Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


492 Chapter 4.7: Outgassing of Materials
balance results in the equation
PnSn + Pn^fnenm^m = ^^nm^m " V(dpjdt) (6)
where
p„
is the partial pressure of the nth gas in torr,
S„
is the net pumping speed
for this gas in liters/sec, A,„ is the exposed area in cm^ of the mth material,
K,,,,^
is
the "free" or intrinsic outgassing rate in torr • liter
s~^cm"^
of this material for
the nth gas, defined as the outgassing rate when readsorption is negligible, t is the
time in seconds, V is the chamber volume in liters,
e„,n
is a sticking or sorption
coefficient for the nth gas on the mth material, and
f, = {RoT/27rM,y^'lO-' (7)
is the "effusion law" factor in units of liters
s ~^
cm~^ at the absolute temperature
T for the nth gas of molecular weight M„ and
RQ
is the molar gas constant
(RQ
=
8.31 X lO'^ergsdeg-^Kmol"^).
The total pressure p in the vacuum chamber at the pumping time t in seconds is
given by [2]
X
K„,„A,„
- Vidpjdt)
P = lPn = I.^ (8)
m
where it is assumed that the partial pressure, p„, of each gas species is known by
measurement with a mass spectrometer or residual gas analyzer
(RGA).
Since out-
gassing rate measurements on individual materials are often made with total pres-
sure gauges, such as an ionization gauge, using the air or nitrogen calibration fac-
tor, in practice the preceding equation can be simplified to
2
K„Am
- V(dp/dt)
P = '^ W
m
where p is the total pressure as measured with the air calibration factor, ^„, is
the air- (or nitrogen-) equivalent free outgassing rate at the time t, ^,„ is the air-
equivalent sorption coefficient,/a is the value of/„ when the molecular weight M„
is taken as 29 (or 28), and S is the net pumping speed for air. When the chamber
is empty of material to be processed and the outgassing from elastomer gaskets
can be neglected, this equation simplifies further to
p = -^S^ "^—^ (10)
^ /aO-A + S ha A + S dt

4.7.1 Relationships Among Pressure, Speed, and Outgassing Rate 493
where a is the effective air-equivalent sorption coefficient for the wall material
(of geometric surface area A), which may be somewhat larger than the ordinary
sticking coefficient because of the porosity of the oxide layer and will depend on
the fraction of adsorption sites that are occupied at time r; A is the chamber wall
area; and
Kn,
= Kn/(t/3600r (U)
where Kfi is the free outgassing rate of the wall material after one hour of pump-
ing and / is in seconds. This equation contains the hidden factor (one hour)" = 1,
which allows for values of a other than 1.
RELATIONSHIP BETWEEN REQUIRED SPEED
AND CHAMBER DIMENSIONS
The volume V in liters of a cylindrical vacuum chamber of length L (cm) and ra-
dius R (cm) with dished heads can be estimated from
V= 10-3 7r/?2(L +0.4/?) (12)
where L is usually about 3R corresponding to V = R^/lOO, The area (in cm^) of
the walls of such a chamber can be estimated from
A = lirRiL +
1.1/?)
(13)
and for L = 3/? the area
A = 26/?2 (14)
For typical industrial vacuum systems filled with material to be processed (such
as vacuum coating) the net air speed, S^, (in liters/sec) of the high-vacuum pump
(diffusion pump or turbomolecular pump) should be about 2.5 times the volume V
of the empty chamber, or
5d = 2.5V=/?V40 (15)
and the roughing pump or backing pump speed, 5r, is usually about 5d/100 or
S, = W40 = /?V4000 (16)
For typical industrial vacuum systems with chambers filled with material to be
processed,
5d/A = /?/1000 (17)
where A is the wall area of the empty chamber.

