Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


2.7.4 Getter Pumps 247
to the use of the titanium sphere is that it must be kept at an elevated temperature
at all times, and this heats up the chamber walls, increasing the rate of outgassing.
SUBLIMATION BY ELECTRON BOMBARDMENT HEATING
For very-high-capacity pumping, titanium evaporation using an electron beam
evaporator can be useful.
2.7.4.3 Control Units for Sublimation Pumps
The simplest form of power supply for a wire or sphere sublimator is simply an
adjustable power source operated from a timer circuit; the time between heating
cycles, and the heating time, can be adjusted to provide an average pumping
speed. This type of regulation does not provide a fixed pumping speed, so that the
pressure in a system slowly rises between periods of sublimation, and in addition,
there is invariably a small pressure spike whenever sublimation starts. It is a triv-
ial matter at pressures below 10"^ torr, because the amount of titanium required
to sustain pumping is often only
1
or 2 grams per year, and the time between sub-
limation periods is so long as to provide a relatively stable pressure environment
for experimentation or process work. However, if a large throughput of gas is
present, then a high rate of sublimation will be needed, and this requires more
careful control to sublime only enough titanium to maintain the required pressure.
Although this process can be accomplished manually, it is far more efficient to
employ a feedback control, with a pressure gauge as the sensing element, com-
bined with careful selection of the periodicity, time, and temperature of sublima-
tion. Under the best of circumstances, the control of a sublimation pump is some-
what crude, as compared to the inherent self-regulation of a sputter-ion pump.
2.7.4.4 Nonevaporable Getter Pump (NEG)
The nonevaporable or bulk getter removes gas by chemical interaction in pre-
cisely the same way as a sublimation pump, but pumping is sustained by the pro-
cess of diffusion into the bulk of the getter material, rather than by the deposition
of fresh getter material. A NEG is often operated at elevated temperature, which
accelerates the rate at which chemisorbed surface species diffuse into the bulk of
the getter. The pumping speed is determined by the rate of diffusion away from the
surface. However, the speed decreases as the concentration of pumped gas in the
solid increases, becoming very slow indeed as saturation is approached.
The early application of bulk getters was largely restricted to use in sealed-
off vacuum devices; an excellent account of these early applications is given by

248 Chapter 2.7: Pumps for Ultra-High Vacuum Applications
Reimann [35]. Titanium and zirconium were frequently selected, using a simple
electrically heated filament mounted in the device, and operated at temperatures
in excess of 800°C, to provide a high rate of gettering for gases such as oxygen,
nitrogen, and the carbon oxides [36,37]. Gettering of these gases produces com-
pounds so stable that they are not decomposed even at temperatures where the
filaments melt. Because the rate of pumping is limited by the rate of diffusion, a
higher speed for most gases except hydrogen is achieved simply by increasing the
temperature, limited only by the danger of filament failure by sagging or melting.
The problem with this approach is that the optimum temperature for pumping hy-
drogen (including that present in water vapor) is 400°C or less [36,37] At higher
temperatures, the dissociation pressure of hydrogen in the metals increases. If the
hydrogen partial pressure in the system is high, the pumping of hydrogen contin-
ues,
but the ultimate pressure to which hydrogen can be pumped is limited by the
dissociation pressure. At 400°C more reactive gases preferentially adsorb on the
surface, displacing most hydrogen already there, and these adsorbed gases only
slowly diffuse into the bulk. Consequently, when using a pure metal such as tita-
nium or zirconium, the pumping of hydrogen —
a
dominant gas in the residual at-
mosphere of many devices—is extremely limited in the presence of most other
reactive gases.
The widespread practical use of NEGs can be traced back to the innovative
work of della Porta and his colleagues [38] in developing an alloy of zirconium,
84%
Zr-16% Al, designated St
101.
