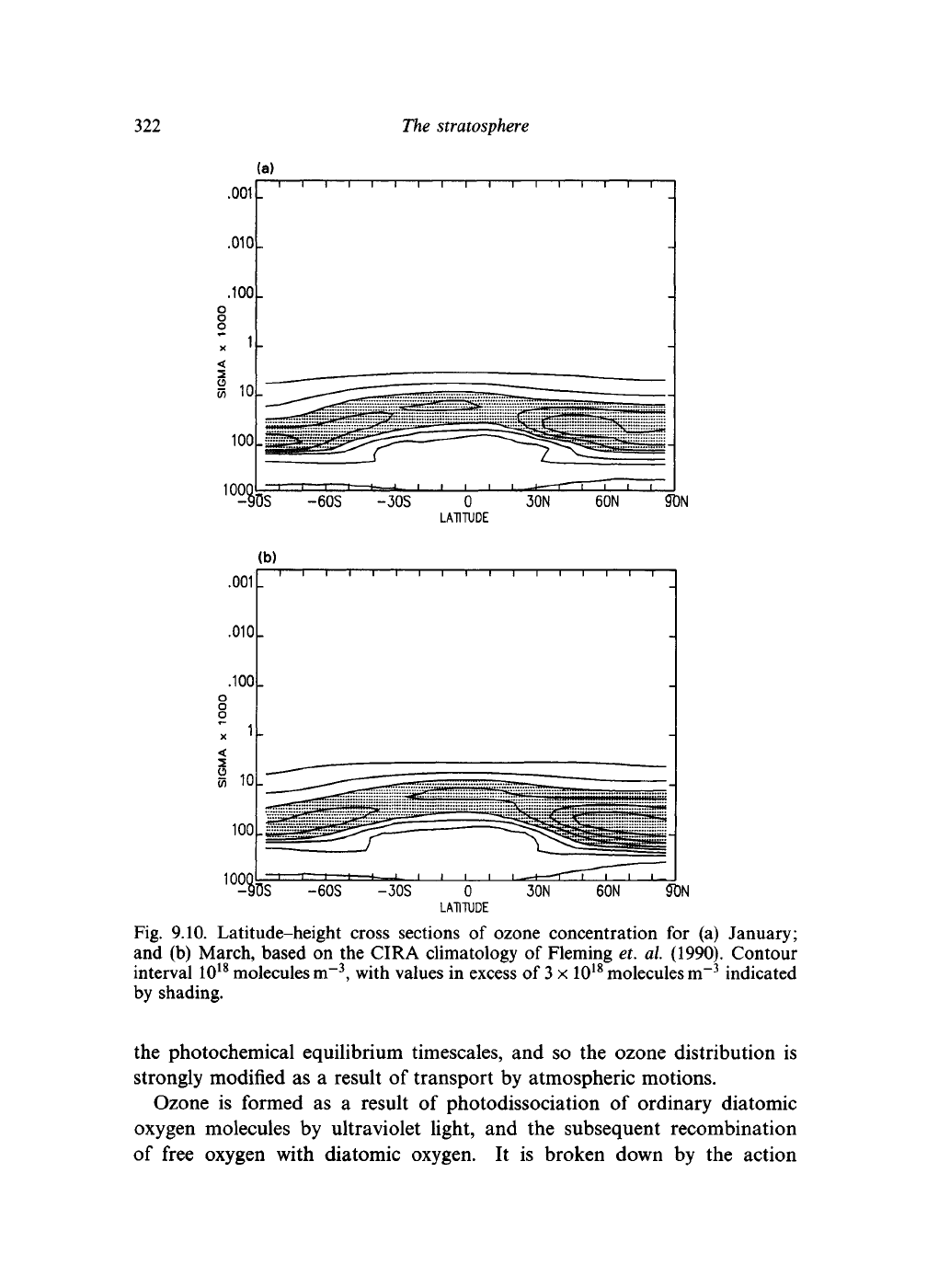
tion. Figure 9.10 shows the global distribution
of
ozone as
a
function of
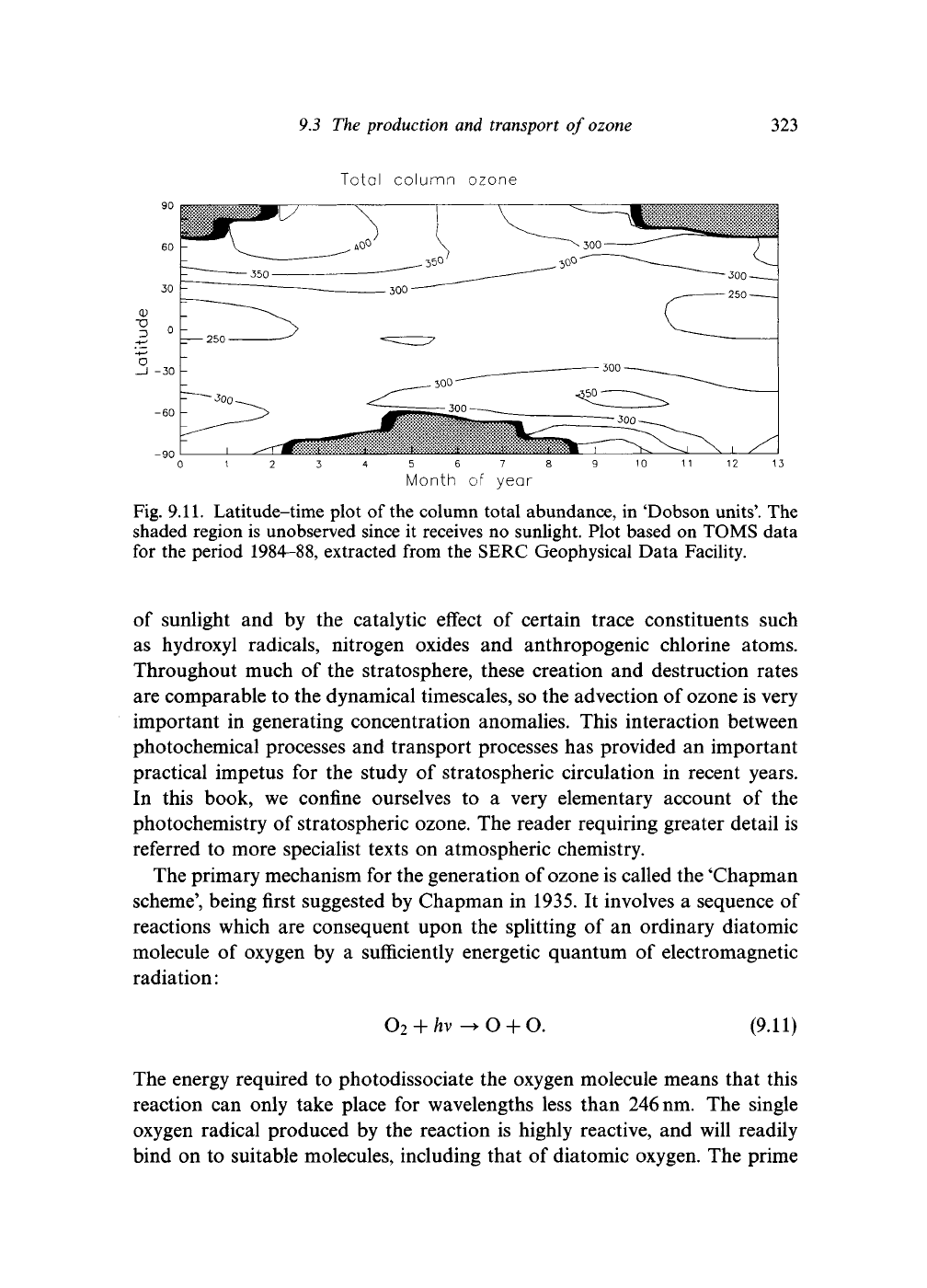
height and latitude, while Fig. 9.11 shows how the total ozone in
a
column
above the Earth's surface varies with latitude and time of year. The maxi-
mum concentration of ozone is generally at heights of 20-25 km and occurs
in the tropics. However, the layer
of
ozone
is
generally rather thicker
in
the winter and spring polar regions, with the result that the column total
ozone abundance reaches its maximum in the spring at high latitudes. The
notorious 'ozone hole' which has recently developed in the southern hemi-
sphere spring is a major depletion of this spring maximum at high southern
latitudes.