494 Chapter 4.7: Outgassing
of
Materials
4.7.2
INITIAL PUMPDOWN FROM ATMOSPHERIC PRESSURE
During the initial pumpdown from atmosphere of the empty chamber with a me-
chanical pump (roughing pump) of net speed
S^,
the outgassing and sorption terms
can be neglected until the pressure produced by the roughing pump alone falls be-
low a value given by the semiempirical formula
p^
= 300 K^A/V (18)
where ^i is the net outgassing rate (including readsorption) at one hour and the
numerical coefficient has the dimensions of time. This pressure p^ is typically
in the range from 10"^ to
10
""^
torr when the ultimate pressure of the roughing
pump is less than 10"^ torr. In the region from atmospheric pressure, po, (about
750 torr) to Pv integration of Equation (10), neglecting the outgassing and sorp-
tion terms, with a roughing pump of maximum speed
Sj.
and ultimate pressure/7ru,
gives
P=Pru+Poexp(-5rf/V) (19)
and the time (in s) to reach the pressure p will be
t, = 2.3 (W5r)logio[(Po -
PruViP
-
Pru)]
(20)
Then the time to reach the pressure p^ will be approximately
r, = 12 V/S, (21)
For L = 3/? the ratio A/Vin Equation (18) equals 2600/R so that
Pv = 7.8 X 10^ K^/R (22)
Since Ky is often of the order of 10"^ torr • liter
s'^cm"-^,
then for R of about
30 cm (1 foot) Pv is about 3 X 10"^ torr. Since oil diffusion pumps can operate
when the forepressure is less than a few tenths of
1
torr, there is no need to wait
until the pressure falls to py to switch the high-vacuum pump into operation by
opening the high-vacuum valve. From Equation (20) and Equation (16), the
roughing time, or time to reach the switchover pressure, p^, for diffusion-pumped
systems of about 0.2 torr will typically be
fv = 8 V/Sr = 320s = 5.3 minutes (23)
For cryopumped systems, the safe switchover pressure depends on the volume of
the chamber, and the recommendations of the manufacturer should be followed.

4.7.3
Pressure
Vs.
Time
During Outgassing 495
4J.3
PRESSURE
VS.
TIME DURING OUTGASSING
4.7.3.1 Pumpdown with Outgassing
After switching to operation with a high-vacuum pump of constant speed
S^^
=
100 ^r, the time to reduce the pressure from p^ to p^ = lO'^p^ (neglecting out-
gassing) will be
r,d = 4.6 V/S^ (24)
or, since these systems typically have a time constant r =
V/S^^
= 0.4 s, the pres-
sure will drop from p^ = 0.2 ton* to p^ = 2 X
10 ""^
torr in about 2 s. When the
pressure reaches
p^^S^/S^^
= 3 X 10~^ torr, the pressure then decreases more
slowly as outgassing, and inleakage become the limiting processes. The term
~[yKfa^^ + S)](dp/dt) can usually be neglected when the pressure decreases
by another factor of
3,
or typically when the pressure with the high-vacuum pump
operating is less than 1 X 10~^ torr. The pressure is then given by
P = K,^A/if,aA + 5d) + /7, = {Kn/h-
)/(f,a
+ S,/A) + p, (25)
where
Pu
is the "ultimate pressure" (lower pressure limit) due to leaks and perme-
ation from the atmosphere, fh is the pumping time in hours, and a is the sticking
probability based on the geometric surface area A. At room temperature (298 K)
the value of/a (for air) is 11.7 liters
s~^cm~^.
For empty chambers with metal
walls that have not been baked, the gas at pressures below
10
"^ torr is about 90%
water vapor. The effusion law factor,/^v, for water vapor at 298 K is 14.8 liters
s~^cm~^.
For stainless steel, as shown below, it can be assumed that a is about 6 X 10"^
after one hour of pumping, so that for chambers with S^/A < I
(or
R < 1000 cm)
the readsorption iermf^a = 0.089 for water vapor cannot be neglected in com-
parison to SJA.
