Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

670 17. Chemoselective Ligation: Bioorthogonal Reagents
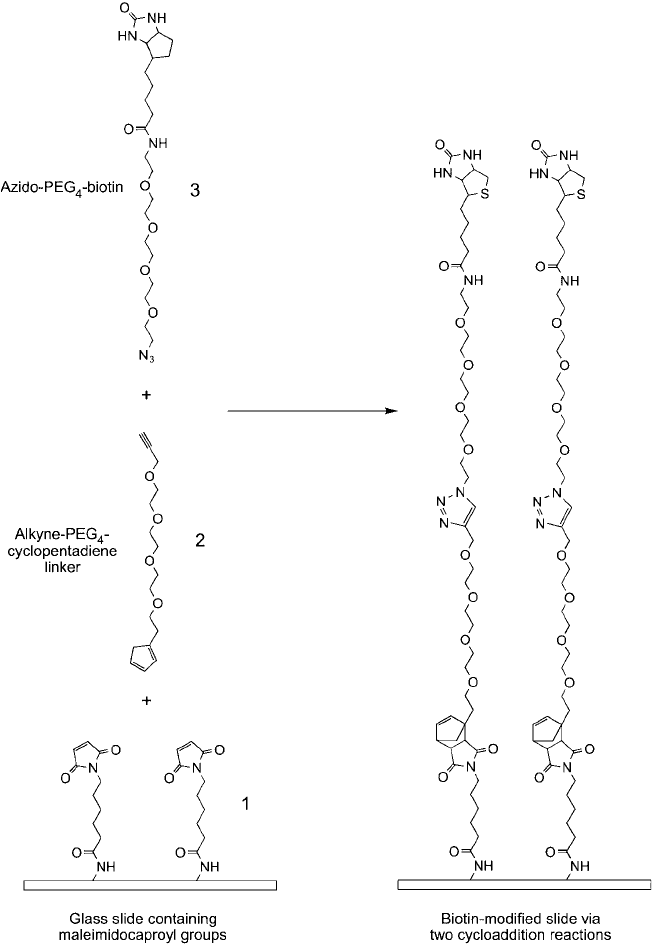
Figure 17.3
Maleimide-modifi ed glass slides (1) can be derivatized using two chemoselective ligation reac-
tions to create biotin modifi cations. In the fi rst step, alkyne–PEG
4
–cyclopentadiene linkers (2) are added to the
maleimide groups using a Diels–Alder reaction. In the second reaction, an azido-PEG
4
–biotin compound (3) is
reacted with the terminal alkyne on the slide using click chemistry to result in another cycloaddition product, a
triazole ring.
successful bioconjugation reactions done with oxidized glycoproteins on cell surfaces or in
lysates (Bayer et al ., 1987a, b, 1990; Bayer and Wilchek, 1990).
The hydrazine–aldehyde reaction has been used intracellularly to deliver non-toxic drug
components, which when linked to form a hydrazone bond in situ, become cytotoxic (Rideout,
1986, 1994; Rideout et al., 1990). This same approach has been used to generate enzyme
inhibitors in vivo, wherein the hydrazine and aldehyde precursors are not active, but when cou-
pled together within cells to form a hydrazone linkage, become active site binders (Rotenberg
et al ., 1991).
