Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

660 16. Mass Tags and Isotope Tags
analysis to multiple samples, because of the shear number of peaks that result in the mass spec
separation.
A new type of mass tag extends the benefi ts of stable isotopic labeling of peptides for mass
spec to the analysis of multiple samples and multiple proteins separated simultaneously within
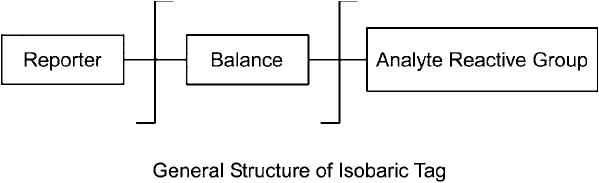
the same mass spec run. An isobaric tag consists of a reactive group for coupling to peptides fol-
lowed by an isotopically labeled mass normalization group, a cleavable linker, and another iso-
topically labeled group, called a mass reporter ( Figure 16.11 ). For every isotopic substitution in
the mass reporter region, the mass normalization group has an inverse mass substitution. Using
this balancing process, the total molecular mass of the entire tag always stays the same–thus the
name “isobaric tag ”. A series of isobaric labels can be created by careful selection of the isotopic
substitutions in the reporter group, which are exactly balanced in the normalization group.
If there are enough potential isotopic substitution sites in the isobaric tag design, a set of
4–10 tags can be created in which each has a different reporter mass, but all of them have the
same total molecular weight. Proteome Sciences, Applied Biosystems, and PerkinElmer/Agilix
each have commercialized isobaric tag sets based on the reporter group/normalization group
blueprint for multiplexed sample analysis. Figure 16.12 shows examples of isobaric tag design,
which were obtained from company advertising, scientifi c publications, or the associated pat-
ents. Most of the tags also contain a tertiary amine group or a guanidino group to aid in the
ionization of the labeled peptide and provide better mass spec signals.
In use, a protein sample fi rst is proteolytically digested and labeled with an individual iso-
baric tag. The most common isobaric tag design contains an amine-reactive NHS ester group
to label each peptide at its N-terminus. Therefore, within a given sample all of the different
peptides after labeling will have a unique isobaric tag modifying them with a characteristic
reporter group mass. Unlike the use of an ICAT tag that targets only cysteine-containing pep-
tides, modifi cation of the N-terminus of every peptide assures 100 percent coverage of the pro-
teome. If multiple samples are being analyzed, then each peptide sample is separately labeled
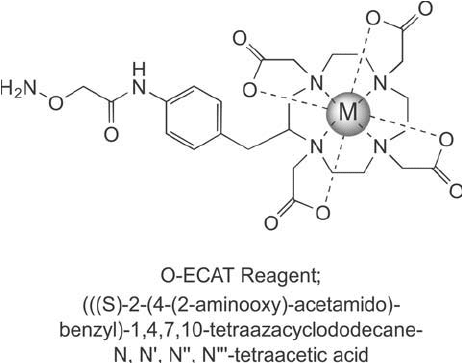
Figure 16.9 The O-ECAT reagent structure contains a DOTA chelating group and a terminal aminoxy group
for coupling to aldehyde and ketone sites of oxidation within biological molecules.
3. Isobaric Tags 661
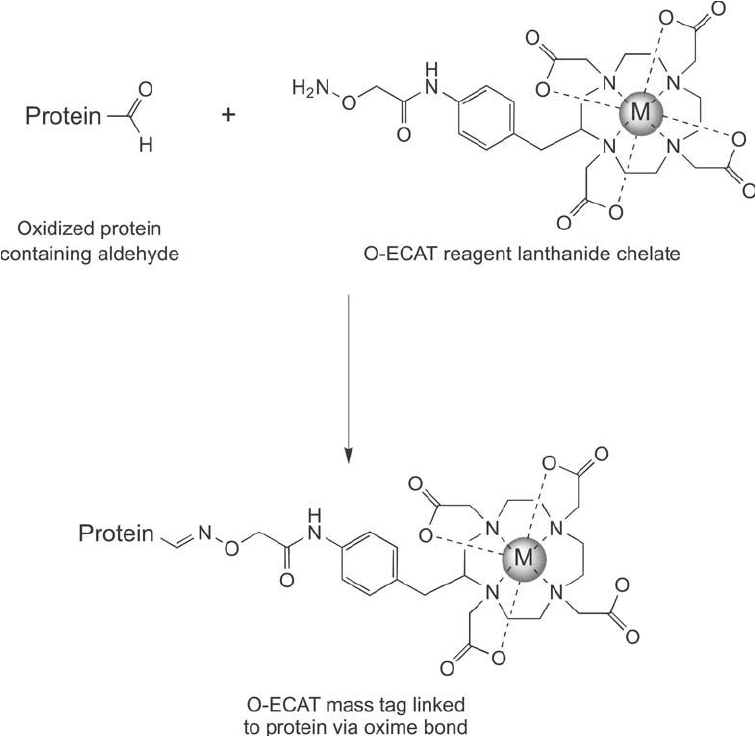
Figure 16.10 The O-ECAT mass tag can covalently link to any oxidized proteins containing aldehydes, forming
an oxime bond.