This alloy has the typical gettering capabili-
ties of zirconium for reactive gases, but with the advantage of significantly lower
gettering temperatures. A second major advantage is that hydrogen is gettered
even in the presence of the more reactive gases. With the commercialization of
this development, NEGs are available that can simultaneously pump all the prin-
cipal, chemically reactive residual gases in a vacuum system when operated at
~400°C. The alloy is produced as a fine powder that is coated, without any binder,
on a substrate [38]. The total quantity of getter is limited, so that the practical ap-
plications are for relatively low throughput conditions.
When a NEG is exposed to the atmosphere, during installation in a system, it
is covered with an adsorbed layer of
gas,
making it unusable for gettering; it must
be activated by heating to a temperature in the 500 to 800°C range, under vac-
uum, to allow the surface layer to diffuse into the bulk. The efficiency of activa-
tion increases with temperature and time. Since the gettering occurs even during
the activation, reducing the amount of getter available for subsequent pumping,
the pressure is kept as low as possible during activation. The published specifi-
cations for a particular getter pump using this material, quote an initial pumping
speed for nitrogen of —110 liters/sec, when operating at 400''C, and a practical
pumping capacity of ~25 torr liter before reactivation is required. Reactivation
by heating to ~800°C allows diffusion of the near-surface sorbed gas into the
bulk, restoring the getter to nearly its initial performance. Reactivation can be re-

2.7.4 Getter Pumps 249
peated, giving a total useful pumping capacity of -^255 torr liter,
^
but the perfor-
mance slowly degrades with each successive activation. This getter is particularly
useful for pumping hydrogen and its isotopes, providing pumping speeds a factor
of
5
higher than for nitrogen. Hydrogen pumping is effective even at ambient tem-
perature (but at reduced speed), so long as the partial pressures of gases such as
oxygen remain low.
Following the success of the original zirconium/aluminum getter alloy, NEGs
have been developed that permit the pumping of all common gases, at still lower
temperatures, and useful performance can now be achieved at ambient tempera-
ture.
NEGs having a highly porous structure are very effective at low tempera-
tures,
providing a very large surface area accessible to the gas phase to provide
sustained pumping. The large area compensates for the fact that the rate of diffu-
sion into the bulk is limited at low temperature. These NEGs can be reactivated
by periodically heating to a higher temperature, accelerating diffusion into the
bulk, and freeing the surface for additional gettering. One main advantage of room
temperature operation is obviously the absence of any power input during opera-
tion. One important NEG is an alloy of zirconium, vanadium, and iron, desig-
nated St 707 [39]. The temperature for full activation of these getters can be as
low as 400-500*^C, and even lower temperatures can still provide substantial get-
tering potential. An St 707 getter, capable of pumping nitrogen at approximately
the same speed as the St 101 material just described, would have a practical pump-
ing capacity roughly four to five times greater.
The ability to operate at ambient temperature is important in many large and
complex systems designed to operate well below
10"^^^
torr; in many systems
only mild degassing temperatures can be used, the components being degassed
before assembly; the absence of any heating of the getters minimizes the system
operating temperature, and helps keep the rate of degassing down to acceptable
levels.
For use at higher throughputs, NEGs are also manufactured as self-supported
porous structures, providing greatly increased quantities of accessible getter
material.
2.7.45 Practical Application of NEGS
NEGs are used in a wide range of applications, including small dewars, sealed-off
electronic devices, metal halide discharge lights, and large particle accelerators.
They are particularly valuable for their ability to pump hydrogen, and so have
"^To understand the limited capacity of this getter, compare this performance to that claimed for a
sputter-ion pump, rated at 60,000 hours life at 1 X 10"^ torr, which implies a pumping capacity of
2 X
10**
torr liters.
250
Chapter 2.7: Pumps for Ultra-High Vacuum Applications
been used in fusion test
reactors.
They have also proved successful in particle ac-
celerators, which have very long vacuum chambers and operate at very low pres-
sures,
providing a distributed pumping system that operates at ambient tempera-
ture,
and has no moving parts.