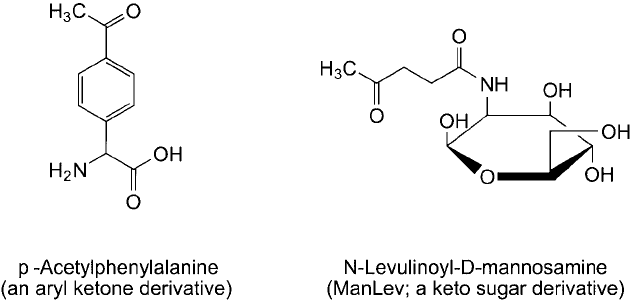
Ketone containing bio-monomer analogs have been used successfully to incorporate these
functionalities into biopolymers, such as proteins and carbohydrates (glycans). For example, a
phenylalanine analog containing a para-substituted aryl ketone group is able to be transformed
into an aminoacyl t-RNA and then introduced with sequence specifi city into a protein by cel-
lular ribosomal machinery (Datta et al., 2002). In addition, the post-translational glycosylation
process within cells is tolerant of certain sugar analogs, and ketone derivatives of monosac-
charides have been used to incorporate these functions into glycans. In particular, growing cells
in the presence of N -levulinoyl- D-mannosamine (ManLev; Figure 17.4 ) was found to result in
the incorporation of this ketone sugar into cell-surface carbohydrates through enzymatic trans-
formation into a keto sialic acid (Mahal et al., 1997; Lemieux and Bertozzi, 1999). Since sialic
acid residues are terminal sugars on most mammalian cell-surface glycoproteins, the addition of
pendent ketone groups allows for specifi c targeting of these glycans with hydrazine (hydrazide/
aminoxy) based reagents.
There are many bioconjugate reagents and probes available with aldehyde or hydrazide func-
tional groups that may be used for hydrazone bond formation. However, the best choices for
making this reaction as chemoselective and bioorthogonal as possible are aromatic aldehyde and
aromatic hydrazine derivatives due to their extremely low reactivity with biological functional
groups, minimal nonspecifi c interactions with biomolecules (charge or Schiff base formation),
and their highly effi cient hydrazone bond formation in complex aqueous solutions (Solulink,
Thermo Fisher). Reagents of this type typically contain reactive groups consisting of either an
aryl aldehyde (benzaldehyde) or a 6-hydrazinium nicotinate (Schwartz et al., 1993, 1995).
Figure 17.4 Ketone derivatives of phenylalanine and mannose can be fed to cells to incorporate the monomers
into proteins and glycans. The resultant modifi cations can be probed using hydrazide-containing reagents.
2. Hydrazine–Aldehyde Reagent Pairs 671
672 17. Chemoselective Ligation: Bioorthogonal Reagents
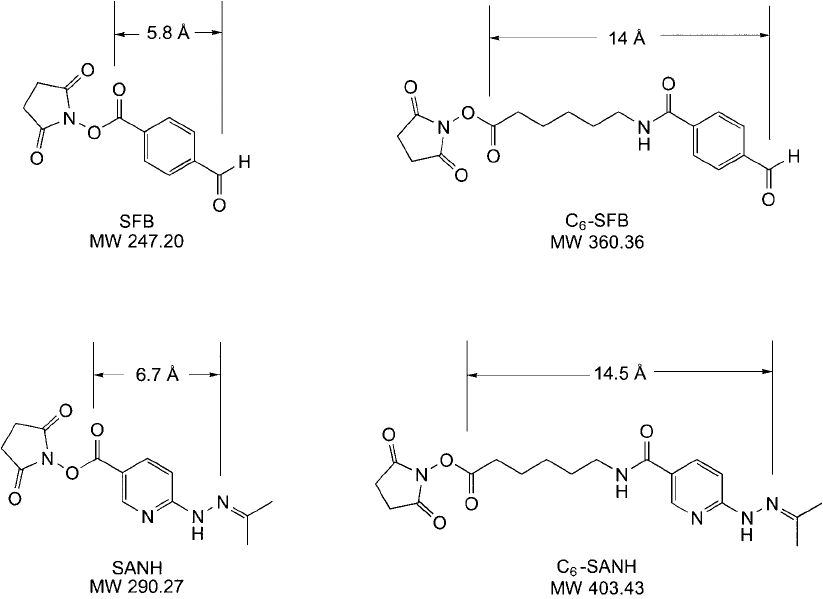
These aldehyde and hydrazine functionalities can be associated with virtually any other
reactive group or tag, such as heterobifunctional crosslinkers, fl uorescent labels, or biotin
compounds. Two reagents frequently used to chemically introduce the aldehyde and hydra-
zine groups include SANH (succinimidyl 4-hydrazinonicotinate acetone hydrazone) and SFB
(succinimidyl 4-formylbenzoate) ( Figure 17.5 ). Long-chain analogs of these two compounds
also exist, which contain either a 6-aminocaproyl spacer arm in the cross-bridge or a short
PEG spacer to promote increased water solubility. The SFB and SANH-NHS ester compounds
are amine-reactive and form amide bonds with the molecules modifi ed by them ( Figure 17.6 ).
