Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.

10.4 Coincidence of projected surfaces 215
T
P
µ
B
β
γ
(γ)
(α)
(β)
(δ)
T
P
(α)
(δ)
(a) (b)
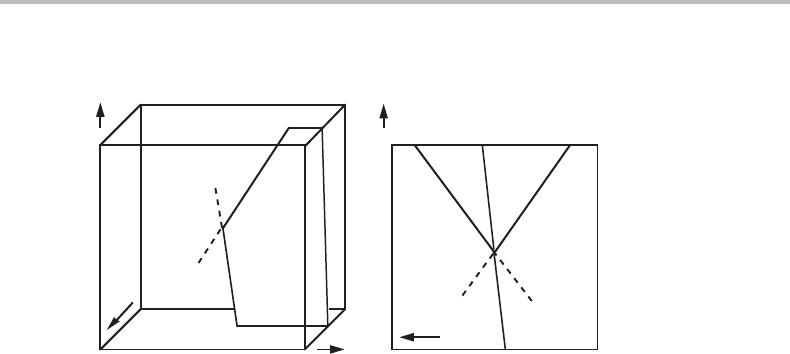
Figure 10.11 Modification of Fig. 10.8 by rotation of the β + γ surface until it is parallel to the
µ
B
axis. In the µ
B
projection the β + γ + δ(α) and α + β + γ + (δ) lines will now coincide
and their extrapolations will not be visible.
On the other hand, if predetermined amounts of C and N are added to a set of specimens
in a capsule, then one could not use Fig. 10.1(b) directly. For each specimen one should
rather consider a molar diagram like Fig. 9.4 and it is evident that the four phases could
occur in the same specimen and this could even happen in more than one specimen in
the same experiment.
10.4 Coincidence of projected surfaces
The method to determine the relative compositions of phases, now to be described, can
be used in higher-order systems as demonstrated in the next section, but a binary system
will be considered first.
Suppose one could gradually change the properties of the system in such a way that
the β + γ surface in Fig. 10.8 would rotate around an axis roughly parallel to the T axis.
One could thus make the two lines (α) and (δ)inthe projection approach each other
without changing the topology of the projected diagram. At the moment of coincidence,
one has a situation such as that illustrated in Fig. 10.11.
The β + γ surface is now parallel to the direction of projection, µ
B
, and a continued
rotation would put the β + γ surface on the other side of the (α) and (δ) lines. Thus,
β and γ would be transposed in Fig. 10.10.Itispossible to conclude that the β and γ
phases have the same value of z
B
if the µ
B
projections of the (δ) and (α) lines coincide
when they meet at the four-phase point. Evidently, before the gradual rotation the β and
γ phases must have been neighbours along the z
B
line in Fig. 10.10. When the lines
coincide, the phases fall on the same point on the z
B
axis. This rule of coincidence is
closely related to Konovalov’s rule which will be discussed in Section 10.8. The relative
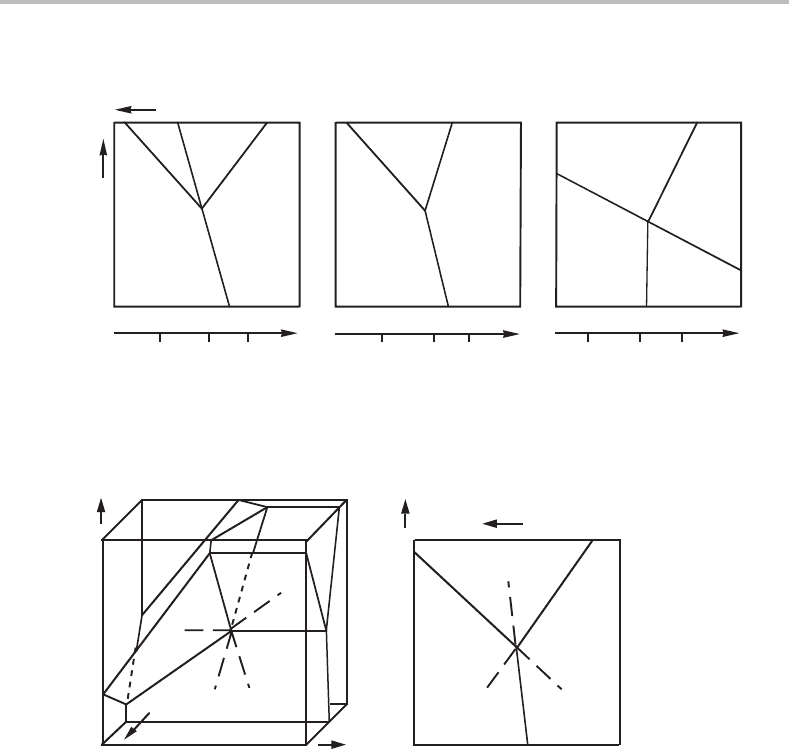
positions of the phases for the various cases of coincidence are shown in Fig. 10.12.
