Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.

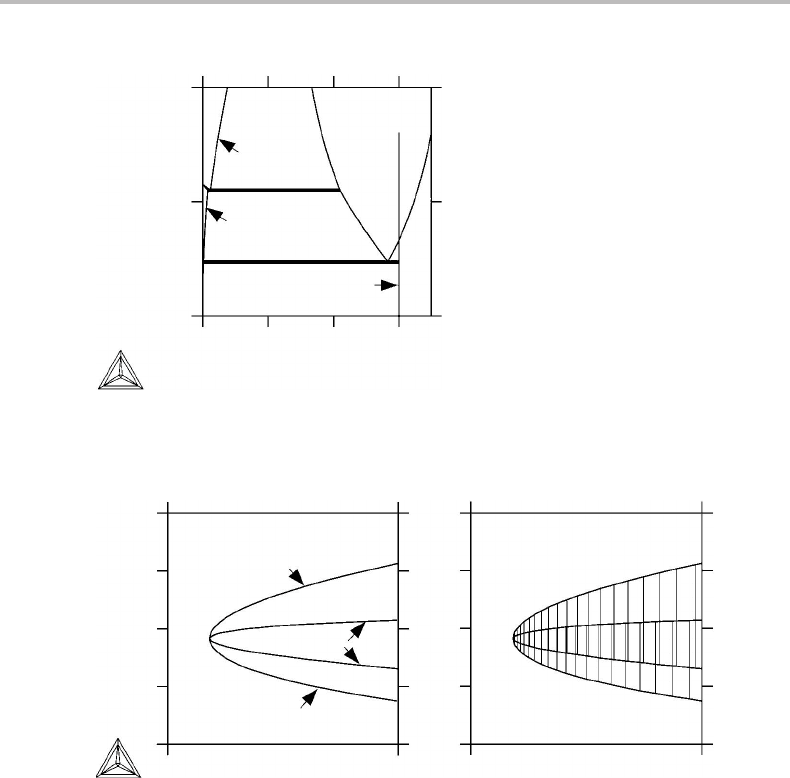
10.7 Selection of axes in mixed diagrams 225
1500
1000
500
0 0.2 0.4 0.6
fcc
bcc
x
Cr
M
3
C
2
M
7
C
3
T (K)
Figure 10.23 T , x
C
diagram for Fe–Cr–C at 1 bar and a
C
= 0.3. This is not a true phase diagram.
6
5
4
3
2
6
5
4
3
2
1.02 1.06
1.02 1.06
a
C
a
C
H (kJ/mol Fe)
fcc
cem
fcc
(a) (b)
Figure 10.24 H
m1
, a
C
diagram for Fe–C at 1 bar. This is not a true phase diagram as revealed by
the overlapping two-phase fields, shown when the tie-lines are included in (b).
conjugate variables. That is revealed by the tie-lines which are included in Fig. 10.24(b).
The reason is that the numerical values used for H
m1
refer to reference states of Fe and
Cat298Kbuta
C
refers to graphite at the actual temperatures. It is evident that one
should also be careful when representing the oxygen potential with P
O
2
.Itisonly under
isothermal conditions that it should be combined with an axis for a molar quantity given
relative to references at 298 K. It should be noted that the diagrams in Figs 8.13 and
8.14 used µ
C
−
o
G
gr
C
or (µ
C
−
o
G
gr
C
)/T as an axis and
o
G
gr
C
was defined at the actual
temperature which varied. That did not cause any problem because there was no molar
axis in those diagrams.
Exercise 10.10
Figure 10.23 showed an incorrect selection of axes. If one really wanted to section at a
constant value of a
C
,what composition axis should one have used?

226 Projected and mixed phase diagrams
Hint
Consult the Tables 9.1, 9.2 and 9.3.
Solution
a
C
represents µ
C
/T which may be combined with −1/ T , −P, and z
Cr
(according to
fifth row in Table 9.2)oru
Cr
(according to fifth row in Table 9.3). Of course, −1/T could
be replaced by T.