Other reactive groups or tags also are available having the benzaldehyde or hydrazinium nico-
tinate groups, including maleimide (thiol-reactive), biotin, and various fl uorescent probes
(Solulink).
The hydrazinium nicotinate group on these reagents commonly is protected against reaction
with the active ester by the addition of acetone to form the acetone hydrazone derivative. This
hydrazone protective group is readily reversible at neutral or mildly acidic pH and will imme-
diately exchange with a benzaldehyde on the corresponding chemoselective partner to form a
stable hydrazone linkage.
Figure 17.5 Structures of aldehyde-containing and hydrazine-containing heterobifunctional crosslinkers that
can be used in chemoselective hydrazone conjugation procedures.
Proteins, other molecules, or surfaces that are activated to contain either the benzaldehyde
functionality or the hydrazinium nicotinate group can be quantifi ed as to the number of these
groups present using certain chromophoric reactants. For instance, 2-hydrazinopyridine can
be reacted with a benzaldehyde-containing molecule to give a UV absorbing derivative with
max
350 nm and an extinction coeffi cient of 18,000 /cm
1
M
1
. Conversely, the hydrazinium
nicotinate-activated molecules can be reacted with p-nitrobenzaldehyde to create another UV
detectable derivative having
max
390 nm with an extinction coeffi cient of 24,000 cm
1
M
1
.
The aromatic nature of the SFB and SANH reactants create a hydrazone structure with
unique absorptivity for monitoring the course of the ligation process. The aromatic hydra-
zone bond has a maximal absorbance at 354 nm and a molar extinction coeffi cient equal to
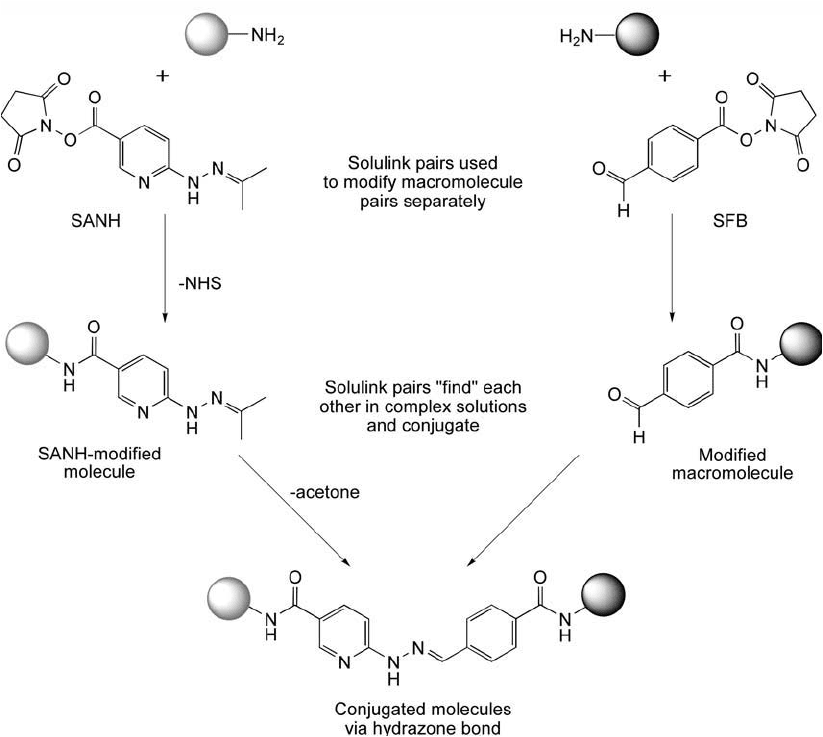
Figure 17.6 The reaction of SANH with amine-containing proteins or other molecules results in amide bond
modifi cations containing terminal hydrazine groups. The reaction of SFB with amine-containing proteins or
other molecules results in amide bond modifi cations containing terminal aldehyde groups. Subsequently, the
two modifi ed molecules can be reacted together to create a conjugate via hydrazone bond formation.