Other cases of coincidence may occur but not until at least one of these has occurred.
Before any rotation, the phases must have been arranged along the z
B
axis as shown in
Fig. 10.10 or in the completely reverse order.
216 Projected and mixed phase diagrams
T
P
(α)
(α)
(α)
(β)
(β)
(β)
(γ)
(γ)
(γ)
(δ)
(δ)
(δ)
αδβ,γ
α γ,δ β α,δ γ β
(a) (b) (c)
z
B
z
B
z
B
Figure 10.12 Three cases of coincidence of three-phase lines in a projected potential phase
diagram obtained by modifying Fig. 10.8.
T
P
µ
B
β
γ
δ
(γ)
(β)
(δ)
P
T
(a) (b)
Figure 10.13 (a) Modification of Fig. 10.8 by rotation of the entire diagram until the
β + γ + δ(α)isparallel to the µ
B
axis. All three surfaces, β + γ, γ + δ and δ + β, are then
parallel to the µ
B
axis. In the µ
B
projection (b), β + γ + δ(α)degenerates to a point and will
thus coincide with all the other lines without them coinciding with one another.
It is interesting to note from Fig. 10.8 that it should be possible to rotate the α + β
surface in such a way that the (γ) and (δ) lines approach each other and finally coincide.
However, the 180
◦
rule prevents this from happening before there is another coincidence.
What happens if three of the four phases β, γ and δ,have the same z
B
value will
now be investigated. The three surfaces representing β + γ, β + δ, γ + δ must all be
parallel to the µ
B
axis and the β + γ + δ(α) line must point in the µ
B
direction. It thus
degenerates to a point. This case is illustrated in Fig. 10.13.
Exercise 10.6
Prove mathematically that the compositions of β and γ must coincide when the µ
B
axis
in a binary system is parallel to the β + γ surface, as in Fig. 10.11.

10.5 Projection of higher-order invariant equilibria 217
Hint
Apply Eq. (8.47)tothe binary case.
Solution
Forabinary system we get (z
β
B
− z
γ
B
)dµ
B
=−(S
β
m1
− S
γ
m1
)dT +(V
β
m1
− V
γ
m1
)dP. When
the β + γ surface is parallel to µ
B
, then we can change µ
B
and stay on the surface
without changing T or P, i.e. with dT = 0 and dP = 0. The coefficient of dµ
B
must
thus be zero, i.e. z
β
B
= z
γ
B
.
Exercise 10.7
Suppose one has measured µ
C
as function of T at 1 bar for a ternary A–B–C system.
What conclusion could be drawn if the diagram looks like Fig. 10.11(b) with µ
C
inserted
instead of P?
Hint
Compared to Fig. 10.11 we have one component more but the dimensionality has been
reduced to the same by keeping P constant. In both cases µ
A
and µ
B
are the potentials
that are not shown in the projection, i.e. those used to reduce the number of axes by
projection from the four-dimensional fundamental property diagram.
Solution
One of µ
A
and µ
B
is the dependent variable and the final projection has been made in the
direction of the other one. From the coincidence of the (α) and (δ) lines we may conclude
that z
β
B
= z
γ
B
if µ
A
is the dependent variable and z
β
A
= z
γ
A
if µ
B
is the dependent one.