with a different isobaric tag in the series. After labeling, all the samples then are combined and
separated chromatographically to reduce the total sample complexity.
Since each isobaric tag in a set is structurally identical except for its isotopic substitution
pattern, all of them perform identically with regard to peptide modifi cation and chromato-
graphic separation. Multiple peptide samples then can be labeled separately with different tags
in a series, the samples combined, subjected to fractionation typically done by multidimen-
sional protein identifi cation technology (MudPIT; Liu et al., 2004), and injected into a mass
spectrometer for analysis. As each peak comes off the MudPIT separation system and goes
into the mass spec, it contains the same sequences of labeled peptides from each sample that
happen to elute under the instant conditions of salt strength and solvent addition being used at
that moment. The only difference in the peptide mixture within a given chromatographic peak,
662 16. Mass Tags and Isotope Tags
then, is the type of isobaric label attached to them, which is indicative of the sample it came
from. Thus, the peptides representing a particular sample will all be labeled with a tag having a
characteristic reporter group mass. Another set of peptides with the same amino acid sequence
but coming from another sample will be labeled with a different isobaric tag having another
reporter group mass signature.
It is only upon tandem mass spec analysis that the isobaric labels can be distinguished and
the peptides identifi ed. For instance, if four samples were each labeled with a different isobaric
tag, in the fi rst dimension of a MS separation a given peptide from each sample will appear in
the same peak, because the peptides will all have the same sequences and the isobaric tags labe-
ling them will all have the same mass signatures. Therefore, their mass/charge ratios will all be
the same and they will be indistinguishable at this point. However, upon MS/MS separation
wherein additional energy is used to promote fragmentation of the labeled peptides within the
peak (usually by collision-induced dissociation (CID) or electron capture dissociation (ECD)),
the peptides will break down into their respective charged amino acids and the isobaric tags
will be cleaved to release their reporter groups. From the peaks that result from this second
stage MS separation both the peptide sequence and the sample from which it came can be iden-
tifi ed from the amino acids and the mass of the respective reporter groups.
There are many publications describing the development and use of isobaric tags. For
instance, Thompson et al. (2003) describes the development of tandem mass tags based on an
isotopically labeled peptide design. A later iteration of this concept uses commercially available
isotopes of alanine to form the reporter group, followed by a piperazine ring (as the charge-
carrying group), a second alanine used as the mass normalization group, and a proline residue,
which functions as the electrospray cleavable linker (Thompson et al., 2007). Ross et al. (2004)
used a set of four isobaric tags designed around an N-methyl piperazine group to study the glo-
bal protein expression of a wild-type yeast strain versus strains defective in certain pathways.
These tags are part of the iTRAQ (isotope tags for relative and absolute quantitation) system
from Applied Biosystems. Figure 16.12 shows some of the major structural characteristics of
commercial isobaric tags. Figure 16.13 illustrates the design of a multiplex isobaric set that
uses a piperidine ring as the reporter group, showing all of the various isotopic modifi cations
done to balance the reporter and normalization group to create six unique tags (TMT system:
Tandem Mass Tags from Proteome Sciences).