2. Hydrazine–Aldehyde Reagent Pairs 673
674 17. Chemoselective Ligation: Bioorthogonal Reagents
29,000/M. If the conjugation reaction is done with suffi cient amounts of benzaldehyde and
hydrazinium nicotinate reactants, then the yield of the reaction can be directly measured by
monitoring the change in absorbance at this wavelength over time.
The bis-aryl hydrazone bond formed by this reaction is stable in aqueous solution over a broad
pH range (pH 2–11) and under elevated temperature conditions (up to 94 °C) (Solulink web site).
Kozlov et al. (2004) used SANH and SFB to create DNA-antibody conjugates for highly sen-
sitive detection in immunoassays using PCR amplifi cation with fl uorescently labeled primers.
They also investigated hydrazone bond formation and its stability in aqueous solution using
3 and 5 labeled DNA pairs, wherein one oligonucleotide contained a benzaldehyde group and
the other contained the hydrazinium nicotinate group. It was found that the most effi cient pH
for the creation of the hydrazone bond was pH 5.0–7.0, with an optimum at about pH 6 and
yields dropping off at lower or higher pH. In addition, once the hydrazone bond was formed
and the conjugate purifi ed, it was stable in PBS buffer, pH 7.2, with or without 10 mg/ml BSA
for 12 weeks at 4 °C. The hydrazone linkage also was found to be stable at pH values between
2.3 and 11.3 without hydrolytic breakdown.
When reactions between the benzaldehyde and hydrazinium nicotinate groups are done in rela-
tively dilute solution, for example using a modifi ed antibody molecule at 1 mg/ml ( 6 M) con-
taining one of the two reactants, then to obtain maximal yield of the hydrazone conjugate, the
second component should be added in suffi cient excess to drive the reaction to completion. Kozlov
et al. (2004) found that at least an 8-fold molar excess or above of the second reactant over the
fi rst reactant is necessary to obtain a yield of about 80 percent hydrazone bond formation.
The practical use of chemoselective ligation reactions for bioconjugation purposes involves
the attachment of one of the reactants to a fi rst biomolecule using a standard coupling chem-
istry (e.g., NHS ester) to an available functional group, while the second reactant is coupled
to a second molecule or a surface, depending on the fi nal conjugate desired. In this regard, the
following protocols for modifi cation of oligonucleotides or proteins can be done with either
the benzaldehyde component or the hydrazine component and the opposite reactant is used to
modify another molecule or surface that will be coupled in the fi nal conjugation step.
Protocol for Modifi cation of Amine-Oligo with SANH or SFB
1. Dissolve a 5 -amine modifi ed oligonucleotide at a concentration of 0.5 mM in 0.1 M
sodium phosphate, 0.15 M NaCl, pH 7.4.
2. Dissolve SANH (or SFB) in DMF at a concentration of 100 mM. Note: both reagents are
soluble in this solvent to about 50 mg/ml concentration.
3. Add a quantity of SANH or SFB solution to the oligo to provide a 40-fold molar excess
of crosslinker over the amount of amine-oligo present. Mix well.
4. React at room temperature for 2 hours to overnight with gentle mixing.
5. Purify the modifi ed oligo from unreacted crosslinker and reaction byproducts using a
micro-spin concentrator with a membrane having a molecular weight cutoff able to
retain the size of the DNA being modifi ed.
A similar method can be used to modify proteins, such as antibodies, to contain either benz-
aldehyde or hydrazinium nicotinate groups for subsequent conjugation with another molecule
modifi ed by the opposite functionality. The following protocol is based on the methods of Thermo
Fisher, Solulink, and Kozlov et al. (2004).