These two results are identical since z
B
= N
B
/N
A
= 1/z
A
.
10.5 Projection of higher-order invariant equilibria
The topological examination may be extended to higher-order invariant equilibria and
adjoining univariant equilibria, although the visibility is then lost. However, it has been
shown by analytical methods [17–19] that the same principles, which have been derived
here by inspection, apply. Three components will yield a four-dimensional phase diagram
and it must be projected twice in order to yield a two-dimensional picture. It may show an
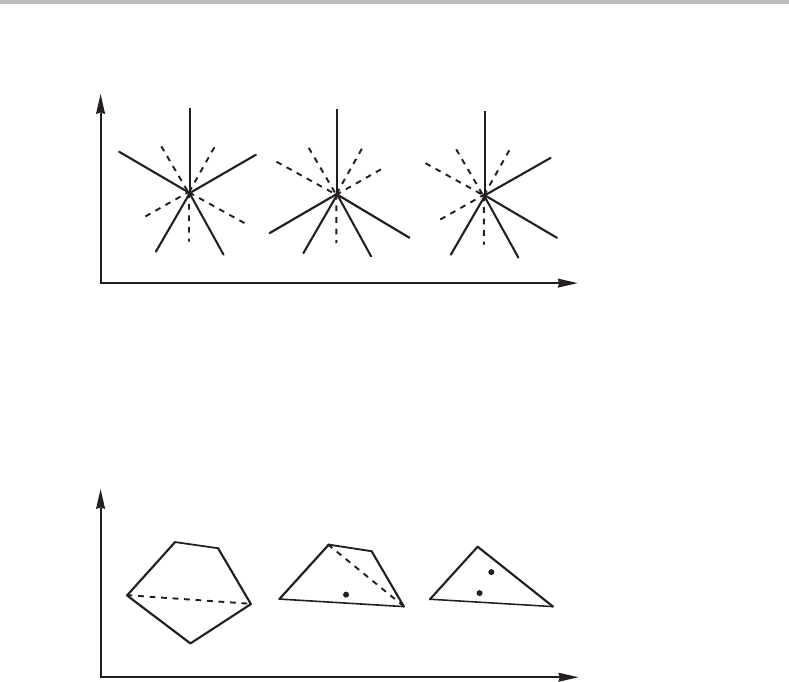
invariant five-phase equilibrium and five adjoining four-phase lines. If no lines coincide,
they can arrange themselves in three different ways, as illustrated in Fig. 10.14.
Foracloser discussion of the compositions of the phases taking part in the five-
phase equilibrium, the two potentials on the axes will be kept constant at the values of
the invariant equilibrium, while the two projected potentials will be replaced by their
218 Projected and mixed phase diagrams
(α) (α) (α)
(β)
(β)
(β)
(γ)
(γ)
(γ)
(δ)
(δ)
(δ)
(ε)
(ε)
(ε)
Y
j
Y
k
(a) (b) (c)
Figure 10.14 Possible Schreinemakers’ projections for a ternary system obtained by projecting the
Y
j
, Y
k
, Y
m
, Y
n
phase diagram in the Y
m
and Y
n
directions. Points represent invariant five-phase
equilibria. The five lines radiating from each point represent four-phase equilibria and are
identified by giving the absent phase in parentheses. Dashed lines are metastable extrapolations.
α
αα
β
β
β
γ
γ
γ
ε
ε
ε
δ
δ
X
n
m1
X
m
m1
δ
.
(a) (b) (c)
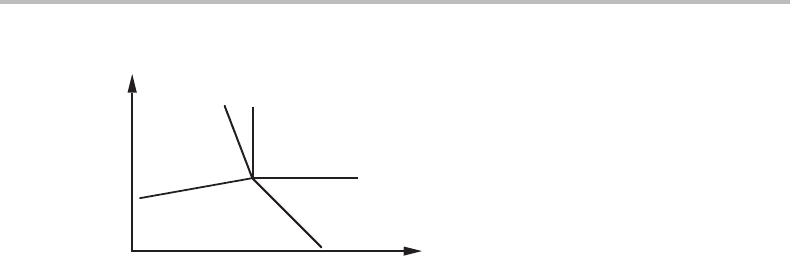
Figure 10.15 Molar phase diagrams at constant Y
j
and Y
k
showing the relative positions of the
five phases in the invariant equilibria in Fig. 10.14. The change occurring when lines (ε) and (β)
in case (a) are rotated to approach each other can be illustrated by moving point α towards the
straight line between δ and γ. Case (b) is obtained by letting the (ε) and (β) lines pass one
another, thus making point α cross the δ − γ tie-line. Case (c) is obtained by letting the (α) and
(δ) lines rotate and pass one another, whereupon ε will cross the β − γ line.