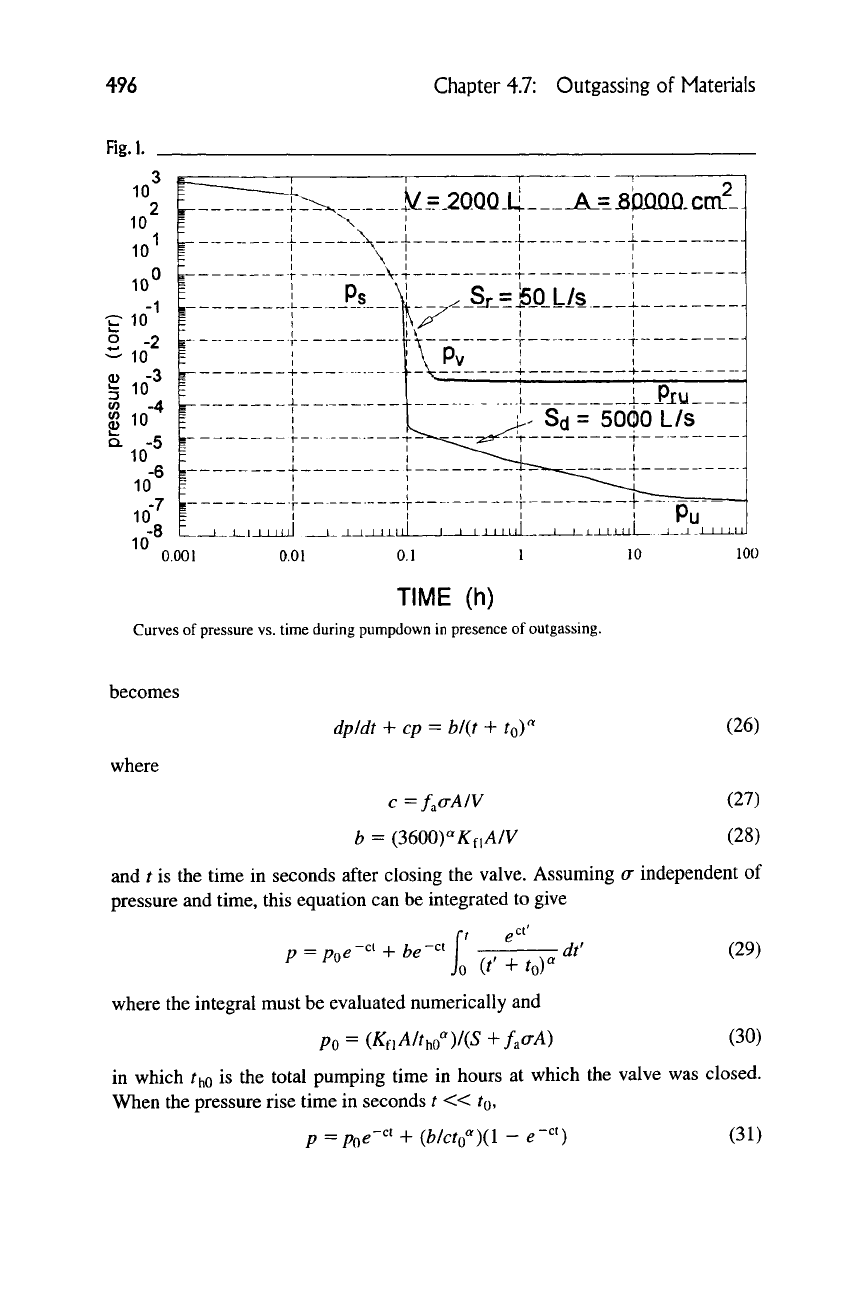
The preceding relationships are illustrated by Figure 1 for the pumpdown
curve of a typical vacuum system with V = 2000 liters, S, = 50 liters/sec, A =
80,000 cm2, 5a = 5000 liters/sec,
K^^
= I X 10"^ torr • liter
s'^cm'^
po =
750 torr,/7s = 0.2 torr,/?^ = 10"-^ torr, and ultimate pressure/7u = 1 X 10~^ torr
with the diffusion pump operating as shown by the dashed line.
4.7.3.2 Rate of Rise of Pressure in Closed Chamber
If the high-vacuum valve is closed after the time to (in seconds), then 5 = 0 and,
using Equation (11), the differential equation. Equation (10), for the pressure
496
Chapter 4.7: Outgassing of Materials
Fig.l.
2
10
10^
t-
10
-1
e-10
2 -2
-- 10
-3
CD
(o -4
s 10
Q. -5
10
10
10'
--8
10
-_..V=-20QQ.li ^=JBQQQQ.cml
\ I
Ps___\[__
^_Sr_=ML/S
:y'
KEZi:::::
i__Pru_.