10.8 Konovalov’s rule
The rule that two one-phase fields are separated from each other by a positive distance,
when the proper molar quantity is introduced instead of a potential, was described in
Section 9.1. That rule is not as trivial as it may appear. It was discovered experimentally
by Konovalov [20]when measuring the vapour pressure of liquid solutions of water and
various organic substances under isothermal conditions. He established that, compared
with the solution, the vapour contains a higher relative content of that component which,
when added to the solution, increases the total vapour pressure. In addition, he found
two cases with a pressure maximum and realized that the liquid and vapour must have
the same composition at such a point. A case of this type is shown in Fig. 10.25, and it
is evident that it is simply due to the fact that the molar quantity which is used, here z
B
,
replaces a potential whose axis happens to be parallel to a line tangential to the linear two-
phase field in the potential diagram. Except for that, the system has no unique properties
at this point. The point is sometimes called a singular point and the equilibrium under
this special condition is called singular equilibrium.
Figure 10.26(a) shows a three-dimensional diagram for the same kind of system but
including both temperature and pressure axes. It was presented in Fig. 8.23 and it was
then concluded that an extremum in P at constant T must lead to an extremum in T at
constant P. The corresponding diagram, where z
B
has been introduced instead of µ
B
,is
shown in Fig. 10.26(b) and it confirms that the two phases have the same composition at
the point of extremum considered. In fact, there is a whole series of such points, marked
as a dotted line. This is the locus of points of tangency for tangents parallel to the µ
B
or z
B
axis. That line represents a singular equilibrium and could be included in the T, P
diagram, obtained by projecting in the µ
B
(i.e. z
B
) direction, Schreinemakers’ projection.
The line representing singular equilibrium is called a singular curve. Singular equilibria
will be further discussed in Sections 12.6 and 13.7 to 13.9.
A major difference between univariant lines and singular curves should be noted. A
univariant line shows exactly where a particular univariant equilibrium occurs. A singular
curve shows the maximum extension of a divariant equilibrium which is otherwise not
shown in the diagram. It would thus be wise to indicate on what side of a singular curve
the particular equilibrium exists. This is done in Fig. 10.27,which is a projection of
Fig. 10.26.
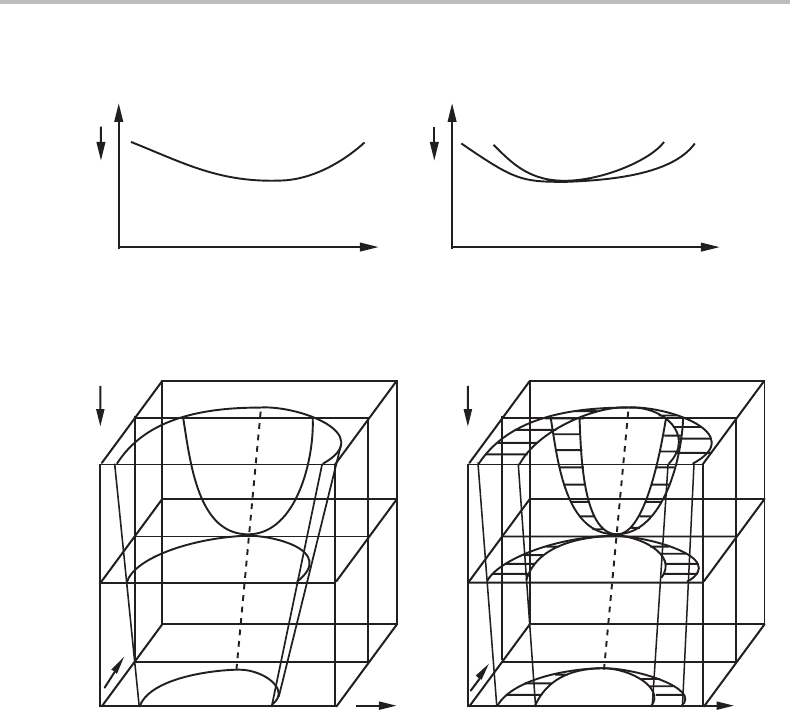
10.8 Konovalov’s rule 227
z
B
P
vapour
vapour
liquid liquid
P
µ
B
(a) (b)
Figure 10.25 An isothermal section of a binary diagram with a singular point for two phases
illustrating Konovalov’s rule.