conjugate molar quantities. The five phases will fall on different points on the plane
formed by the two molar quantities, and Fig. 10.15 illustrates the arrangement of the
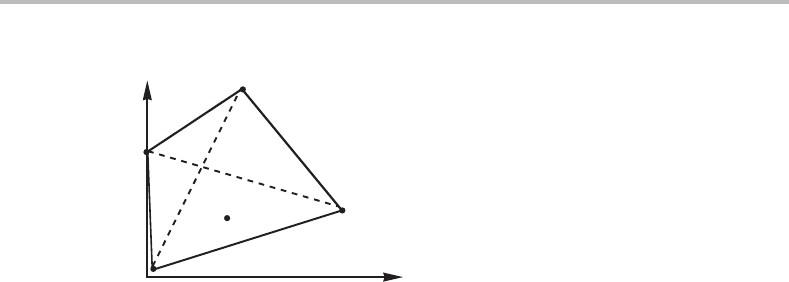
phases in the three different cases. Three phases may here be regarded as neighbours
if their points can be connected to form a triangle with no other point inside and if the
triangle can be changed into a line without any one of its points first moving inside any
other such triangle. If two lines in the projected potential phase diagram coincide, then
the three phases they have in common will fall on a straight line in the molar diagram.
Four components yield a five-dimensional phase diagram and it must be projected
three times in order to yield a two-dimensional picture. A six-phase equilibrium will
be invariant and represented by a point from which six lines will radiate, representing
univariant equilibria with one phase absent in each. The relative positions of the six lines
will reveal how the six phases are arranged in the three-dimensional compositional space
formed by three molar quantities. However, this is not easy to visualize. The rule relating
10.5 Projection of higher-order invariant equilibria 219
(L)
(w)
T
(met)
logP
O
2
(gas)
(fay)
Figure 10.16 See Exercise 10.8.
the coincidence of lines in a projected potential phase diagram to the positions of the com-
mon phases in the space formed by the molar quantities has been called the ‘coincidence
theorem’ [19]. The theorem can be generalized as demonstrated by the following exam-
ple. Suppose that two of the points, α and δ,inthe left-hand picture of Fig. 10.15 coincide.
Then δ, α and γ fall on a line, and lines (β) and (ε)inFig.10.14 should coincide. However,
β, δ and α would also fall on a line, and lines (γ) and (ε)inFig. 10.14 should also coin-
cide. As a consequence all three lines, (β), (γ) and (ε), should coincide. It is thus possible
to generalize the coincidence theorem as follows. Consider a two-dimensional projection
of an r-dimensional potential diagram. It may have an invariant equilibrium involving
r + 1 phases. From this point, r + 1 univariant equilibria, each with r phases, radiate. The
theorem concerns the positions of the phases in the (r − 2)-dimensional space formed
by the molar quantities conjugate to the projected potentials. If t of the phases fall in a
(t − 2)-dimensional section through that space, then all the univariant equilibria, which
contain the t phases, coincide in the two-dimensional projection. There would be
r + 1 − t such equilibria.