Protocol for Modifi cation of Protein or Antibody with SANH or SFB
1. Dissolve the protein or antibody to be conjugated in 0.1 M sodium phosphate, 0.15 M
NaCl, pH 7.4. The antibody solution should be as concentrated as possible, given the
amount of antibody available for modifi cation. This general protocol will work for
protein or antibody concentrations ranging from about 0.5 mg/ml to 10 mg/ml, but
an increase in the molar excess of either SANH or SFB may have to be done at lower
antibody concentrations to provide the same modifi cation yields obtained at higher
concentrations.
2. Dissolve SANH or SFB in DMF at a concentration of 2 mg/100 l DMF.
3. Add a quantity of the crosslinker solution of choice (SANH or SFB) to the antibody solu-
tion to obtain the desired molar excess of reagent over the antibody. Typically, antibody
modifi cation procedures are done with 10- to 20-fold molar excess, but for dilute anti-
body concentrations, this may have to be doubled, depending on how many hydrazine or
aldehyde groups are desired to be introduced on the modifi ed antibody.
4. React for 2–3 hours at room temperature with gentle mixing.
5. Purify the modifi ed antibody by use of size exclusion chromatography or dialysis, using a
molecular weight exclusion limit of 5,000–10,000 Daltons. The modifi ed antibody may
be stored at 4°C or frozen until used to make a conjugate. The protected hydrazine on
SANH is stable for several months and the SFB benzaldehyde group is stable indefi nitely.
For longer storage, the modifi ed antibody can be lyophilized.
Once a molecule is modifi ed with a hydrazine reagent and another molecule is modifi ed with
the benzaldehyde compound, they may be combined to form the fi nal conjugate, which will
result in a hydrazone linkage between the two molecules. In addition, chemoselective ligation
using aldehyde/hydrazine reactions may be done to immobilize biomolecules. In this regard,
one modifi ed component may be a surface and the other one an antibody, protein, or oligonu-
cleotide destined for immobilization onto the surface.
Conjugation Using the Aldehyde/Hydrazine Reaction
1. Separately dissolve the SFB-modifi ed molecule and the SANH-modifi ed molecule in cit-
rate buffer (100 mM sodium citrate, 150 mM NaCl, pH 6.0) at concentrations of at least
1 mg/ml. Note that pH 6 is an optimal pH for the formation of the hydrazone bond, but
pH values slightly lower (to pH 4.7) or higher (to pH 7.4) also may work, but they will
result in lower reaction rates and lower yields.
2. Mix a portion of the SFB-modifi ed molecule with the SANH-modifi ed molecule to obtain
a molar ratio that will give the desired conjugate properties. The optimal ratio of reactants
may have to be determined experimentally by performing a series of conjugations using
different molar ratios and testing the performance of the fi nal conjugate in the intended
application.
3. React for at least 2 hours at room temperature, longer if pH values other than pH 6 are
used.
4. To purify the conjugate from reactants that did get incorporated into the conjugate, size
exclusion chromatography may be used with resins having a molecular exclusion limit
able to accommodate both the labeled molecules and the fi nal conjugate.
2. Hydrazine–Aldehyde Reagent Pairs 675
676 17. Chemoselective Ligation: Bioorthogonal Reagents
3. Boronic Acid–Salicylhydroxamate Reagent Pairs
Phenylboronic acid (PBA) groups can interact with a variety of polar constituents on adjacent
or nearby carbon atoms to result in a complex consisting of a 5- or 6-member heterocyclic ring.
This process has been used for the affi nity chromatographic purifi cation of carbohydrates, gly-
coproteins, RNA, AMP (from cAMP), glycated proteins (such as glycated hemoglobin formed
in diabetes; Klenk et al., 1982), and a range of small molecules containing 1,2- or 1,3-diols,
1,2- or 1,3-hydroxy acids, 1,2- or 1,3-hydroxylamines, 1,2- or 1,3-hydroxyamide, 1,2- or
1,3-hydroxyoxime, as well as various sugars containing these species (Weith et al., 1970;
Rosenberg and Gilham, 1971; Rosenberg et al., 1972; Pace and Pace, 1980; Singhal et al .,
1980). For a review on the use of PBA in affi nity separations, see Scouten (1983). In addition,
bioconjugate labeling reagents containing a PBA group also have been used as probes of these
species in biological molecules, including fl uorescent reagents for targeting glycans on cell sur-
faces (Burnett et al ., 1980; O ’Shannessy and Quarles, 1987).