Figure 16.11 The general structure of an isobaric mass tag reagent. The reactive group facilitates coupling to
discrete sites on peptides, such as amines. The reporter group creates a unique mass signal in MS
2
analysis and
its total mass is exactly balanced by the balance group by changing the stable isotopic labels to provide the
opposite mass differential as that of the reporter group. The result is that all isobaric tags have the same initial
molecular mass, but upon fragmentation in MS
2
, the reporter group is released and it provides the unique mass
signal to identify the sample being analyzed.
3. Isobaric Tags 663
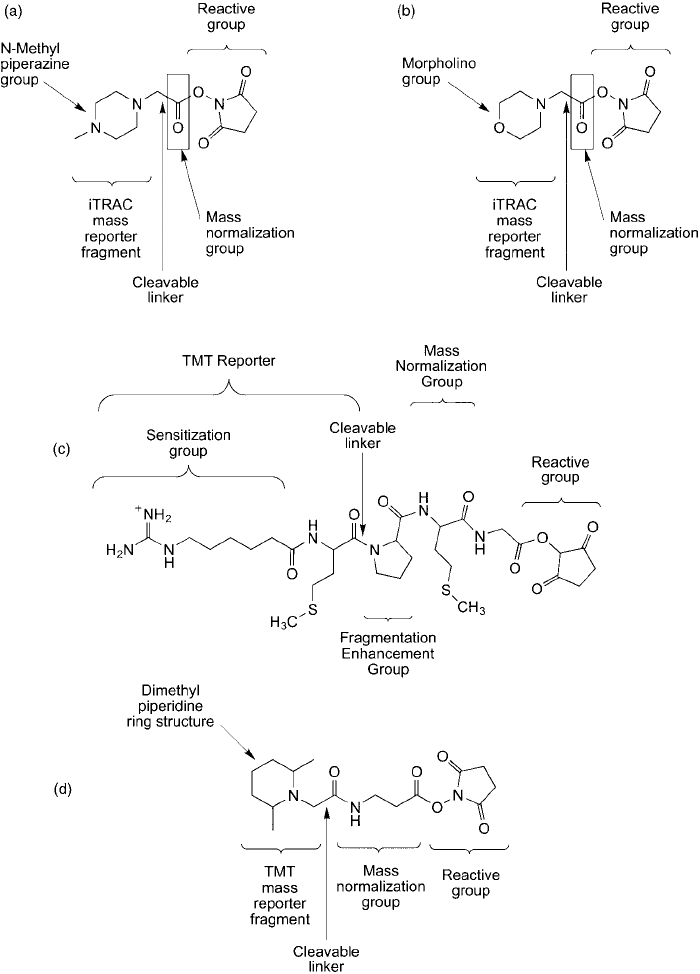
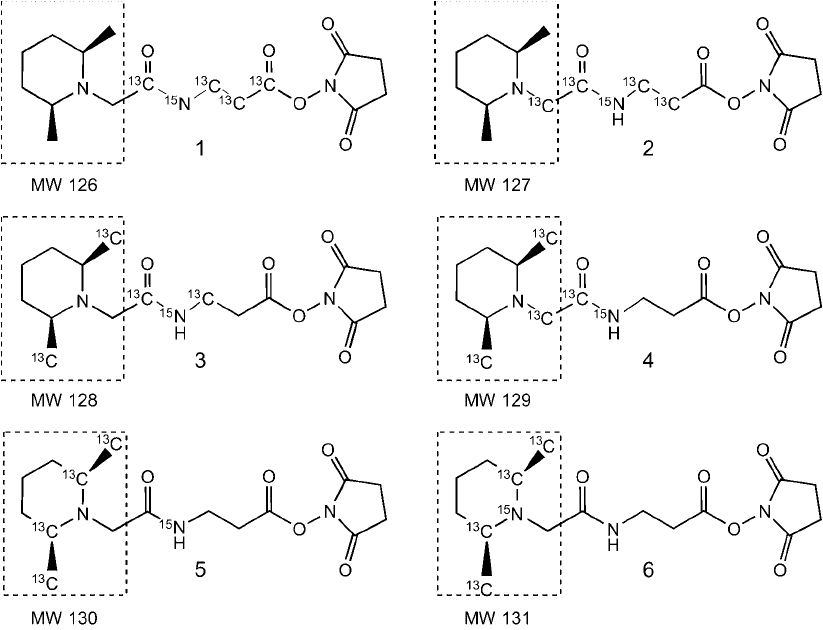
Figure 16.12 A number of isobaric tags have been developed having different structural motifs. (a and b):
Isobaric tags used as part of the iTRAC reagents (Applied Biosystems). (c) An early design of isobaric tags using
a core of amino acid derivatives (Proteome Sciences). (d) The TMT tag design as commercialized by Proteome
Sciences. All of these isobaric tags contain a sensitization group to enhance mass spec detection, which is repre-
sented either by a tertiary amine that can be protonated to carry a positive charge or a guanidino group.