Exercise 10.8
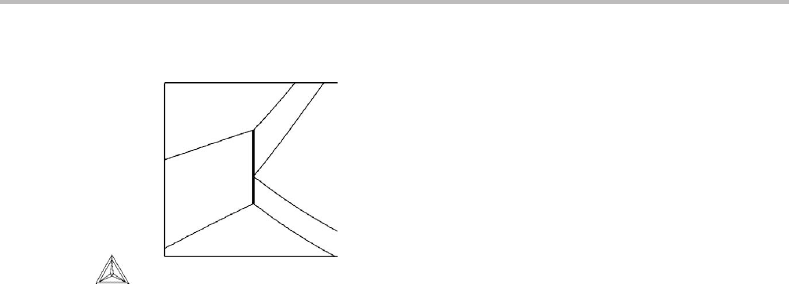
Figure 10.16 is part of the potential phase diagram for the Fe–Si–O system, showing the
five-phase equilibrium, metallic melt (met), liquid melt (L), w¨ustite (w), fayalite (fay)
and gas (gas). Use the information in the diagram to decide how the composition of L
falls relative to the other phases.
Hint
By extrapolating the lines we find that our case corresponds to case (b) in Fig. 10.14 with
L identified as α. Our diagram can be regarded as obtained by projecting the fundamental
potential diagram in the directions of P, µ
Fe
and µ
Si
, leaving T and µ
O
as axes in our
diagram. The first projection produces a complete potential phase diagram and, since
we are interested in comparing compositions, we shall regard P as subjected to the
first projection. We should thus write the Gibbs–Duhem relation as dP = (S/ V )dT +
(N
i
/V )dµ
i
. The conjugate variable to the next two potentials to be projected would
thus be N
Fe
/V and N
Si
/V . They should appear in our diagram as in Fig. 10.15(b).
220 Projected and mixed phase diagrams
met
fay
gas
W
N
Si
/
V
N
Fe
/V
L
Figure 10.17 Solution to Exercise 10.8.
Solution
Since L is identified as α,itwill have to form within the quadrangle formed by the other
four phases. Fayalite is 2FeO · SiO
2
,w¨ustite is approximately FeO and the metallic melt
is mainly Fe. The gas has a very large volume and will thus fall close to the origin in the
diagram. Since the extension of the (L) line in our potential phase diagram falls between
(gas) and (fay), corresponding to (γ) and (δ), its composition falls close to the gas +fay
join, corresponding to γ + δ join, as illustrated in Fig. 10.17.
10.6 The phase field rule and mixed diagrams
The number of axes in a complete phase diagram, whether a potential one or a molar
one, is r = c + 1. For a closed system one has fixed the composition and has actually
sectioned at c −1 molar axes. The number of remaining axes is r = c + 1 − (c − 1) = 2.
Foraclosed system the equilibrium state is thus uniquely defined by choosing values
for T and P or their conjugate variables independent of how many phases it has. This is
called Duhem’s theorem.
In the most common type of phase diagram there is a temperature axis and a com-
position axis. It is thus an example of phase diagrams with a mixture of potential and
molar axes. Such diagrams are more complicated and due to the large variety no general
description will be given. However, it is worth discussing how the phase field rule can
be generalized to such diagrams but first it should be emphasized that the discussion
only concerns true phase diagrams, i.e. diagrams obtained from a single set of conjugate
pairs of variables. The nine possibilities were discussed in Section 3.5 and they resulted
in 27 sets when molar variables were introduced in Section 9.2.Asaconsequence, all
the variables in a mixed diagram, including those that have been projected or sectioned,
must come from one of the 27 sets in Tables 9.1, 9.2 and 9.3.
Figures 8.1 and 8.2 give the impression that the degrees of freedom increase by
one unit for each molar axis that is introduced instead of its conjugate potential axis.
However, it should be remembered that Gibbs’ phase rule was derived by considering
potentials and not molar quantities. The freedom to vary the amounts of the phases by
moving along a tie-line without varying the compositions of the individual phases is

10.6 The phase field rule and mixed diagrams 221
not regarded as a degree of freedom in Gibbs’ phase rule because the potentials do not
vary. Instead of redefining Gibbs’ phase rule we have thus decided to also to work with
a parallel concept, the dimensionality of a phase field. That was the main reason why the
phase field rule was introduced in Section 8.5. The effect of molar axes on Eq. (8.23)
yields
d = υ − n
s
+ n
m
= c + 2 − p − n
s
+ n
m
, (10.5)
where n
s
is the total number of sectioned quantities, potentials as well as molar quantities,
and n
m
is the total number of molar quantities used, i.e. sectioned molar quantities, n
ms
,
as well as molar axes in the final diagram, n
ma
.Ofcourse, n
m
= n
ms
+ n
ma
.Onthe other
hand, if we project a phase diagram in the direction of an axis, then it does not matter
what kind of variable was chosen on that axis, a potential or its conjugate molar quantity.