In
this section, we will consider how the major features
of
the
ozone distribution are to be understood, beginning with
a
consideration of
the photochemical processes which produce ozone. But in the lower strato-
sphere, the meridional advection timescales are quite short compared with
322
The stratosphere
.001 _
0 30N
LATITUDE
.001-
0
LATITUDE
Fig. 9.10. Latitude-height cross sections of ozone concentration for (a) January;
and (b) March, based on the CIRA climatology of Fleming et. a\. (1990). Contour
interval 10
18
molecules m~
3
, with values in excess of
3
x 10
18
molecules m~
3
indicated
by shading.
the photochemical equilibrium timescales, and so the ozone distribution is
strongly modified as a result of transport by atmospheric motions.
Ozone is formed as a result of photodissociation of ordinary diatomic
oxygen molecules by ultraviolet light, and the subsequent recombination
of free oxygen with diatomic oxygen. It is broken down by the action
93 The production and transport of ozone
323
Total column ozone
0123456789
Month of year
Fig. 9.11. Latitude-time plot of the column total abundance, in 'Dobson units'. The
shaded region is unobserved since it receives no sunlight. Plot based on TOMS data
for the period 1984-88, extracted from the SERC Geophysical Data Facility.
of sunlight and by the catalytic effect of certain trace constituents such
as hydroxyl radicals, nitrogen oxides and anthropogenic chlorine atoms.
Throughout much of the stratosphere, these creation and destruction rates
are comparable to the dynamical timescales, so the advection of ozone is very
important in generating concentration anomalies. This interaction between
photochemical processes and transport processes has provided an important
practical impetus for the study of stratospheric circulation in recent years.
In this book, we confine ourselves to a very elementary account of the
photochemistry of stratospheric ozone. The reader requiring greater detail is
referred to more specialist texts on atmospheric chemistry.
The primary mechanism for the generation of
ozone
is called the 'Chapman
scheme', being first suggested by Chapman in 1935. It involves a sequence of
reactions which are consequent upon the splitting of an ordinary diatomic
molecule of oxygen by a sufficiently energetic quantum of electromagnetic
radiation:
O
2
+
hv
-• O + O. (9.11)
The energy required to photodissociate the oxygen molecule means that this
reaction can only take place for wavelengths less than
246
nm.
The single
oxygen radical produced by the reaction is highly reactive, and will readily
bind on to suitable molecules, including that of diatomic oxygen. The prime

324 The stratosphere
reaction for producing ozone is:
O + O
2
+ M -• O
3
+ M. (9.12)
where M is any third molecule, generally N2 or O2. This third molecule
plays a catalytic role. It is needed to carry off excess momentum, enabling
the O and O2 molecules to bind during a collision while conserving both
kinetic energy and momentum. Chapman also identified ozone destruction
reactions; the balance between the production and destruction processes
should define the observed ozone concentrations. The first process is the
photolysis of ozone, effectively reversing reaction (9.12) by solar radiation
with wavelengths of less than 1140nm:
O3 +
/1V
-»O
2
+ O. (9.13)
Finally, a reaction involving both O and O3 will destroy both forms of free
oxygen:
O3+O-+2O2. (9.14)
Because the concentrations of both O3 and O are low, this last reaction is
relatively slow.
These reactions can be used to estimate a photochemical equilibrium
concentration of ozone. This is based on the law of mass action, which
states that the rate of reaction of two constituents is proportional to the
product of their concentrations. That is, for a simple reaction of the form:
A + B^C (9.15)
the rate of production of molecules of C is given by:
^ =
kn
A
n
B
.