NEGs are available
as
self-contained pumping
units,
fitted
with integral heaters
that range from tiny pellets with dimensions of the order of millimeters, having
pumping speeds of a liter/sec or less, to pumps with speeds up to 750 liter/sec.
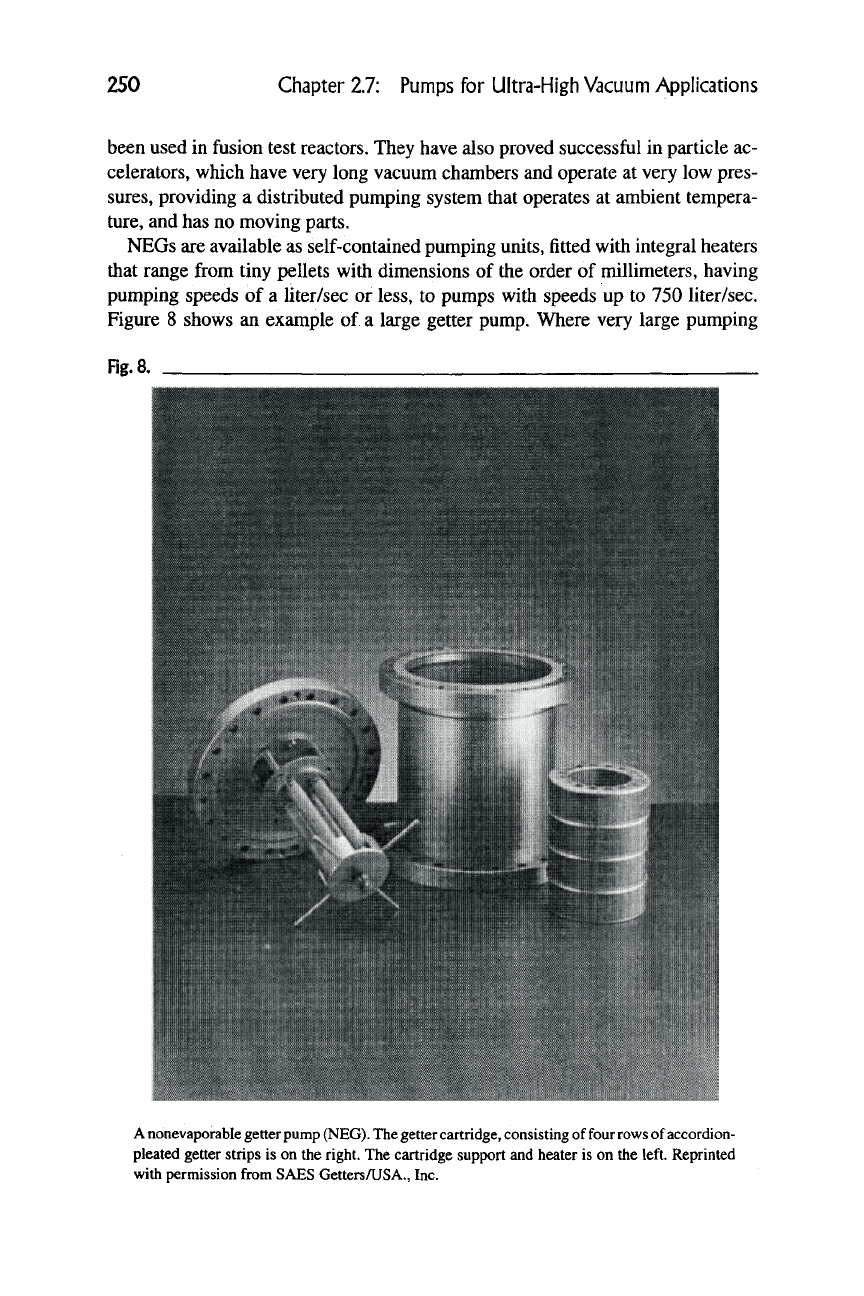
Figure 8 shows an example of a large getter pump. Where very large pumping
Fig.
a
A nonevaporable getter
pump
(NEG).