The projected phase diagram will look the same and all the projections will thus have
the same effect on the phase field rule. The number of projected molar quantities should
not be included in n
m
.
As before, the number of axes in the phase diagram will be given by Eq. (10.2),
r = c + 1 − n
s
− n
pr
, and Eq. (10.5) can thus be written as
d = c + 2 − p − n
s
+ n
m
= 1 + r − p + n
pr
+ n
m
for p ≥ 1 + n
pr
+ n
m
. (10.6)
This expression is valid only as long as p ≥ 1 + n
pr
+ n
m
because it yields d = r for
p = 1 + n
pr
+ n
m
. This is a critical value because when the number of projections or
molar axes is increased further Eq. (10.6)would yield d > r which is impossible. For
each one of further projections and molar axes both the phase field and the diagram will
lose one dimension and retain the relation d = r.For less phases we obtain instead of
Eq. (10.4),
d = r = c + 1 − n
pr
− n
s
for p ≤ 1 +n
pr
+ n
m
. (10.7)
Afew more considerations of the properties of mixed diagrams should be added. The
lowest possible dimensionality of a phase field will occur for the maximum number of
phases. In a potential phase diagram that dimensionality will be zero but it is evident
from the preceding discussion that it will increase by one unit for each molar axis and
the lowest possible dimensionality will thus be equal to the number of molar axes in the
final diagram, n
ma
, and this will occur at the maximum number of phases. By inserting
n
ms
+ n
ma
= n
m
we obtain
n
ma
= d
min
= c + 2 − p
max
− n
s
+ n
ma
+ n
ms
= r + 1 − p
max
+ n
pr
+ n
ma
+ n
ms
(10.8)
p
max
= c + 2 − n
s
+ n
ms
= r + 1 + n
pr
+ n
ms
. (10.9)
The MPL boundary rule can be applied to mixed diagrams but only with caution. It
is important first to distinguish between phase fields and phase boundaries. The rule
concerns two adjoining phase fields separated by a phase boundary. As an example, we
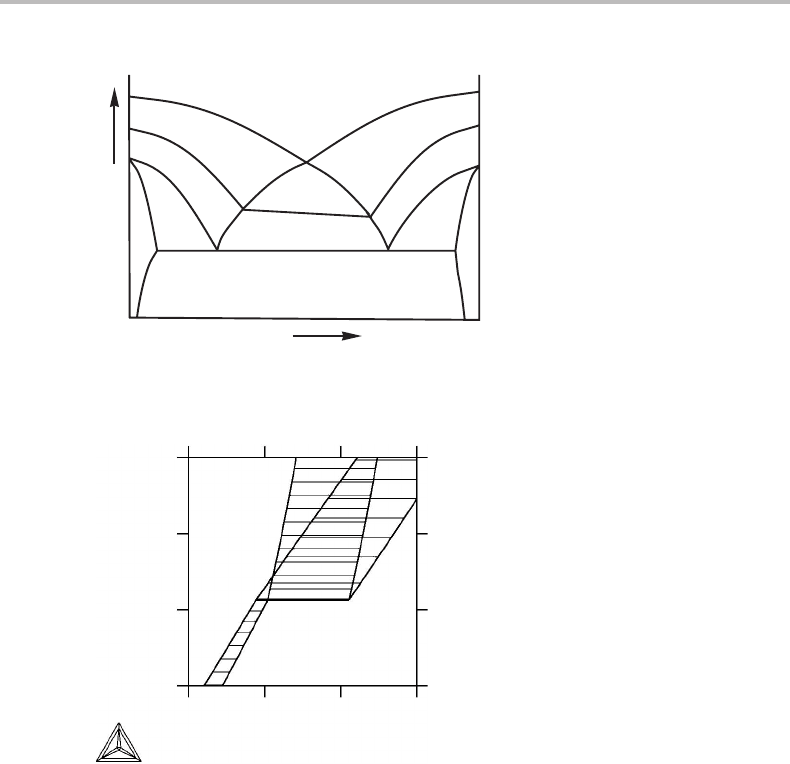
may examine the case illustrated in Fig. 9.2(c).Itisreproduced in Fig. 10.18 without
tie-lines and with the three-phase field bcc + fcc + hcp marked with a thick line. All the
other lines are phase boundaries. The MPL rule cannot be applied to the contact between
222 Projected and mixed phase diagrams
70
64
500 1000
T (K)
V
m
⋅10
7
(m
3
)
bcc
fcc
hcp
Figure 10.18 Mixed phase diagram from Fig. 9.2(c), reproduced without tie-lines. The thick line
represents the three-phase field. All other lines are phase boundaries.