P
T
µ
B
α
α
β
P
T
α
α
β
z
B
(a) (b)
Figure 10.26 (a) A two-phase equilibrium in a binary system illustrated with the complete
three-dimensional potential phase diagram. Points of tangency for lines parallel to the µ
B
axis
are marked with a dotted line. (b) After µ
B
has been replaced by its conjugate molar quantity,
z
B
, the phases still coincide along the dotted line where the two phases have the same
composition, expressed through z
B
.
Points of extremum in P and T were discussed in Section 8.9 and Konovalov’s rule was
actually derived there in an analytical way, using the ordinary molar quantities, S
m
, V
m
and x
i
.InChapter 8 and the present one we have mainly used molar quantities defined by
dividing the integral quantities with the content of a certain component, N
A
for instance.
We denote these quantities with S
m1
, V
m1
and z
i
.However, if all the molar quantities we
are interested in are molar contents, then the results look the same in both notations. As
an example, the insertion of x
i
= x
1
z
i
in the result for p = c in Section 8.9 yields
0 =
1 x
β
2
..x
ε
c
=
x
α
1
x
β
2
..x
ε
c
=
1 z
β
2
..z
ε
c
· x
α
1
x
β
1
...x
ε
1
(10.10)
0 =
1 z
β
2
..z
ε
c
. (10.11)
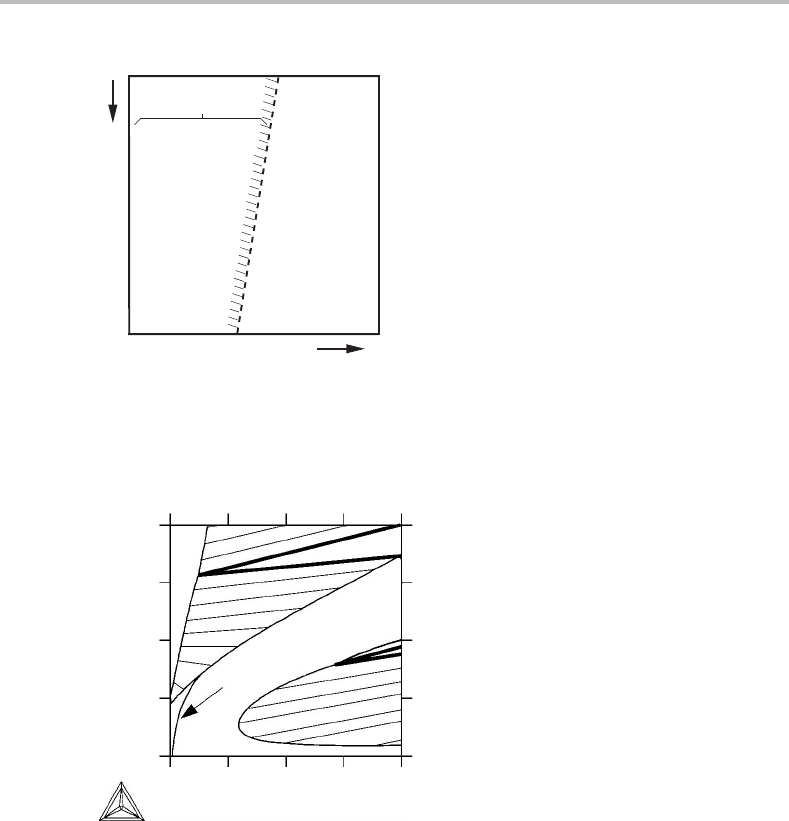
228 Projected and mixed phase diagrams
P
α
β
T
α+β
Figure 10.27 Singular curve showing the maximum extension of the α + β equilibrium in
Fig. 10.25. Projected in the z
B
direction. The α + β surface is folded and to the left of the curve
one would intersect that surface twice by moving in the projected direction, i.e., perpendicular
to the picture.
40
30
20
10
0 0.5 1.0 1.5 2.0
0
mass-% N
mass-% Cr
a
N
= 0.75
γ
α
Figure 10.28 See Exercise 10.11.