Sd = 5000 L/s
0.001
TIME (h)
Curves of pressure vs. time during pumpdown in presence of outgassing.
becomes
where
dpidt + cp = hl(t + t^Y
(26)
c=/aCrA/V (27)
b = {3600r K^^A/V (28)
and t is the time in seconds after closing the valve. Assuming a independent of
pressure and time, this equation can be integrated to give
P=Poe
be'
Jo {f + t^r
"^^
(29)
where the integral must be evaluated numerically and
Po = (/i:fiA/rho")/(5+/acrA) (30)
in which
t^Q
is the total pumping time in hours at which the valve was closed.
When the pressure rise time in seconds t « t^,
p=Poe-'' + {b/cton(l-e-'') (31)

4.7.4
The
Outgassing Rate
of
Elastomers and Plastics
497
where
blc
=
{?>6mrKnlho-
(32)
From Equation (31) the rate-of-rise equation
is
dpidt
=
[(b/ton
-
cpo]e-''
(33)
and
the
initial
{t = 0)
rate-of-rise times
the
chamber volume
V
divided
by the
chamber wall area A
is
(V/A) dpIdt
=
(Kn/tuon/(l ^hcrAIS)
(34)
which
is
just
the net
(measured) outgassing rate (including readsorption)
at the
pumping time
(in h)
f
no
when
the
valve
was
closed. When SI A
» f^a, one ob-
tains
the
free outgassing rate
at
rno- This
is one
method
of
measuring
the
out-
gassing rate. The equations are based
on
the assumption that the time exponent
a
is constant over the times involved
in
the measurements, which
is
usually approx-
imately true for typical values of a and times up to f
hm
^s
defined in Section 4.7.1.2
(p.
485)
and
Section 4.7.4.1
(p.
500).
Of
course, when
the
system contains layers
of oil or solids with volatile plasticizers, the evolution of vapors from these sources
will not follow Equation (31).
47.4
THE OUTGASSING RATE OF ELASTOMERS AND PLASTICS
4.7.4.1 Outgassing
by
Diffusion
of
Absorbed
Gas
to Exposed Surface
It
has
been found experimentally
[2]
that
the
outgassing rate
of
elastomers
and
plastics, once the volatile organic matter (plasticizer) has been removed,
is
deter-
mined mainly
by
the diffusion
of
absorbed gas and water molecules from the bulk
of the material
to the
exposed surface, where
the
molecules
can
escape directly
from open pores
or
move
by
surface diffusion
to
adsorption sites
on the
exposed
surface from which they soon evaporate. Nitrogen and oxygen molecules initially
adsorbed
on the
directly exposed (geometric) surface
are
desorbed
in
less than
a
second
at
room temperature because
the
energy
of
adsorption
on
elastomers
and
plastics
is
less than
10
kcal/mol. Similarly, water molecules
on the
exposed sur-
face
are
desorbed quickly from most elastomers
and
plastics
(but not
from sili-
cone elastomers). However,
air and
water molecules that have dissolved
in the
bulk material
by
diffusion through capillary pores before evacuation
are
only
re-
moved slowly
at
room temperature because
of
the small diameter
of
the capillar-

498 Chapter 4.7: Outgassing of Materials
ies and the "random walk process," which is the nature of diffusion along a capil-
lary pore.
The diffusion of gas into or out of a solid is controlled by Pick's law [8]
d ( dC\ d ( dC\ d ( dC\ dC
dx\ ' dxj dy\ ^ dyj dz\ dzj dt
where C is the concentration (in molecules cm~^) of gas in the solid at the loca-
tion (x, y, z), Di, D2, and D3 are diffusion coefficients (in cm^s"^), which may
vary with the location
(jc,
y, z) and the direction of the concentration gradient, and
t is the time (in s). For material in sheet form of sufficient thickness, w,„, that it
can be treated as a semiinfinite solid with a uniform concentration
CQ
at time zero
and a constant diffusion coefficient
D„„j
for the nth gas, when the exposed surface
is suddenly exposed to a very low pressure by fast pumping of the vacuum cham-
ber containing the material, the concentration distribution along the jc axis per-
pendicular to the surface at the time / will be given by
C(x, t) = Co erf[;c/2(A„„0^^^] (36)
where erf refers to the error function, which is tabulated in various mathematical
handbooks [9].