The interaction of PBA derivatives with molecular species having the above functional
groups occurs optimally in the pH range of 8–9, but it is typically reversible at acid pH or in
the presence of a high concentration of competing ligand. However, the heterocyclic boronic
acid complex is relatively stable under optimal conditions of formation.
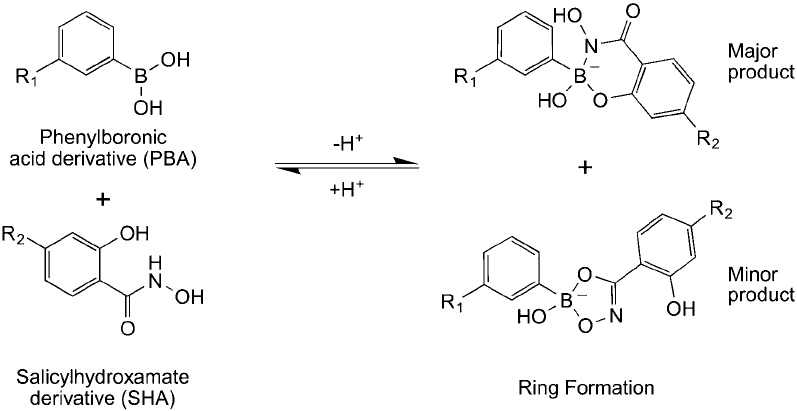
Stolowitz (1997) exploited this interaction potential in the design of a new chemoselective
bioconjugation reagent pair consisting of a PBA group on one reagent and a salicylhydroxamic
acid (SHA) group on a second reagent. Each reactant of the pair can be used to modify biomole-
cules, surfaces, or other compounds for subsequent conjugation or immobilization through spe-
cifi c PBA–SHA ring formation (Springer et al., 2002). The major product of this reaction forms
a 6-membered ring structure consisting of the PBA ’s boron atom along with one of its oxygens
coordinated with the SHA ’s hydroxyl oxygen and hydroxamate nitrogen atoms ( Figure 17.7 ).
It also is possible that a 5-membered ring structure can form from interaction of the PBA boron
with the hydroxamate hydroxyl and carbonyl oxygens on SHA, but this is a minor product as
proven by NMR (Stolowitz et al., 2001).
A signifi cant advancement in the PBA reagent was to add another boronic acid group to
the phenyl ring and thus allow two cycloaddition products to form from a single complex-
ation. The phenyldiboronic acid (PDBA) group effectively increases the affi nity constant
of the interaction if reacting with SHA-modifi ed molecules or surfaces that have more than
one near-neighbor SHA group available. The resultant formation of two 6-membered rings
per conjugation reaction assures that the bond won ’t hydrolyze even under high or low pH
conditions. This is particularly useful for using the reaction to couple proteins or other mol-
ecules to surfaces for arrays, because multiple linkages maintain stability of the immobilized
molecule without the possibility for leaching off. A single SHA–PBA bond reportedly has an
affi nity constant in the range of 10
6
M
1
, which is relatively weak for affi nity binding proper-
ties. Two such bonds, however effectively raise the avidity to an observed affi nity constant of
10
10
M
1
(Lonza product information). Note that multiple single PBA groups also can com-
bine with multiple SHA groups when conjugating proteins together or proteins to surfaces and
thus increase the effective avidity of the resultant bonding interaction. Springer et al. (2003)
describes the use of SHA membranes in this process for the immobilization of PDBA-modifi ed
molecules, including nucleic acids and proteins.
Complex formation between PBA or PDBA group and the SHA group occurs in a wide
range of buffer types from pH 5 to 9, and it can tolerate high salt conditions (to 1.5 M) or the
presence of moderate amounts of water-miscible solvents, detergents, chaotropes, and denatur-
ing agents commonly used when working with protein solutions.