The
getter
cartridge,
consisting of four rows of accordion-
pleated getter strips is on the right. The cartridge support and heater is on the left. Reprinted
with permission from SAES GettersAJSA., Inc.

2.7.4 Getter Pumps 251
Hg.9.
Getter modules with integral heaters, for mounting inside the vacuum chamber. Reprinted with
permission from SAES Getters/USA., Inc.
speeds are required, they are available as modules, such as those shown in Fig-
ure 9, which can be mounted inside the vacuum chamber so as to provide pump-
ing close to the source of the gas load; in this function they have been used in
place of cryogenic panels, to provide very high total pumping speeds.
A NEG can only pump reactive gases, so that supplementary pumping must be
provided to remove noble gases, or methane. A positive characteristic is that they
do not contribute any significant impurities in most applications.
The efifective pumping speed is very dependent on the operating temperature
and on the particular gas species to be pumped. Where hydrogen or its isotopes
are the principal gas load, high pumping speeds can be maintained at ambient tem-
perature, and large amounts of gas can be pumped. For such applications, the life
of
the
getter is limited by embrittlement of
the
getter, rather than by the saturation.
Operation at elevated temperature provides more sustained pumping for all
gases.
Conversely, operating porous getters at low temperatures may require peri-
odic heating to reactivate the getter by allowing the pumped gas to diffuse from
the near-surface region into the bulk getter. The speed of pumping of all getters
decreases with the quantity of
gas
that has been pumped, so careful attention must
be paid to the effect of this decline for each application.

252 Chapter 2.7: Pumps for Ultra-High Vacuum Applications
The combination of a NEG with a sputter-ion pump provides a significant
pumping speed for hydrogen, which is particularly advantageous for very-low-
pressure applications, and is also a very satisfactory method of pumping large
quantities of hydrogen. The NEG is built into the envelope of some sputter-ion
pumps, but a separate NEG unit can be combined with any sputter-ion pump to
provide the same advantages. Hydrogen is reversibly sorbed by all getter materi-
als,
and hence the getter can be regenerated by heating, while using a supplemen-
tary pumping
system.
The
regeneration feature
makes NEG
pumping of hydrogen,
and its isotopes, very useful even where large quantities of
gas
must be pumped.
For such cases, self-supporting thicker cross sections of getter are preferable to
those forms in which the active material is coated as a thin layer on a substrate.
Note that NEG pumps, like sublimation pumps, have limited capability for
pumping very large quantities of gas, especially as compared to the essentially
unlimited capacity of
a
turbomolecular or diffusion pump.
Because the getter material in a NEG is only used up as gas is pumped, the
pumps are self-regulating, in the same way as a sputter-ion pump. This places
the NEG at a distinct advantage to the sublimation pump, where optimum life of
the pump requires careful management of the rate of sublimation.
REFERENCES
1.
D. Alpert,
Handbuch
der Phys., 12, (1958) 609.
2.
L. D. Hall,
Rev.
Set
Instr.
29, (1968) 367.
3.
K. M. Welch, Capture
Pumping Technology
(Pergamon Press, NY. 1991).
4.
W. J. Lange, /.
Vac.
ScL
Technoi 7, (1969) 228.
5. F. M. Penning, Physica 4, (1937) 71.
6. W. M. Brubaker,
Trans.
6th
Nat.
Vac.
Symp. (1959) (Pergamon Press, New York, 1960), p. 302.
7. T Tom and B. D. James,
J.
Vac.
Sci. Technoi, 6, (1969) 304.
8. R. L. Jepsen, Proc. 4th
Int.
Vac.
Congress (1968) (Institute of Physics and the Physical Society,
London, 1968), p. 317.
9. J. A. Vaumoron and M. P DeBiasio,
Vacuum
20, (1970) 109.
10.
A. R. Hamilton, Trans. 2nd
Int.
Cong, and 8th Nat.