(9.16)
dt
Here, n
A
, n
B
and n
c
are the so-called number densities, that is, the number
of molecules per unit volume, of the reactants A, B and C respectively, and
k is the reaction rate, which will generally be a function, possibly a rather
sharply varying function, of temperature. Applied to the Chapman scheme,
the law of mass action predicts the equilibrium number density of ozone
molecules,
713,
to be:
(9.17)
where n
2
and n
M
are the number density of the oxygen molecules and the
catalyst molecules M respectively, k
2
and k
3
are the reaction rates of re-
actions (9.12) and (9.14), respectively, and J
2
, J3 are the photodissociation

93 The production and transport of ozone 325
rates associated with reactions (9.11) and (9.13), respectively. These photo-
dissociation rates themselves depend upon the ozone distribution, since they
measure the amount of ultraviolet light of the appropriate wavelengths
reaching the location under consideration. Some of this ultra-violet light will
have been absorbed by O3 and O2 as it passes through higher layers of the
atmosphere. The rate J
2
increases more rapidly with height than does /
3
,
with the result that Eq. (9.17) predicts a maximum of the number density
of ozone molecules in the stratosphere. However, the scheme predicts rather
too much ozone generally, and certainly cannot account for features of the
distribution such as the spring maximum. Rather, photochemical arguments
alone would suggest that the ozone distribution should correlate strongly
with the distribution of short wave radiation.
Two important modifications to the simple photochemical equilibrium
theory need to be introduced. First, further chemical reactions, often involv-
ing trace atmospheric constituents, can modify the chemistry of
the
Chapman
scheme. Second, atmospheric transports can redistribute the ozone.
The most important modifications to the chemistry arise through catalytic
destruction of ozone by trace constituents. Denote such a constituent by X.
Then the ozone destruction process can be written:
X + O
3
->XO + O
2
, (9.18a)
O^X + O
2
. (9.18b)
The net effect is to combine O and
O3
to form two molecules of
O2,
leaving X