For gas molecules that are adsorbed on the walls of capillary pores in a solid
with adsorption lifetime, r^, and that diffuse along the capillary by desorption fol-
lowed by collision with the wall and readsorption, the diffusion coefficient is
given approximately by [2]
D„„, = (A2/4)/[(A/M„„) + rj (37)
where
A
is the mean distance (in cm) in the
JC
direction traveled by a molecule
bewteen collisions with the capillary wall and
M,„„
is the average molecular veloc-
ity at the temperature
T,„
of the solid.
The free outgassing rate in molecules
s ~ ^
cm
"^^
is then given by
N(t) = D„„,[dC(x,t)/dxh^o (38)
where the concentration gradient is evaluated at
;c
= 0 corresponding to the ex-
posed surface and t > 7rA^/16D,„„. Substituting Equation (36) in Equation (38)
gives
Nit) = Co{D„j7rty^^ (39)
and the free outgassing rate in ton*
•
liter
s"^cm"^
is then
K^ = (RJ/60N,)Co(D,J^t^y^^ (40)
where R^ = 62.36 torr
•
liter K"
Ms
the molar gas constant in vacuum engineer-
ing units, T is the absolute temperature of the gas in the vacuum chamber,
A^a
=
6.02 X 10^^ is Avogadro's number (the number of gas molecules in one mole).
4.7.4 The Outgassing Rate
of
Elastomers and Plastics
499
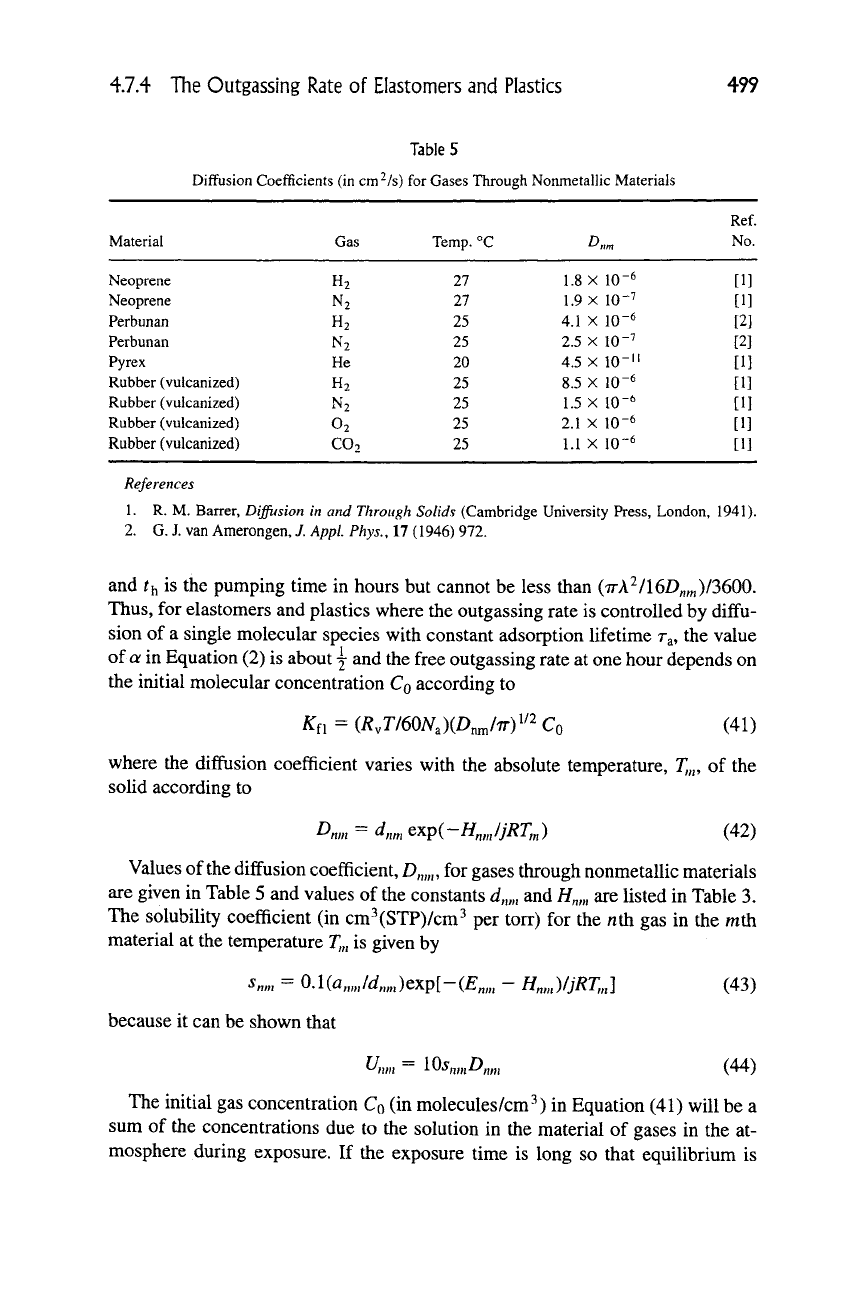
Table 5
Diffusion Coefficients (in cm^/s) for Gases Through NonmetalHc Materials
Material
Neoprene
Neoprene
Perbunan
Perbunan
Pyrex
Rubber (vulcanized)
Rubber (vulcanized)
Rubber (vulcanized)
Rubber (vulcanized)
Gas
H2
N2
H2
N2
He
H2
N2
02
C02
Temp.
°C
27
27
25
25
20
25
25
25
25
D„„
1.8 X 10"^
1.9 X 10"^
4.1 X 10-6
2.5 X 10"^
4.5 X lO-'i
8.5 X 10-6
1.5 X 10-6
2.1 X 10-6
1.1 X 10-6
Ref.
No.
[1]
[1]
[2]
[2]
[1]
[1]
[1]
[1]
[1]
References
1.
R. M. Barrer,
Diffusion
in and
Through
Solids (Cambridge University Press, London, 1941).
2.
G. J. van Amerongen, 7.
Appl.
Phys., 17 (1946) 972.
and ^h is the pumping time in hours but cannot be less than (7rA^/16Z)„,„)/3600.
Thus,
for elastomers and plastics where the outgassing rate is controlled by diffu-
sion of a single molecular species with constant adsorption lifetime r^, the value