AVS
Symp. (Pergamon Press, New York,
1962),
p. 388.
11.
M. Pierini and L. Dolcino, /
Vac.
Sci.
Technoi.
Al, (1983) 140.
12.
J. H. Singleton,
J.
Vac.
Sci.
Technoi.
6, (1969) 316.
13.
S. L. Rutherford and R. L. Jepsen,
Rev.
Sci.
Instr.,
32, (1961) 1144.
14.
J. H. Singleton,
J.
Vac.
Sci. Technoi, 8, (1971) 275.
15.
R della Porta and B. Ferrario,
Vuoto
1, (1968) 2.
16.
Thomas Snouse,
J.
Vac.
Sci. Technoi, 8, (1971) 283.
17.
R.
L.
Jepsen,
Cooling Apparatus for
Cathode
Getter
Pumps,
U.S. Patent 3,331,975, July 16,1967.
18.
J. E. Kelly and T. A. Vanderslice,
Vacuum,
11, (1961) 205.
19.
David Lichtman,
J.
Vac.
ScL
Technoi, 1, (1964) 23.
20.
A. K. Gupta and J. H. Leek,
Vacuum
25, (1975) 362.
21.
J. H. Singleton, unpublished measurements.

References 253
22.
R. Zaphiropoulos and W. A. Lloyd, Proc. 6th Nat AVS
Vac.
Symp, 1969 (Pergamon Press, New
York, 1960), p. 307.
23.
M. Pierini and L. Dolcino, J.
Vac.
Sci. x Technoi, Al, (1983) 140.
24.
Donald J. Santeler, private communication. Formerly at Process Applications, Inc., Oak Ridge,
Tennessee.
25.
ULTEK Bulletin SIB No. 200-9A, September 10, 1968.
26.
D. R. Denison, J.
Vac.
Sci.
TeclinoL,
4, (1967) 156.
27.
R A. Redhead, J. R Hobson, and E. V. Kornelsen, The Physical Basis of Ultrahigh Vacuum
(Chapman and Hall, London, 1968), pp. 209-216.
28.
M. V. Kuznetsov, A. S. Nasarov, and G. F. Ivanovsky, J.
Vac.
Sci. Technol. 6, (1969) 34.
29.
D. R. Denison, Proc. 4th. Int.
Vac.
Cong. (Institute of Physics and the Physical Society, London,
1968),
p. 377.
30.
D. J. Harra, J.
Vac.
Sci. Technol. 13, (1976) 471.
31.
R. Steinberg and D. L. Alger, J.
Vac.
Sci. Technol. 10, (1973) 246.
32.
G. M. McCracken and M. A. Pashley, J.
Vac.
Sci. Technol. 3, (1966) 96.
33.
R. W. Lawson and J. W. Woodward, Vacuum 17, (1967) 205.
34.
D. J. Harra and T. W. Snouse, J.
Vac.
Sci. Technol. 9, (1972) 552.
35.
Arnold L. Reimann, Vacuum Technique (Chapman and Hall, London, 1952).
36.
V. L. Stout and M. D. Gibbons, J. Appl. Phys. 26, (1955) 1488.
37.
J. H. Singleton, Residual Gases in Electron Tubes (Academic Press, London and New York,
1972),
p. 213.
38.
R della Porta, T. Giorgi, S. Origlio, and F Ricca, Trans. 2nd
Int.
Cong, and 8th Nat. AVS Symp.
(Pergamon Press, New York, 1962), p. 229.
39.
C. Pisani and R della Porta, Suppl. Nuovo Cimento 5, (1967) 261.
CHAPTER 3.1
The Measurement
of Low Pressures
Ron Goehner
Electron
Technology Division
Emil Drubetsky
The
Televac Division
Howard M. Brady
Electron
Technology Division
William
H.BaylesJr.
The
Televac Division
Electron
Technology and Televac are Divisions
of
the Fredericks Company
Measurements at or below atmospheric require knowledge of the expected pres-
sure range and the accuracy and/or repeatability required by the processes taking
place in the vacuum chamber.