We shall continue to use z
i
but it should be remembered that the results hold for x
i
as
well.
The importance of Konovalov’s rule stems from the fact that composition is often
used as an experimental variable. A system with a composition at a point of maximum
or minimum undergoes an azeotropic or congruent transformation on passing through
it and such a point is often given a special name, azeotropic (actually meaning ‘boiling
unchanged’) or congruent.
Exercise 10.11
The phase diagram (Fig. 10.28)isanisobarothermal section at 1273 K and 1 bar of the
Fe–Cr–N phase diagram under conditions where N
2
gas does not form. An isoactivity
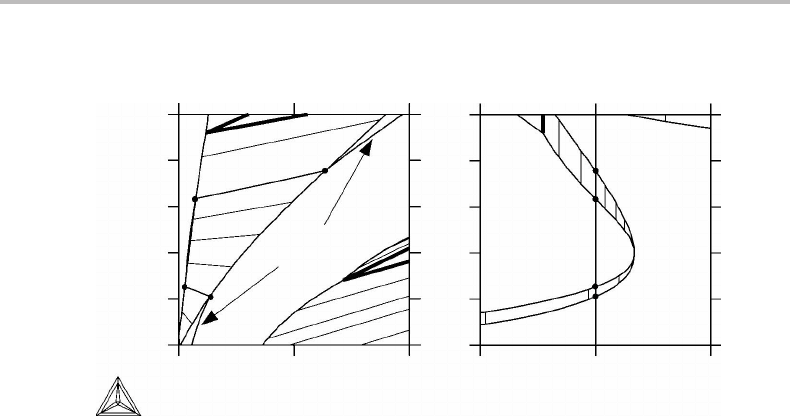
10.9 General rule for singular equilibria 229
0.35
(a) (b)
0.30
0.25
0.20
0.15
0.10
0 0.04 0.08 0.50 0.75 1.00
a
N
= 0.75
α
α
γ
γ
m
N
m
Cr
a
N
Figure 10.29 Solution to Exercise 10.11.
line for N has been drawn in the γ phase field. Show a reasonable continuation of it after
first sketching the corresponding µ
Cr
, a
N
phase diagram.
Hint
Notice that there is a tie-line for which the α and γ phases have the same Cr content. It
should be a point of extremum for the N potential (or N activity).
Solution
The solution is given in Fig. 10.29.
10.9 General rule for singular equilibria
It is evident that Konovalov’s rule does not only apply to composition. It may thus be
generalized. Suppose that a linear two-phase field in a Y
k
, Y
j
diagram, determined at
constant values of all the other potentials except Y
l
,which is chosen as the dependent
potential, shows a Y
j
maximum or minimum. At the point of extremum the two phases
must have the same value of X
k
m−1
. Furthermore, if Y
j
is kept constant and another
potential is allowed to vary, it will also have an extremum at the same value of X
k
m−1
.
Let us now consider a two-phase equilibrium in an isobaric potential diagram for a
ternary system, which is three-dimensional. Thus, p = c − 1. Suppose there is a point
of tangency for a plane parallel to the µ
B
, µ
C
plane (i.e. an isothermal plane) as shown
in Fig. 10.30(a) which is a reproduction of Fig. 8.24.Asdemonstrated in Fig. 10.30(b),
the two phases thus have the same composition and the point of extremum is a congruent
transformation point. This was already proved in Section 8.9 using an analytical method.
230 Projected and mixed phase diagrams
T
µ
C
α
β
T
z
B
µ
B
z
C
Figure 10.30 (a) Isobaric potential phase diagram of a ternary system with a doubly singular
point on a divariant phase field. Thin lines represent points of tangency for lines parallel to the
µ
B
or µ
C
axis. Their intersection is a point of tangency on a µ
B
,µ
C
plane. It gives an extremum
of T. (b) By replacing µ
B
and µ
C
with their conjugate molar quantities, z
B
and z
C
,itisshown
that the two phases in the point of T extremum must have the same z
B
and the same z
C
value.
The point of extremum thus defines the composition of an alloy which can transform
congruently between the two phases.
The point of extremum in Fig. 10.30 may be characterized as a doubly singular point.