664 16. Mass Tags and Isotope Tags
Isobaric labels thus permit quantitative information regarding protein expression levels in
multiple samples analyzed simultaneously by MS. The multiplexed capability of these reagents
allows the measurement of peptides and proteins in diseased samples, treated samples, and nor-
mal samples all in the same experiment. In addition, since all peptides from a given protein get
labeled at their N-termini, the MS analysis generates more than one peptide signal, which can be
used to confi rm protein identity with greater confi dence than using a cysteine label, like ICAT.
The protocol for using isobaric tags differs from that described previously for the ICAT or
ECAT type reagents. In the following method, the proteins are denatured and the disulfi des
reduced and then alkylated to block them permanently. This eliminates disulfi de re-association
and also prevents the isobaric tags from forming thioester modifi cation with cysteine thiols.
Next, the proteins are digested with trypsin and then modifi ed with an isobaric tag. Each sam-
ple is labeled with a different isobaric compound so that the samples can be differentiated upon
MS/MS analysis.
Figure 16.13 Isobaric tags allow multiplexed analysis of different samples by changing the mass of the reporter
group and balancing that change by the opposite change in the balance group. This fi gure illustrates the 6-plex
tag system from Proteome Sciences, which contains six different reporter groups (boxed areas). All of the rea-
gents contain amine-reactive NHS esters for modifying lysine side chains in proteins and peptides.
The following protocol illustrates the modifi cation reaction and the handling of the protein
samples, but it is not meant to be instructive of mass spec techniques.
Protocol
1. Grow cells to 70–80 percent confl uence and harvest by scraping the cells into 5 ml of
0.1 M sodium borate, pH 7.5. Avoid the use of amine-containing buffers, as these will react
with the NHS esters on the isobaric tags. Aliquot cell counts of approximately 2.5–5 10
6
for processing. Lyse cells using a detergent lysis buffer (e.g., Poppers, Thermo Fisher) or by
mechanical means. Centrifuge and discard the cellular debris. Measure the total protein
concentration using the BCA assay (Thermo Fisher) and adjust the protein concentration
to 1 mg/ml using 0.1 M sodium borate, 0.1 percent SDS, pH 7.5. A total of 100 g of each
protein sample can be used in this protocol. Separately process test samples and a control
sample made up of different cell populations. Simultaneous measurement can be done on a
total number of samples equal to the number of different isobaric tags available.
2. Reduce disulfi des in the protein sample by the addition of 2 l of 50 mM TCEP (Thermo
Fisher) to each 100 l aliquot of protein solution (fi nal concentration 1 mM). Cover and
boil the samples for 10 minutes in a water bath to completely denature and reduce the
proteins. Alternatively, reduction may be done at 60°C for 1 hour. Avoid the use of thiol-
containing reductants, such as DTT, as these will react with the thiol blocking agent used
in the next step.
3. Add 6 l of iodoacetamide to each sample solution and react with mixing for 30 minutes
at room temperature.
4. Prepare a solution of TCPK-trypsin in 0.1 M sodium borate, pH 7.5, at a concentration
of 400 ng/ l. Add 10 l of the trypsin solution to each sample and incubate at 37°C for
12–16 hours or overnight with mixing.
5. Dissolve the isobaric tagging reagents in acetonitrile or ethanol at a concentration of
50 mM (or according to the manufacturer ’s recommendations). Use a fume hood to han-
dle organic solvents.
6. Add a quantity of the appropriate isobaric tag solution to each sample to provide a fi nal
concentration of 10–20 mM. This quantity of reagent will assure a large molar excess
of reagent over the concentration of peptides present in order to modify completely all
peptides at their N-terminus. Note that the -amino groups of lysine residues also will be
modifi ed by this procedure. React for 1 hour at room temperature.