of a in Equation (2) is about y and the free outgassing rate at one hour depends on
the initial molecular concentration
CQ
according to
Kn = {R,T/60N,){D^/7r)
'^^
Q (41)
where the diffusion coefficient varies with the absolute temperature, 7,,,, of the
solid according to
Dnm
=
d„„,
Qxp(-H,JjRTJ (42)
Values of the diffusion coefficient,
D,„„,
for gases through nonmetallic materials
are given in Table 5 and values of the constants
d,,„,
and //„,„ are listed in Table 3.
The solubility coefficient (in cm^(STP)/cm^ per torr) for the nth gas in the mth
material at the temperature r,„ is given by
s„,,
= OA(a„Jd„jQxp[-(E„„, - H„J/jRTJ (43)
because it can be shown that
U„„
= 10^„,„A„„ (44)
The initial gas concentration
CQ
(in molecules/cm"^) in Equation (41) will be a
sum of the concentrations due to the solution in the material of gases in the at-
mosphere during exposure. If the exposure time is long so that equilibrium is
500
Chapter 4.7: Outgassing of Materials
reached at the prevailing temperature, the concentration of the nth gas will be
Qo = (760/273) X lO~\NJR,)s„oP„o'^^ (45)
where
5„o
is the value of the solubility coefficient
s^^^
for the nth gas at the initial
pressure P„o and temperature r,„ =
TQ.
Of course, in addition to the atmospheric
gases dissolved in the solid elastomer or plastic there will usually be some resid-
ual plasticizer, but the vapor pressure of the plasticizer may be so low that the out-
gassing rate is determined mainly by the dissolved gas content. Table 6 lists val-
ues of the solubility coefficients for common gases in typical solids.
Since experiments have shown [2] that the sorption probability, £,„„, for the
common gases colliding with the exposed surface of an elastomer and diffusing
some distance into the bulk through the entrance to an open capillary pore, is
less than 1 X 10"^ so that/„g„,„A„, is negligible compared to 5„ when
S,JA,„
>
1 X
10 ~'^,
then ^fi is approximately equal to the net outgassing rate from elasto-
mers in most systems.