It would also appear in a diagram with a P axis under a constant value of T equal to the
extreme value. In order to show in one diagram that this point is an extremum for P as
well as T, one would need a fourth dimension. It is evident that the doubly singular point
in Fig. 10.30 would fall at a different T value if the constant P value was different and in
a P, T projection all such points would form a line, a doubly singular curve.
From Section 8.9 it is evident that Konovalov’s rule is just a special case of a more
general rule. In fact, for the ternary case, p = c = 3, it was formulated by von Alkemade
[21]. His rule was originally formulated for a liquid which solidifies to two solid phases
and P is regarded as constant. It may be stated as follows, ‘The direction of falling
temperature of the liquid in equilibrium with two solid phases is always away from the
tie-line between the solid phases. If the liquid falls on the tie-line, then the three-phase
equilibrium is at a temperature maximum.’ Figure 10.31 illustrates von Alkemade’s rule.
It is evident that Alkemade neglected the possibility of having a temperature minimum.
The reasoning applied to Konovalov’s rule can also be applied to von Alkemade’s rule.
If T is kept constant at the extreme value and P is varied with the three phases present,
then one will find that P also has an extremum. At a different constant value of P, the
T extremum would occur at a different value. The locus of these three-phase equilibria
would also give a line in Schreinemakers’ projection, a singular curve.
From the mathematical study of conditions of extrema given in Section 8.9 it is evident
that Konovalov’s rule can be applied to two-phase equilibria and von Alkemade’s rule to
three-phase equilibria in systems with c > p, although they were originally formulated
for c = p.Konovalov’s rule: T at constant P and P at constant T have extreme values for a
10.9 General rule for singular equilibria 231
X
k
m1
X
I
m1
Y
m
L
L
α
α
β
β
Figure 10.31 Ter nary phase diagram at constant P and with two molar axes showing a
three-phase equilibrium with an extremum of T (here represented by Y
m
), illustrating von
Alkemade’s rule. The triangles are parallel to the X
k
m1
, X
l
m1
plane.
two-phase equilibrium if the two phases have the same composition, i.e. fall on the same
point; von Alkemade’s rule: T at constant P and P at constant T have extreme values for
a three-phase equilibrium if the compositions of the three phases fall on a straight line.
We can combine these cases into a general rule for singular equilibria: T at constant
P and P at constant T have extreme values for an equilibrium between p phases if their
compositions fall on a point for p = 2(Konovalov’s rule), on a line for p = 3(von
Alkemade’s rule), on a plane for p = 4, etc. In all these cases a curve representing the
locus of these equilibria can be plotted in the T , P diagram obtained by Schreinemakers’
projection. For p = c such a line is called a singular line, for p = c − 1adoubly singular
line, etc. The connection between such lines will be demonstrated in Fig. 12.15.
Finally, it maybe instructive to applythe phase field rule to the diagram in Fig. 10.25(b).
For the two-phase field liquid + vapour we get
d = c =+2 − p − n
s
+ n
m
= 2 + 2 − 2 − 1 + 1 = 2,
because we have sectioned once, n
s
= 1, by keeping temperature, which is a potential,
constant. There is one molar variable, used as axis in the P, z
B
diagram, n
m
= 1. The
result agrees with the diagram because it shows a two-dimensional phase field for the
two phases. However, if we section once more, at a constant value of z
B
, then n
s
= 2
and we get d = 2 +2 −2 − 2 + 1 = 1. The phase diagram is now just a vertical line
and in general it will show that the two-phase field extends over a range of P values in
agreement with the calculated d = 1. However, the special section, going through the
point of extremum (the singular point), will show the two phases coexisting at a point,
and one should thus have expected to obtain d = 0. It is evident that one should exercise
special care when applying the phase field rule to systems with singular points. This
problem will be discussed further in Chapter 13.
Exercise 10.12
Trytoconstruct a diagram similar to Fig. 10.31 for a case where α falls between L and
β at the maximum.
232 Projected and mixed phase diagrams
L
L
α
α
β
β
Figure 10.32 Solution to Exercise 10.12.
Solution
The solution is given in Fig. 10.32.