Table
1
gives an indication of the pressure categories often encountered in vac-
uum applications. It is useful to remember that
1
gram molecular mass of any gas
occupies 22.4 liters of volume at a standard temperature of 0° C and
1
atmosphere
pressure. Also, 1 gram molecular mass contains 6.02 X 10^^ molecules. Thus at
this standard temperature and pressure 1 cm-^ of any gas will contain 2.5 X 10^^
molecules. At reduced pressure, there will be proportionally fewer molecules.
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
525.00 y^ll rights of reproduction in any form reserved.
257
258
Chapter
3.1:
The Measurement of Low Pressures
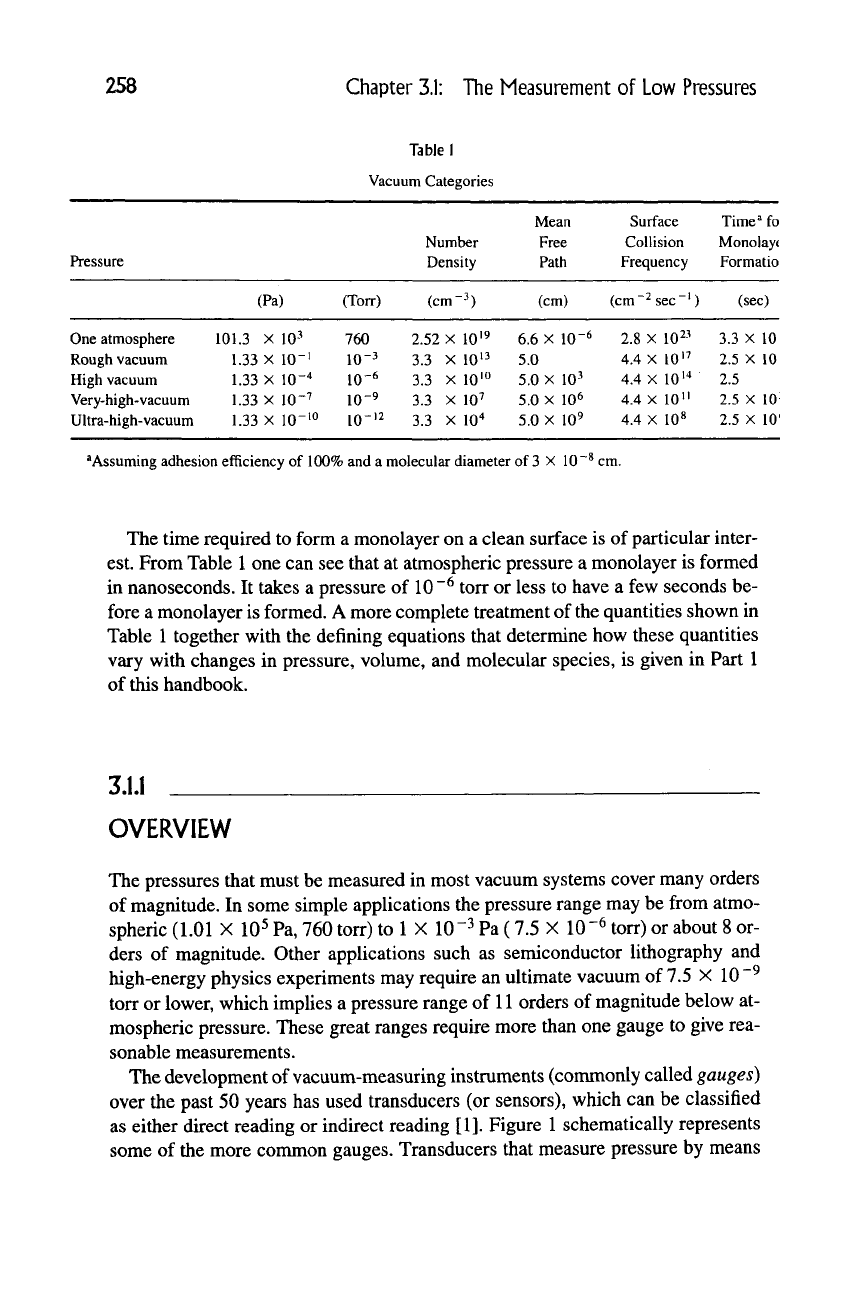
Table I
Vacuum Categories
Pressure
Number
Density
Mean
Free
Path
Surface
Collision
Frequency
Time'* fo
Monolayt
Formatio
(Pa)
(Torr)
(cm-3)
(cm) (cm
^
sec ')
(sec)
One atmosphere
Rough vacuum
High vacuum
Very-high-vacuum
Ultra-high-vacuum
101.3 X 10-^
1.33 X 10"'
1.33 X 10-^
1.33 X 10-7
1.33 X lO-'o
760
10-3
10-6
10-9
10-'2
2.52 X 10'9
3.3 X 10^3
3.3 X 10'"
3.3 X 10^
3.3 X lO'^
6.6 X 10-6
5.0
5.0
X
10^
5.0 X 10^
5.0 X 10^
2.8 X 10^-^
4.4
X
10'7
4.4 X 10'^
4.4 X 10"
4.4 X 10«
3.3 X 10
2.5 X 10
2.5
2.5 X 10
2.5 X 10'