7. To eliminate acylation products at tyrosine residues, add 1 l of 15 M hydroxy-
lamine solution in water to each protein sample and incubate for 30 minutes at 37°C
(Zappacosta et al., 2006).
8. Combine the contents of each labeled sample into a single tube and mix by vortexing,
then centrifuge.
9. Before LC–MS/MS analysis, the sample must be cleaned up to remove excess salts, SDS,
reducing agent, and cell lysis buffer components. This typically is done by using a strong
cation exchange matrix. Protocols may be found for this procedure in the instruction
booklets related to the use of isobaric tagging reagents (e.g., Proteome Sciences, Applied
Biosystems, or Perkin Elmer/Agilix).
3. Isobaric Tags 665

666
17
The many dozens of reactions that are available for bioconjugation purposes generally are
designed to work with biological molecules and the functionalities they contain. The main goal
of most bioconjugate techniques is to use the functional groups on biomolecules to label with
another type of biomolecule or link to synthetic probes. However, it is often desirable to cou-
ple one molecule to another without the potential for cross-reactivity with biomolecules. The
term “chemoselective ligation ” has been coined to describe the coupling of one reactive group
specifi cally with another reactive group without side reactions in aqueous solution or in the
presence of biological material (Lemieux and Bertozzi, 1998).
Unfortunately, the incredible diversity of biomolecules in cells and organisms presents prob-
lems for this goal of total chemoselectivity and bioorthogonality, as the number and variety of
reactive sites on the molecules of life is extraordinary. Even the best crosslinkers or labeling
reagents designed to be somewhat site-specifi c in their reactions often display cross-reactivity
with functional groups on biomolecules other than the ones intended for coupling. For instance,
N-hydroxysuccinimide (NHS) esters usually are considered amine reactive, but they also react
with cysteine, histidine, serine, threonine, and tyrosine side-chain groups. Maleimide groups too
are touted as being thiol specifi c, but amines also can react with maleimides given the right
conditions.
Bioorthogonal reagents ideally should contain a reactive group that only will react with
another specifi c reactive group without any potential for cross-reactivity with biomolecule func-
tionalities. In other words, a bioorthogonal reactive group could be added to a complex mixture
of biological molecules in aqueous solution without reacting with any of them. Moreover, the
ideal bioorthogonal system should be immune to instability in aqueous solutions, such as the
tendency to hydrolyze or easily oxidize. True bioorthogonal reagents of this type will link to each
other and only each other in the presence of intracellular environments or in cell lysates or in
defi ned biomolecule solutions.
The reality is that there are few options in this category of bioconjugate reagents and each of
the systems or reactant pairs that have been developed for bioorthogonal applications have dif-
fering degrees of how well they perform in this task. The following sections describe the major
chemoselective ligation reactions, which can be considered to have a degree of bioorthogonal
characteristics. In each system, the chemoselective pair of reactants can be separately linked
or built into the design of crosslinkers or labeling reagents and used to modify biomolecules,
Chemoselective Ligation: Bioorthogonal Reagents
surfaces, particles, or organic compounds. Subsequently, these labeled components can be
brought together, even in complex solutions, to facilitate conjugation between the two
bioorthogonal reacting species.
In addition, for several reactant strategies in chemoselective ligation, one of the reagent pairs
can be designed into a biological monomer that can be utilized by cellular processes to become
incorporated into biopolymers. This advantage provides a unique in vivo labeling capability
through the feeding of monomer analogs to cells or organisms, such as modifi ed amino acids
or sugar derivatives, which then get selectively added into proteins, carbohydrates, or lipids.
Thus, the ultimate application of bioorthogonal chemoselective ligation is to label specifi cally
the molecules of life directly within living systems and without cross-reactions with other bio-
logical functional groups. For a review on the use of chemoselective reactions in living systems,
see Prescher and Bertozzi, (2005).