11
Direction of phase boundaries
11.1 Use of distribution coefficient
In this chapter we shall examine in more detail the direction of phase boundaries in molar
and mixed phase diagrams. As an introduction we shall first discuss some approximate
calculations based upon the use of the distribution coefficient of a component between
two phases but later we shall use a more general method.
In multinary systems one is often interested in the distribution of a particular com-
ponent between two phases. One may for instance define a distribution coefficient (also
called partition coefficient) which can be used to represent experimental data and to
carry out calculations of phase boundaries and changes in chemical potentials.
Let us consider the equilibrium between two solution phases, α and β,which exist
already without an element B. On adding B one finds that it partitions between the two
phases in a characteristic manner, which can be derived from the equilibrium condition
G
α
B
= G
β
B
.Byapplying a general model for a solution phase we obtain
o
G
α
B
+ RT ln x
α
B
+
E
G
α
B
=
o
G
β
B
+ RT ln x
β
B
+
E
G
β
B
, (11.1)
in which
E
G
α
B
and
E
G
β
B
represent the deviation from ideal solution behaviour. We may
define a distribution coefficient K
α/β
B
as
K
α/β
B
= x
α/β
B
/x
β/α
B
= exp
1
RT
o
G
β
B
−
o
G
α
B
+
E
G
β
B
−
E
G
α
B
. (11.2)
In many cases the distribution coefficient is relatively independent of composition. This
occurs when the composition dependence of the partial Gibbs energy of each phase is
mainly given by the RT lnx term. In such cases the distribution coefficient may be a useful
tool. As an example we may consider the case where both phases are dilute solutions in
a major component A. The excess Gibbs energy terms may then be approximated by a
regular solution parameter L and we find, for low B contents,
K
α/β
B
= exp(G
B
/RT)where G
B
=
o
G
β
B
−
o
G
α
B
+ L
β
− L
α
, (11.3)
It should be emphasized that G
B
, being a Gibbs energy, may be represented as H
B
−
T S
B
and we thus obtain
K
α/β
B
= K
o
exp(H
B
/RT), (11.4)

234 Direction of phase boundaries
in which K
o
and H
B
are often approximated as constant. When there are several minor
components, we can define a distribution coefficient for each one
x
α
j
x
β
j
≡ K
α/β
j
. (11.5)
For the major component we obtain, from G
α
A
= G
β
A
,
o
G
α
A
+ RT ln x
α
A
+
E
G
α
A
=
o
G
β
A
+ RT ln x
β
A
+
E
G
β
A
, (11.6)
but it is not useful to define a distribution coefficient for this component. Instead we can
apply another approximation if the total content of alloying elements is small,
lnx
A
= ln(1 − x
j
)
∼
=
−x
j
. (11.7)
For dilute solutions we may neglectthe excess Gibbs energy for this component, obtaining
x
β
j
−
x
α
j
=
o
G
β
A
−
o
G
α
A
RT. (11.8)
Forabinary system we thus have two equations derived from the equilibrium conditions
for the two components. For any temperature and pressure we can calculate two unknown
quantities, i.e. the compositions of the two phases. The temperature dependence of the
various parameters will give the directions of the two phase boundaries in a T, x diagram.
In an isobarothermal section of a ternary system there will be three equations and each
of the two phase boundaries will be represented by a line. With the approximations used
here we have been able to simplify all the equilibrium equations to linear equations
and the phase boundaries will thus be approximately straight lines as far as the dilute
solution approximation is valid. It is thus possible to construct the A-rich corner of a
ternary diagram from the binary diagrams by simply using a ruler. Two examples are
given in Fig. 11.1 and it should be noticed that the construction of the second one is based
upon an extrapolation of the phase boundaries in one of the binary systems to negative
compositions. This is non-physical but in accordance with the form of the mathematical
equations.
Exercise 11.1
Fe has two allotropic modifications, γ(fcc) and α(bcc). At 1423 K γ is more stable by
71 J/mol but α can be stabilized by alloying with 5 atom % Si. Estimate how much Si
is required if the alloy also contains 0.5 atom % Ni, which has a distribution coefficient
between γ and α of 1.3.
Hint
First evaluate the distribution coefficient for Si from the information.