fcc and bcc + hcp phase fields because they are not connected by a phase boundary but
separated by the three-phase field bcc +fcc +hcp. For the contact between fcc and bcc +
fcc + hcp the rule gives
b = r − D
+
− D
−
= 2 − 2 − 0 = 0,
in agreement with the fact that these two phase fields meet at a point, only. For the contact
between bcc + hcp and bcc + fcc the rule gives
b = r − D
+
− D
−
= 2 − 1 − 1 = 0.
This is also correct because these two phase fields do not make contact along the thick
horizontal line, where they are separated by the bcc + fcc + hcp phase field. They
only make contact at the upper end-point of the thick line. Cases like this can be easily
analyzed by imagining that the one-dimensional phase field is a very thin triangle [12].
That method is also helpful if one wants to draw zero-phase-fraction lines. Each one-
dimensional phase field will have one such line on each side.
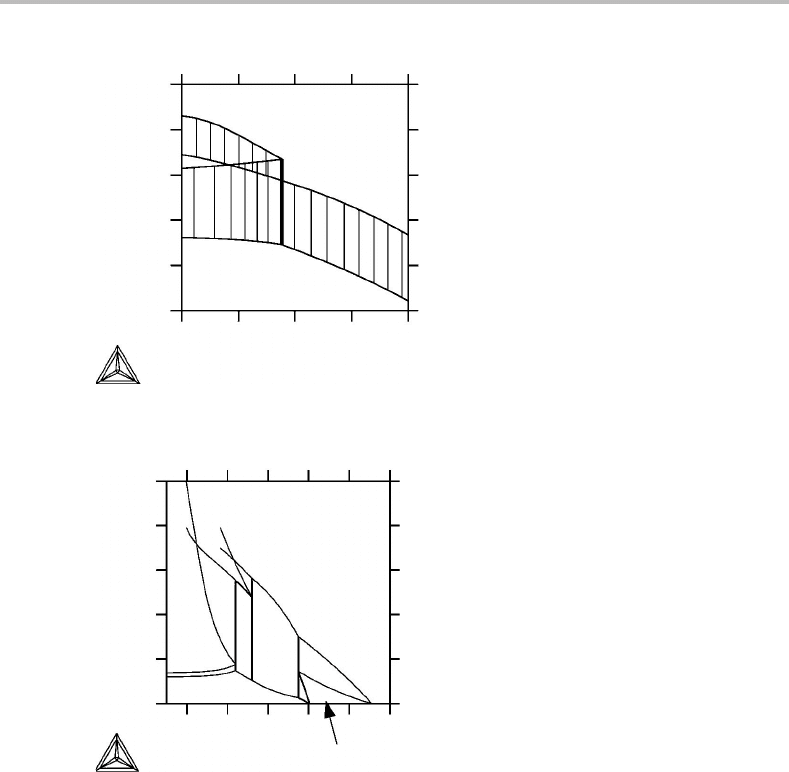
Exercise 10.9
The T,%Cphase diagram (Fig. 10.19) is for a quaternary A–B–C–D system at 20% B
and 20% D and at 1 bar. Test it with the phase field rule.