1. Diels–Alder Reagent Pairs
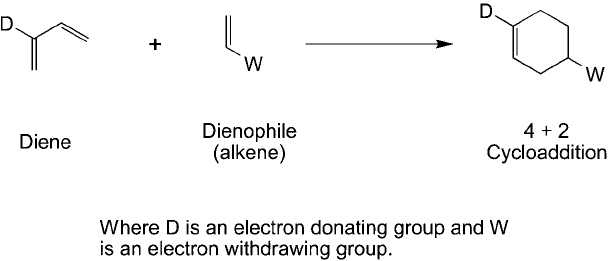
The Diels–Alder reaction has long been a staple for forming carbon–carbon bonds in organic
synthesis (Smith and March, 2007). The typical reaction proceeds through the 2 4 cycloaddi-
tion of a double bond (alkene) and a diene to give a 6-member ring product. The double bond
reactant is often called a dienophile, and electron-withdrawing constituents next to the alkene
are used to accelerate the reaction (such as COOH, CHO, and COR groups, among others).
Conversely, electron-donating groups on the diene are important for increasing reaction rates
(Figure 17.1 ).
Some reports indicated that the Diels–Alder reaction could be done in aqueous environ-
ments with a potential for accelerated reaction rates under the right conditions (Rideout and
Breslow, 1980; Blokzijl and Engberts, 1992; Pai and Smith, 1995; Otto et al., 1996; Wijnen
and Engberts, 1997), and the addition of InCl
3
was determined to act as a catalyst in aqueous
environments (Loh et al., 1996). For a review of organic reactions that can be done in aqueous
media, see Li (2005).
Figure 17.1 A general Diels–Alder reaction consists of a 4 2 cycloaddition between a diene and an alkene,
often called a dienophile. The reaction rate and yield increase if the diene contains an electron-donating group
and the alkene contains an electron-withdrawing group.
1. Diels–Alder Reagent Pairs 667
668 17. Chemoselective Ligation: Bioorthogonal Reagents
In addition, it has been discovered that there are naturally occurring enzymes that facilitate
Diels–Alder type reactions within certain metabolic pathways and that enzymes are also instru-
mental in forming polyketides, isoprenoids, phenylpropanoids, and alkaloids (de Araujo et al .,
2006). Agresti et al. (2005) identifi ed ribozymes from RNA oligo libraries that catalyzed multiple-
turnover Diels–Alder cycloaddition reactions.
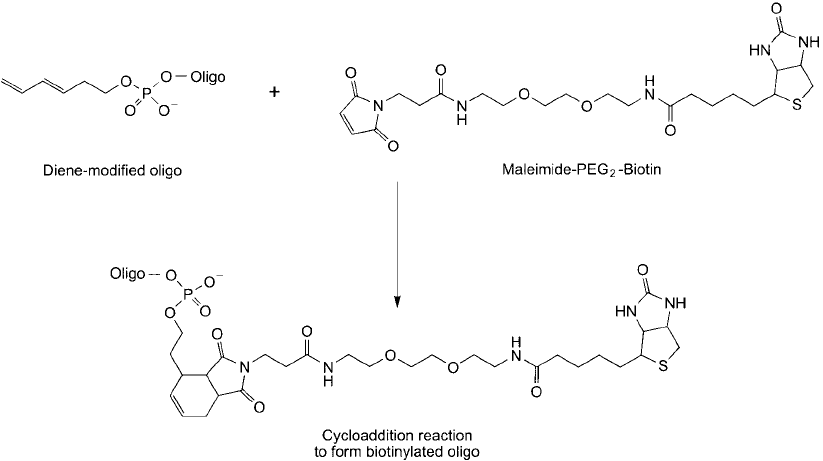
In 2001, Hill et al. extended the use of aqueous phase Diels–Alder reactions for the bio-
conjugation of diene-modifi ed oligonucleotides. The dienophile that was used consisted of a
simple maleimide derivative, which is present on a broad range of commercially available bio-
conjugation reagents. Modifi ed oligonucleotides were prepared by solid phase synthesis using
a 3,5-hexadiene phosphoramidite derivative, which could be incorporated into the oligo at the
5 end. Maleimide compounds investigated for oligo bioconjugation include N -ethylmaleimide,
biotin–BMCC, fl uorescein–maleimide, coumarin–maleimide, maleimide–PEG
2
–biotin, and an
mPEG–maleimide. Figure 17.2 shows the reaction of maleimide–PEG
2
–biotin with the diene-
modifi ed oligo to yield the cycloaddition product.