unconsumed and ready to participate in further ozone destruction. The most
important catalysts are OH, NO and Cl. The OH is formed naturally by the
photodissociation of water vapour. The NO also has natural sources, such as
lightning and photodissociation of ammonia and other biologically produced
nitrogen compounds. It is also found in the exhaust gases of aircraft flying
in the stratosphere, and there have been concerns that unrestricted growth
in the number of commercial supersonic flights could have a significant
impact on the ozone layer. A much more serious threat to ozone is now
thought to come from Cl, which is almost entirely artificial in origin, being
formed by a complex sequence of heterogeneous reactions, starting with
the photodissociation of man-made chemicals such as chlorofluorocarbons.
It is responsible for the large ozone depletions observed in the southern
hemisphere spring and for the smaller depletion now becoming apparent in
the northern hemisphere.
A full quantitative account of
the
photochemical equilibrium concentration
326 The stratosphere
.001
.010
.100
D
D
100 _
100Q
1
—'—'—'—'—'—'—'—'—'—'—'—'—'—'—'—i—
L
-
-9DS -60S -30S 0 30N 60N SDN
LATITUDE
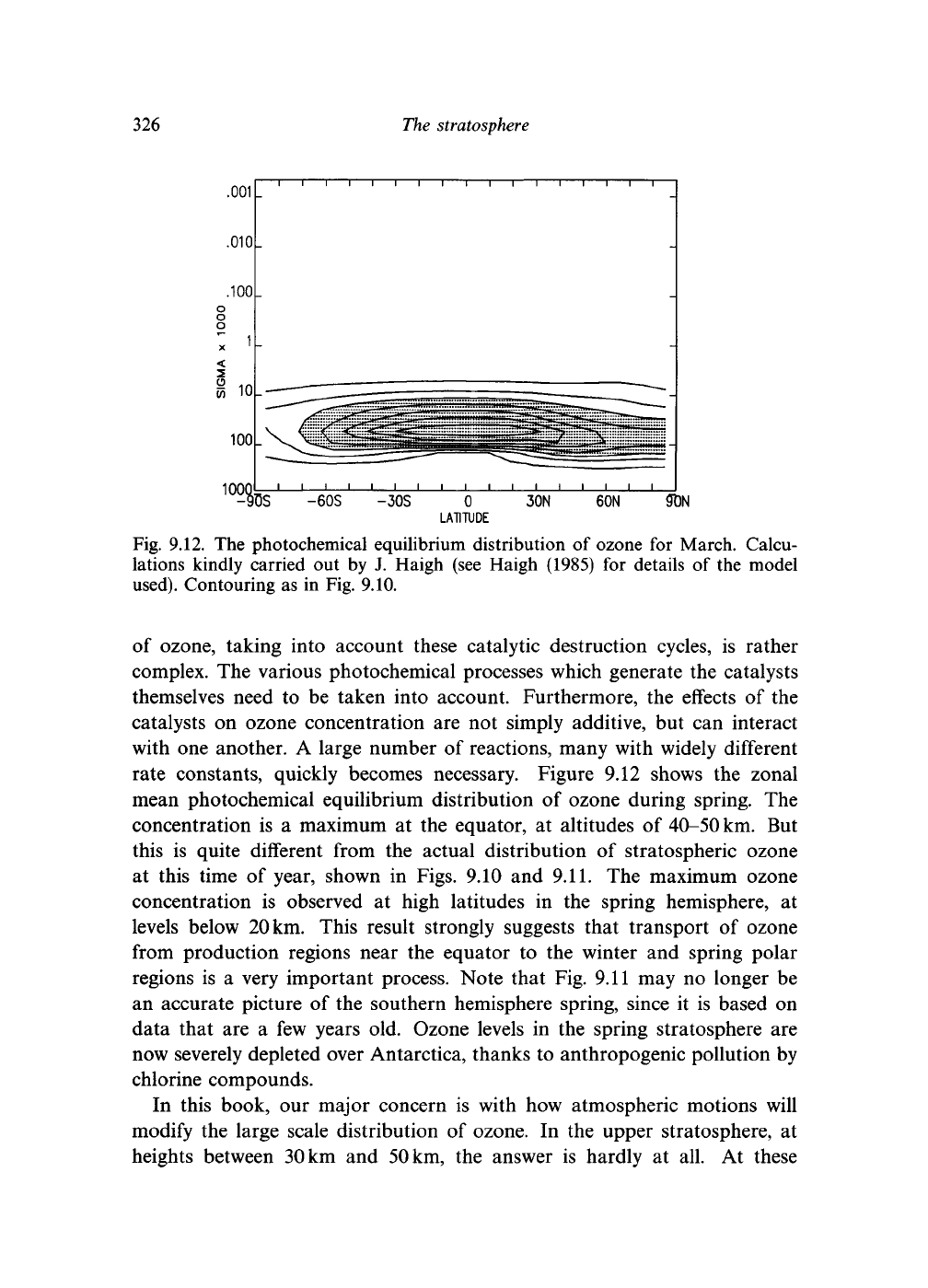
Fig. 9.12. The photochemical equilibrium distribution
of
ozone
for
March. Calcu-
lations kindly carried
out by J.
Haigh
(see
Haigh (1985)
for
details
of
the model
used).
Contouring
as in
Fig. 9.10.
of ozone, taking into account these catalytic destruction cycles,
is
rather
complex. The various photochemical processes which generate
the
catalysts
themselves need
to be
taken into account. Furthermore,
the
effects
of the
catalysts
on
ozone concentration
are not
simply additive,
but can
interact
with one another.
A
large number
of
reactions, many with widely different
rate constants, quickly becomes necessary. Figure
9.12
shows
the
zonal
mean photochemical equilibrium distribution
of
ozone during spring.
The
concentration
is a
maximum
at the
equator,
at
altitudes
of
40-50
km.
But
this
is
quite different from
the
actual distribution
of
stratospheric ozone
at this time
of
year, shown
in
Figs.
9.10 and
9.11.
The
maximum ozone
concentration
is
observed
at
high latitudes
in the
spring hemisphere,
at
levels below
20
km.