Ring formation between SHA groups coupled to solid phase matrices and ligands containing
either PBA or PDBA groups have been investigated for the immobilization of affi nity molecules
for protein purifi cation (Wiley et al., 2001). Using the dimeric PDBA group, immobilization of
modifi ed alkaline phosphatase onto SHA-agarose resulted in good retention of enzyme activity
plus high stability of the coupled protein. The immobilized protein also was stable to elution con-
ditions of acidic (pH 2.5) or basic (pH 11) buffers without cleavage of the boronic acid complexes
or leakage of coupled protein. This indicates that multivalent attachment points using PDBA
instead of PBA result in high tolerance for extreme environmental conditions without hydrolysis.
Bergseid et al. (2000) also reported on the use of SHA-activated chromatography supports
for the coupling of boronate-containing affi nity ligands. In this case, immobilized RNase A was
used to purify anti-RNase antibodies from antiserum samples. RNase A was modifi ed with an
NHS–PDBA crosslinker at a molar ratio of 100:1 (crosslinker:protein), purifi ed to remove excess
crosslinker, and then coupled to an SHA-agarose support in 0.1 M sodium bicarbonate, pH 8.0.
Prolinx originally developed the technology utilizing the SHA–PBA interaction and later por-
tions of it were commercialized by Invitrogen and then Lonza (Cambrex; Versalinx reagents).
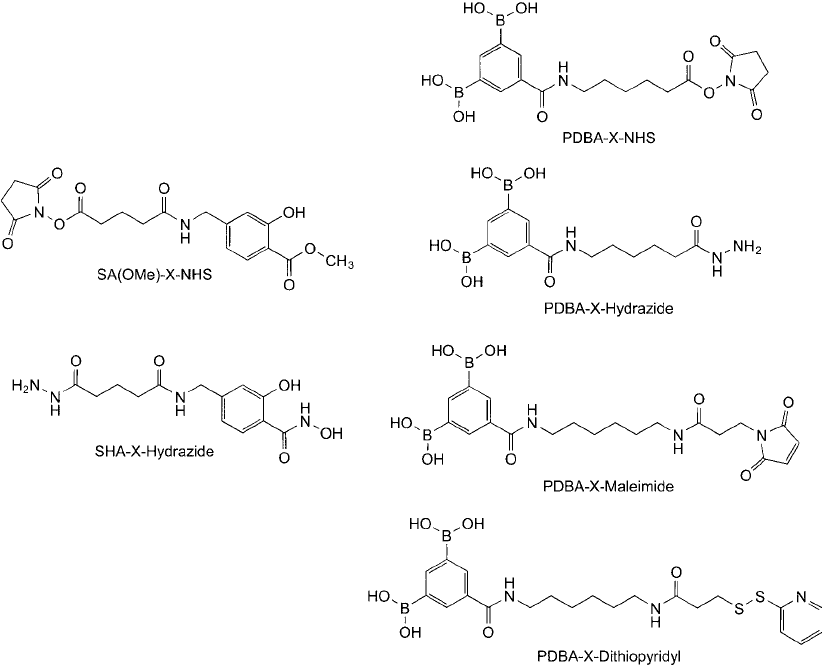
A number of PDBA crosslinking agents now are available to introduce the diboronic acid group into
biomolecules. These include heterobifunctional compounds containing an NHS ester (amine reac-
tive), a hydrazide group (aldehyde reactive), a maleimide group (thiol reactive), a pyridyl disulfi de
group for reversible linkage to thiols or for thiolation of target molecules, and an iodoacetyl group
for creating thioether bonds. SHA crosslinkers include a hydrazide-containing compound, an NHS
ester, and a bis-SHA compound containing a hydrazide group. Some examples of these reagents are
shown in Figure 17.8 .
Figure 17.7 PBA derivatives react with salicylhydroxamate derivatives to form 5-membered or 6-membered
ring structures, with the 6-membered ring the major product.