^Assuming adhesion efficiency of 100% and a molecular diameter of
3
X 10
^
cm.
The time required to form a monolayer on a clean surface is of particular inter-
est. From Table
1
one can see that at atmospheric pressure a monolayer is formed
in nanoseconds. It takes a pressure of 10"^ torr or less to have a few seconds be-
fore a monolayer is formed. A more complete treatment of
the
quantities shown in
Table 1 together with the defining equations that determine how these quantities
vary with changes in pressure, volume, and molecular species, is given in Part 1
of this handbook.
3.1.1
OVERVIEW
The pressures that must be measured in most vacuum systems cover many orders
of magnitude. In some simple applications the pressure range may be from atmo-
spheric (1.01 X 10^ Pa, 760 torr) to
1
X
10
"^ Pa (7.5 X 10'^ torr) or about 8 or-
ders of magnitude. Other applications such as semiconductor lithography and
high-energy physics experiments may require an ultimate vacuum of 7.5 X 10"^
torr or lower, which implies a pressure range of
11
orders of magnitude below at-
mospheric pressure. These great ranges require more than one gauge to give rea-
sonable measurements.
The development of vacuum-measuring instruments (commonly called gauges)
over the past 50 years has used transducers (or sensors), which can be classified
as either direct reading or indirect reading [1]. Figure 1 schematically represents
some of the more common gauges. Transducers that measure pressure by means
3.I.I
Overview
259
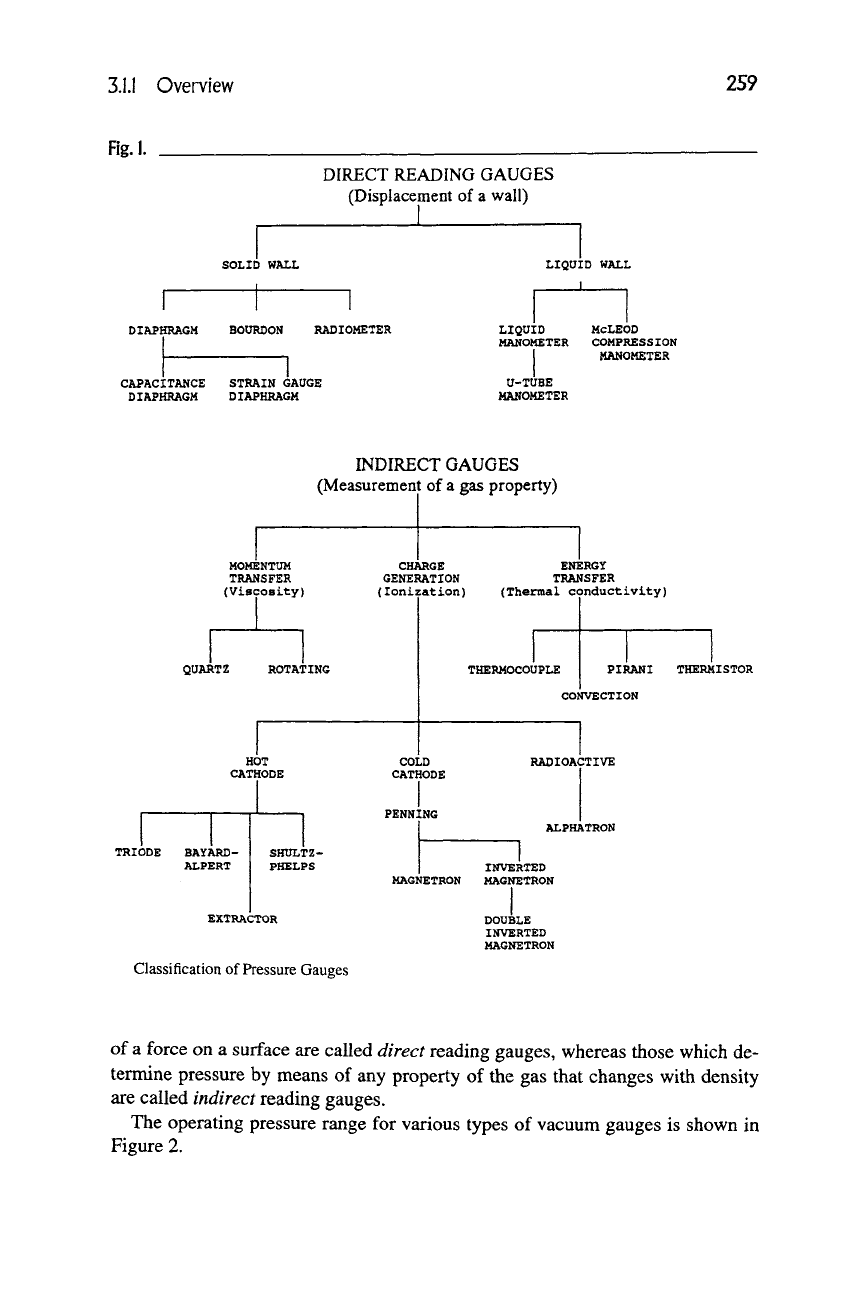
Fig.l.
DIRECT READING GAUGES
(Displacement of a wall)
SOLID WALL
DIAPHRAGM BOURDON RADIOMETER
CAPACITANCE STRAIN GAUGE
DIAPHRAGM DIAPHRAGM
LIQUID WALL
1
LIQUID
MANOMETER
1
U-TUBE
MANOMETER
McLEOD
COMPRESSION
MANOMETER
INDIRECT GAUGES
(Measurement of
a
gas property)
MOMENTUM
TRANSFER
(Viscosity)
QUARTZ
HOT
CATHODE
TRIODE BAYARD-
ALPERT
SHULTZ-
PHELPS
EXTRACTOR
Classification of Pressure Gauges
CHARGE
GENERATION
(Ionization)
ENERGY
TRANSFER
(Thermal conductivity)
THERMOCOUPLE | PIRANI THERMISTOR
CONVECTION
COLD
CATHODE
PENNING
RADIOACTIVE
ALPHATRON
INVERTED
MAGNETRON MAGNETRON
DOUBLE
INVERTED
MAGNETRON
of a force on a surface are called direct reading gauges, whereas those which de-
termine pressure by means of any property of the gas that changes with density
are called indirect reading gauges.
The operating pressure range for various types of vacuum gauges is shown in
Figure 2.