This result strongly suggests that transport
of
ozone
from production regions near
the
equator
to the
winter
and
spring polar
regions
is a
very important process. Note that Fig. 9.11 may
no
longer
be
an accurate picture
of
the southern hemisphere spring, since
it is
based
on
data that
are a few
years
old.
Ozone levels
in the
spring stratosphere
are
now severely depleted over Antarctica, thanks
to
anthropogenic pollution by
chlorine compounds.
In this book,
our
major concern
is
with
how
atmospheric motions will
modify
the
large scale distribution
of
ozone.
In the
upper stratosphere,
at
heights between
30
km
and
50
km,
the
answer
is
hardly
at all. At
these

9.3 The
production
and
transport
of
ozone
327
levels,
the ozone concentration reaches its photochemical equilibrium in
quite a short time, certainly short compared to the time for atmospheric
motions to advect ozone to regions where the temperature, pressure or
radiative regime is significantly different. In the lower stratosphere, the
photochemical reaction rates become small, and so the time required to
reach photochemical equilibrium becomes several weeks, much longer than
the typical dynamical timescale. In the lower stratosphere, then, ozone is
advected around almost as a conserved tracer. In fact, the largest abundances
of ozone are found in the lower stratosphere, suggesting that the transport
of ozone from production regions is a very important process. Furthermore,
the column integrated ozone amount is a maximum at high latitudes in the
spring, where photochemical production rates would be expected to be very
low; there are simply not enough sufficiently energetic photons available
to produce the observed abundances of ozone. Once more, there must be
significant transport of ozone from lower latitudes.
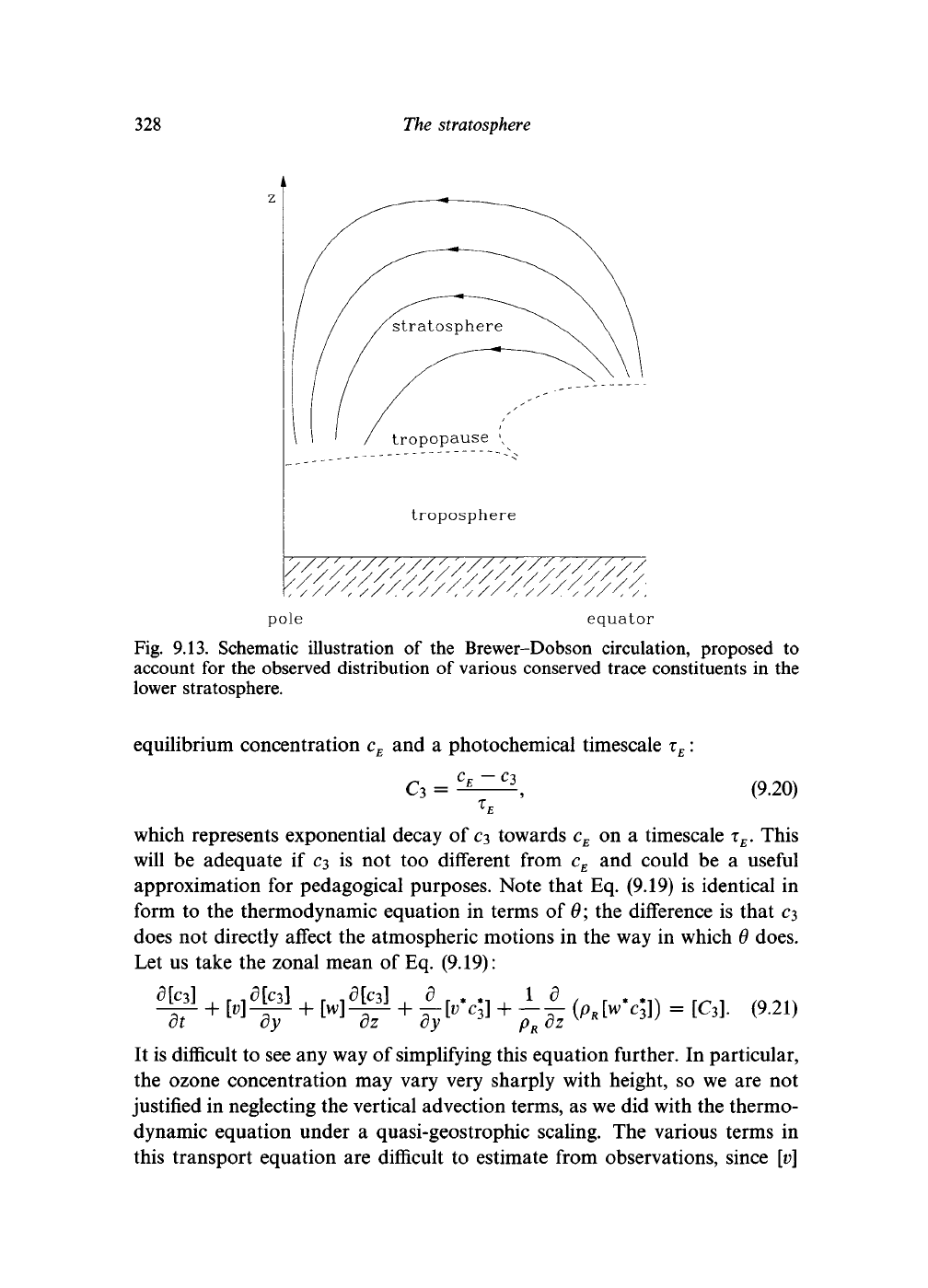
The 'Brewer-Dobson' circulation was proposed in the 1940s to account for
the observed distribution of ozone and other conserved trace constituents
in the lower stratosphere. It is illustrated in Fig. 9.13 and consists of a
meridional circulation in each hemisphere, with air rising into the strato-
sphere in the tropics, moving poleward, with descent and entrainment back
into the troposphere at high latitudes. Such a mass circulation will trans-