Equation (39) should be valid until t^ >
^hm*
where
^hm = [7r(w,/20)2/16D,„J/7200 (46)
in which
w^^
is the thickness (in mm) of a sheet of elastomer exposed to the vac-
uum on both sides [2]. When f h >
^hm»
the outgassing rate should begin to decay
more or less exponentially with pumping time as the gas content of the elastomer
becomes more than half depleted.
Table
6
Solubility Coefficients for Common Gases in Typical Materials (in units of
cm^(STP)/cm^
per torr)
Material
Iron (a)
Neoprene G
Neoprene G
Neoprene G
Neoprene
Perbunan
Perbunan
Perbunan
Pyrex
Rubber (natural)
Rubber (natural)
Gas
H2
H2
N2
02
A
H2
N2
O2
He
N2
O2
Temp.
°C
300
25
25
25
36
25
25
25
500
25
25
s„^
2.9 X 10-^
3.8 X 10-5
4.7 X 10-5
9.9 X 10-5
2.0 X 10-^
3.7 X 10-5
4.6 X 10-5
1.0 X lO""*
1.1 X 10-5
6.8 X 10-5
1.3 X 10-^
Ref.
No.
[3]
[2]
[2]
[2]
[1]
[2]
[2]
[2]
[1]
[2]
[2]
References
1.
R. M. Barrer,
Diffusion
in and
Through
Solids (Cambridge University Press, London, 1941).
2.
G. J. van Amerongen,
J.
AppL
Phys., 17 (1946) 972.
3.
C. J. Smithells (ed.), "Metals Reference Book," 5th ed. (Butterworths, London, 1976).

4.7.5 The Outgassing Rate of Metals and Ceramics 501
4.7.4.2 Experimental Data on Outgassing
of Elastomers and Plastics
Measured values of the outgassing rate
K„ji
and the exponent a
j
after one hour of
pumping are listed in Table 2 for common elastomers and plastics. Also listed are
the area A,„ (in cm^) of the outgassing material and the net pumping speed, 5a, for
air at 25 °C in the chamber containing the outgassing material. Although the ratio
SJA,n was smaller than 0.01 in some experiments, the values of
K,„i
can be con-
sidered to be the free outgassing rate because the sorption coefficient for solution
of gas in the bulk is sufficiently low, as noted earlier.
Data are also available on the percentage of different gases evolved obtained by
mass spectrometer measurement for some materials [10,11], but in general the
main constituent is water vapor, together with some nitrogen, oxygen, and carbon
dioxide, when the material has been exposed for some time to the atmosphere and
not baked under
vacuum.
Carbon monoxide sometimes shows up in the mass spec-
trometer, but this may be due to interaction of H2O with carbon-contaminated hot
filaments in the gauges [12].
4.7.5
THE OUTGASSING RATE OF METALS AND CERAMICS
4.7.5.1 Experimental Data on Outgassing
of Metals and Ceramics
Measured values of the outgassing rate
K^^i
and the exponent a
j
after one hour of
pumping are listed in Table 7 for conunon metals and ceramics. Also listed is the
area
A,„
(in cm^) of the outgassing material and the net pumping speed, 5a, for air
at 25 °C (usually limited by an orifice) in the test chamber containing the out-
gassing material. Since the outgassing of unbaked metals is mainly due to evolu-
tion of water vapor from the oxide layer on the surface, and the sorption probabil-
ity for water vapor in the oxide layer in general varies from about
10 ""^
to 10"^
depending on the fraction of adsorption sites occupied and surface heterogeneity,
the listed values of
K^ni
cannot be considered as free outgassing rate constants but
may depend on the ratio A,„/5w, where 5^ is the net pumping speed for water va-
por at 25°C, as well as the partial pressure,
p^.,
of water vapor in the atmosphere
and the time of exposure, fg, before pumpdown.
Table 1 gives reported outgassing rates at one hour, K,n\, for aluminum alloy
and stainless steel for various values of A JS^, p^ and t^ as well as the free out-
gassing rate (where readsorption would be zero) calculated from the semiempiri-