3. Boronic Acid–Salicylhydroxamate Reagent Pairs 677
678 17. Chemoselective Ligation: Bioorthogonal Reagents
Conjugation reactions between phenylboronates and salicylhydroxamates entail the forma-
tion of a low affi nity interaction involving a reversible heterocyclic ring formation. Conjugates
formed as a result of this reaction are relatively stable provided the pH is maintained within
the optimal range and there are no competing species in solution, which may exchange for the
SHA group. This may include sugars or carbohydrates containing diols or other organic con-
stituents mentioned previously. The creation of dimeric or multivalent interactions with two or
more SHA groups and a phenylboronate-modifi ed molecule (using PBA or PDBA) will dramat-
ically increase the stability of the linkage over that observed with a single PBA–SHA linkage.
Wiley et al. (2001) found that modifi cation of alkaline phosphatase with PBA or PDBA for
subsequent immobilization on an SHA-agarose support resulted in stable immobilization if
6 PBA groups were present on each alkaline phosphatase molecule or the equivalent of 3 dimeric
PDBA groups. This difference refl ects the dimeric nature of the PDBA group in forming more
than one boronate ring structures per modifi cation.
Figure 17.8 Heterobifunctional crosslinking agents containing the PBA or salicylhydroxamate group for chem-
oselective conjugation purposes.
The use of the PBA–SHA conjugation in a bioorthogonal mode probably will be problem-
atic due to the potential side interactions that could occur involving the phenylboronate groups
with biological molecules in cells or cell lysates. In addition, there are no reports of using SHA
or PBA bio-monomer analogs (e.g., amino acids containing these groups) to incorporate into
biopolymers in vivo as there are with other chemoselective reacting groups. However, the use
of the PBA–SHA reaction as a chemoselective immobilization process for attaching proteins or
other molecules to surfaces may have advantages over other bioconjugation reactive groups,
because the reactive groups are very stable and won ’t hydrolyze in aqueous solution. In addi-
tion, the PBA–SHA reaction can be used with success for the conjugation of two pure biomol-
ecules, such as in the creation of a protein–protein conjugate (Le Roch et al ., 2000).
The following protocol describes the immobilization of a protein on an amine-surface using
the P(D)BA–SHA chemistry.
Protocol
Preparation of P(D)BA-Modifi ed Protein
1. Dissolve a fi rst protein to be modifi ed at a concentration of 1–10 mg/ml in 0.1 M sodium
bicarbonate buffer, pH 8.5. PBS buffer at physiological pH also may be used as the reac-
tion medium.
2. Add a quantity of PDBA–NHS to the protein solution to provide the desired molar
excess of crosslinker over the protein. A suggested starting point is to use a 10- to
15-fold molar excess of reagent, but the optimal amount to be added should be deter-
mined by experimentation to provide a fi nal conjugate having the best possible proper-
ties for the intended application. The PDBA-NHS may be fi rst dissolved in DMF as a
concentrated stock solution and then an aliquot added to the reaction mixture. Mix well
to dissolve.
3. React for at least 1 hour at room temperature with gentle mixing.
4. Purify the modifi ed protein by gel fi ltration or dialysis using a molecular weight cutoff
that will be appropriate for the protein being modifi ed.
Preparation of SHA-Modifi ed Surface
An amine-containing surface, such as an APTS-modifi ed glass slide (see Chapter 13, Section 2),
may be modifi ed with a long-chain NHS-salicylic acid methyl ester derivative (Lonza), which
then can be converted to the SHA group for coupling to a P(D)BA-modifi ed protein. The fol-
lowing protocol describes this method.
1. Thoroughly wash the amine-slide with D.I. water and then with 0.1 M sodium bicarbon-
ate, pH 10 (coupling buffer).
2. Add a quantity of NHS-salicylic acid methyl ester reagent dissolved in coupling buffer to
the slide surface to provide at least a 2-fold molar excess of crosslinker over the quantity
of amines present on the surface. The surface of the slide may be coated with a minimum
solution volume of the crosslinker by layering the solution over the surface. Slide masks
or gaskets may be used to isolate only certain regions for modifi cation. Alternatively,
the slide may be immersed in the crosslinker solution. The NHS-salicylic acid methyl
ester may be fi rst dissolved in DMF as a concentrated stock solution and then an aliquot
3. Boronic Acid–Salicylhydroxamate Reagent Pairs 679