port ozone from the tropical production regions and accumulate it towards
the poles, accounting for the spring polar maximum. Of course, such a
circulation, deduced from the observed concentrations of trace constituents,
is a Lagrangian circulation. Attempts to deduce a meridional circulation
from observed heating rates and eddy fluxes of heat and momentum, as was
described in Chapter 4, yield quite a different Eulerian mean circulation.
Because of this misunderstanding, the 'Brewer-Dobson' circulation was re-
garded for a long time as incorrect. In more recent times, there has been
renewed interest in the Lagrangian mean circulation and in approximations
to it, such as the isentropic mean circulation or the residual circulation
introduced in the last section.
The concentration of ozone in an air parcel can be written in the form:
^ w^ = C
3
. (9.19)
cz
Here,
c?,
denotes the mixing ratio of ozone, while C3 denotes the photo-
chemical production or destruction rate of ozone. Note that if
C3
represents
a volume mixing ratio, it is proportional to the molecular number density
H3 given in Eq. (9.17). Conceptually, C3 could be written in terms of an
328
The stratosphere
troposphere
pole equator
Fig. 9.13. Schematic illustration
of
the Brewer-Dobson circulation, proposed
to
account for the observed distribution of various conserved trace constituents in the
lower stratosphere.
equilibrium concentration c
E
and
a
photochemical timescale x
E
:
(9.20)
which represents exponential decay of
C3
towards c
E
on
a
timescale x
E
. This
will
be
adequate
if
C3
is
not too different from
c
E
and could
be a
useful
approximation for pedagogical purposes. Note that Eq. (9.19) is identical in
form to the thermodynamic equation in terms of 9; the difference is that C3
does not directly affect the atmospheric motions in the way in which 9 does.
Let us take the zonal mean of Eq. (9.19):
It is difficult to see any way of simplifying this equation further. In particular,
the ozone concentration may vary very sharply with height,
so
we are not
justified in neglecting the vertical advection terms, as we did with the thermo-
dynamic equation under
a
quasi-geostrophic scaling. The various terms
in
this transport equation are difficult
to
estimate from observations, since [v]