Solution
There are four components, c = 4. The complete phase diagram has been sectioned
three times, n
s
= 3, but two of the sections were for the molar quantities %B and %D,
n
ms
= 2. In the final diagram there is one molar axis, n
ma
= 1. There is no projection,
n
pr
= 0. In the diagram we can see a horizontal line. Let us test if it is a phase field or
just a boundary between two-dimensional phase fields. A line has the dimensionality
1 and it thus gives 1 = d = c + 2 − p − n
s
+ n
ma
+ n
ms
= 4 + 2 − p − 3 + 1 +2 =
6 − p; p = 5. If the horizontal line is a phase field, it should have five phases. From
the neighbouring phase fields we find α + β + γ + δ + L. We may conclude that this
10.7 Selection of axes in mixed diagrams 223
α+β+γ+δ
α
+
β
+
δ
β
+
γ
+
δ
L +β+γ+δ
L +α+β+δ
L
L+α
L+γ
L+γ+δ
L+ α+γ
L+α+δ
L+α+γ+δ
% C
T
Figure 10.19 See Exercise 10.9.
850
800
750
700
48 52
S
m
(J/molK)
56 60
bcc
T (K)
hcp
fcc
Figure 10.20 T , S
m
diagram for Fe. This is not a true phase diagram.
line is a phase field. The diagram is two-dimensional, r = 2. Let us now check for
what number of phases a field should be two-dimensional, p ≤ 1 + n
pr
+ n
ma
+ n
ms
=
1 + 0 + 1 + 2 = 4. This is also confirmed by the diagram.
10.7 Selection of axes in mixed diagrams
For mixed diagrams it is particularly important to pay attention to how the axes are
selected. As already emphasized, they must all come from a set of conjugate pairs
of variables and one from each conjugate pair. The various possibilities are listed in
Tables 9.1, 9.2 and 9.3.Anumber of examples will now be given in order to demonstrate
what could otherwise happen.
Figure 10.20 shows part of the T, S
m
diagram for Fe. Two two-phase fields overlap
which is made possible by the fact that T and S
m
do not represent different conjugate
pairs in any of the sets in the tables. This is not a true phase diagram.
224 Projected and mixed phase diagrams
100
90
80
70
60
50
−40 −35 −30 −25 −20
µ
Pb
(kJ/mol)
liq
fcc
bct
S
m
(J/mol K)
Figure 10.21 S
m
,µ
Pb
diagram for Pb–Sn. This is not a true phase diagram.
1.0
0.8
0.6
0.4
0.2
−3 −2 −1010
0
M
23
Cr
6
M
7
Cr
6
bcc
fcc
cem
log(a
C
)
X
Cr
Figure 10.22 x
Cr
, a
C
diagram for Fe–Cr–C. This is not a true phase diagram.
Figure 10.21 shows the S
m
,µ
Pb
diagram for the Pb–Sn system at 1 bar. Two two-phase
fields overlap because S
m
and µ
Pb
do not come from the same set of conjugate pairs.
This is not a true phase diagram. One should have combined S
m
with µ
Pb
− µ
Sn
or S
m1
with µ
Pb
.
Figure 10.22 shows the x
Cr
, a
C
diagram for Fe–Cr–C at 1 bar and 1200 K. The inter-
secting phase boundaries in the upper left corner, forming two ‘swallow-tails’, indicate
that this is not a true phase diagram. The activity a
C
can be regarded as an expression
for µ
C
/T and should have been combined with u
Cr
or z
Cr
but not x
Cr
.
Figure 10.23 shows the T, x
Cr
diagram for Fe–Cr–C at 1 bar and a
C
= 0.3, relative to
graphite. The two intersecting phase boundaries on the right-hand side indicate that this is
not a true phase diagram. The two axes, T and x
Cr
,dobelong to the same set of conjugate
variables but one must also consider the sectioned axes. In this case one has sectioned
at constant P and a
C
, i.e. µ
C
/T .However, µ
C
/T and x
Cr
do not belong to the same set.
Figure 10.24(a) shows the H
m1
, a
C
diagram for Fe–C at 1 bar. This is not a true phase
diagram although H
m1
and µ
C
/T, here represented by a
C
, come from the same set of