The reaction kinetics between a maleimide derivative and a 3,5-hexadiene derivative var-
ies depending on the maleimide compound being reacted. Cycloaddition yields of greater than
80 percent and often as much as 90–95 percent can be expected within 1–18 hours at room
temperature or slightly elevated reaction conditions (e.g., 30 °C).
The conjugation of oligonucleotides with peptides also can be done using Diels–Alder cyclo-
additions in water (Tona and Häner, 2005). Marchan et al. (2006) used the same 3,5-hexadiene
phosphoramidite derivative as Hill et al. (2001), but in this case used a maleimide-modifi ed
Figure 17.2 Maleimide groups provide good dienophiles for a Diels–Alder reaction. Biotin–PEG
2
–maleimide
can react with an oligo-diene molecule to form a covalent cycloaddition product, which adds the biotin tag to
the oligo.
peptide sequence. A mild, aqueous Diels–Alder reaction between them resulted in the forma-
tion of the cycloaddition product.
The Diels–Alder reaction for bioconjugation also has been used for the chemoselective liga-
tion of peptides and proteins in aqueous solution (de Araujo et al., 2006). Peptides modifi ed
using a 2,4-hexadienyl ester were derivatized to contain a diene and were found to be reac-
tive toward other peptides containing an N-terminal maleimide group to give the cycloaddition
product in high yield. The hexadienyl group also was attached to biotin and allowed to interact
with streptavidin, which then could be conjugated with peptides containing a maleimide group.
Diels–Alder cycloaddition reactions also have been used to link covalently carbohydrates
to proteins (Pozsgay et al., 2002) as well as for the immobilization of oligonucleotides on
glass surfaces to create arrays (Latham-Timmons et al., 2003). In an application that used two
chemoselective ligation reactions, Sun et al. (2006) employed sequential Diels–Alder and azide–
alkyne (click chemistry) cycloaddition reactions to immobilize protein, biotin, or carbohydrate
ligands on solid surfaces. In this case, glass slides containing maleimidocaproyl groups were
used as the dienophiles and a PEG
4
spacer containing an alkyne on one end and a cyclopen-
tadiene at the other end was the reactive linker. A Diels–Alder reaction coupled the maleimide
groups to the cyclopentadiene groups on the spacer, while the alkyne groups at the other end
were used in a click chemistry reaction to attach azide-containing ligands ( Figure 17.3 ). The
cycloaddition reaction between the maleimide groups on the slides and the cyclopentadiene
group on the spacer was done in a 1:1 solution of water: tert-BuOH at room temperature for
12 hours. After washing with the same water/solvent mixture, the slides contained hydrophilic
spacers terminating in alkyne groups, which then could be coupled with the azide-containing
ligands using click chemistry (see Section 4, this chapter).
Chemoselective ligation reactions using the Diels–Alder cycloaddition process offer another
bioconjugation route using a reactive component available commercially (maleimide-containing
reagents). However, their use as true bioorthogonal reactants is limited due to the cross-reactivity
of maleimides toward thiols. For instance, in the modifi cation of a Rab protein, a cysteine resi-
due had to be protected prior to cycloaddition using a maleimide compound (de Araujo et al .,
2006); otherwise, the maleimide would have coupled to the sulfhydryl, too.
2 . Hydrazine–Aldehyde Reagent Pairs
The reaction between an aldehyde or ketone and a hydrazide or hydrazine derivative to form a
hydrazone bond has been frequently used for bioconjugation purposes (Chapter 2, Section 5.1).
The reaction is appealing from a bioorthogonal perspective, because natural biopolymers don ’t
normally contain these reactive groups. Although aldehydes may form temporary Schiff base
interactions with amines on proteins and other biomolecules, in aqueous solution they are fully
reversible and will rapidly exchange with a hydrazide or hydrazine, if present. This also holds
true of aminoxy (hydroxylamine) derivatives, which form stable oxime bonds with aldehydes,
although the number of reagents available with an aminoxy functionality is limited.
The only potential problem of cross-reactivity for this chemoselective reaction pair with
molecules of biological origin might occur from a hydrazine reacting with aldehyde or ketone
containing metabolic intermediates, reducing sugars, or similar small organic molecules present
within cells or cell lysates. However, if the hydrazine reagent is added in suffi cient excess,
the desired coupling reaction still will occur in such environments, as evidenced by the many
2. Hydrazine–Aldehyde Reagent Pairs 669
