Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

640 15. Buckyballs, Fullerenes, and Carbon Nanotubes
ratio is so high for nanotubes, bundles often are observed to be knotted masses, which are very
diffi cult to unravel. The best that can be done is to disrupt the bundle and form a dispersion of
the nanotubes in a solvent medium.
Some of the better solvents for pure SWNTs are the amide-containing ones, like DMF or
N-methylpyrrolidone, but they still do not permit full dissolution, just dispersion (Boul et al .,
1999; Liu et al., 1999). The addition of surfactants to carbon nanotube suspensions can aid in
their solubilization, and even permit their complete dispersion in aqueous solution. The hydro-
phobic tails of surfactant molecules adsorb onto the surface of the carbon nanotube, while the
hydrophilic parts permit interaction with the surrounding polar solvent medium.
2.2. Nanotube Functionalization
An important route to solubilization of carbon nanotubes is to functionalize their surface
to form groups that are more soluble in the desired solvent environment. It has been shown
that acid treatment of nanotube bundles, particularly with HCl or HNO
3
at elevated temper-
atures, opens up the aggregate structure, reduces nanotube length, and facilitates dispersion
(An et al., 2004; Kordás et al., 2006). Nitric acid treatment oxidizes the nanotubes at the
defect sites of the outer graphene sheet, especially at the open ends (Hirsch, 2002; Álvaro et al .,
2004), and creates carbonyl, carboxyl, and hydroxyl groups, which aid in their solubility in
polar solvents.
Such carbonyls may be further oxidized using potassium permanganate (KMnO
4
) and per-
chloric acid (HClO
4
) to convert all of these groups into carboxylic acids. Once functionalized
in this manner, the nanotubes can be fully dispersed in aqueous systems. Kordás et al. (2006)
used these derivatives to print nanotube patterns on paper or polymer surfaces to create con-
ductive patterns for potential use in electronic circuitry. The carboxylates also may be used as
conjugation sites to link other ligands or proteins to the nanotube surface using a carbodiimide
reaction as previously discussed (Section 1, this chapter; Chapter 2, Section 1.11; Chapter 3,
Section 1).
Untreated carbon nanotubes nonspecifi cally adsorb protein in an irreversible manner
much like the noncovalent adsorption of protein onto hydrophobic surfaces (Chen et al .,
2003). It is essential, therefore, to modify the surfaces of nanotubes to prevent hydropho-
bic binding of biomolecules, especially if the resultant conjugates are to be used in biological
assays or systems. Two main strategies have been used to make nanotubes biocompatible: (1)
the noncovalent modifi cation of the graphene surface with amphipathic molecules, which have
functional groups that can be used for conjugation or (2) the covalent modifi cation of the outer
nanotube surface to promote hydrophilicity and create functional groups for coupling other
molecules.
2.3. Detergent or Lipid Modifi cation of Carbon Nanotubes
Detergents have been used for simple solubilization of SWNTs in aqueous solution. Ionic deter-
gents such as SDS will coat the nanotube surface and expose the negatively charged sulfonate
groups to the surrounding aqueous environment, thus allowing SWNT dispersion in aqueous
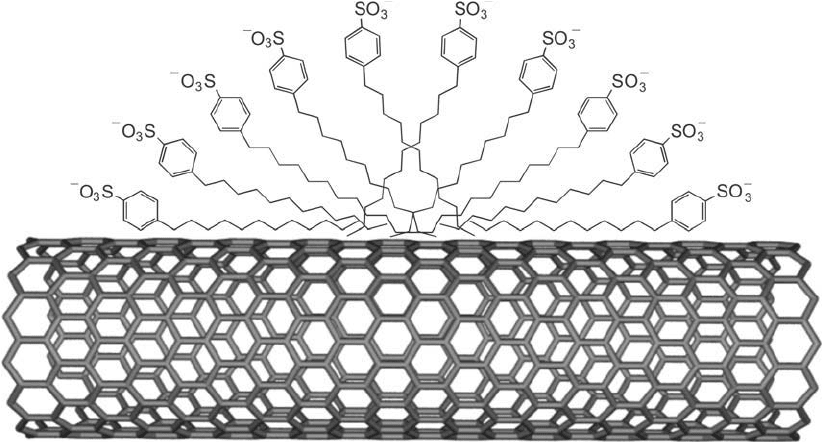
environments. Similarly, nonionic detergents such as Triton X-100 will coat the tubes and
present their hydrophilic groups to the aqueous phase. Fischer et al. found that coating SWNTs
with sodium dodecylbenzene sulfonate resulted in the best solubilization properties with long-
term stability of the nanotubes in aqueous buffers (Zhou et al., 2004). The detergent molecules
don’t merely lie in a random pattern on the nanotube surface; they coat the SWNT in a series
of half-micelle structures, which create small knobs along its length ( Figure 15.12 ).
Detergents also have been exploited in the noncovalent modifi cation of carbon nanotubes
by using modifi ed detergents containing a coupled affi nity ligand, which is linked to the
hydrophilic part of the detergent molecule. Detergents that contain both a hydrophobic por-
tion and a hydrophilic part with at least one terminal functional group for conjugation can be
used in this process. The hydrophobic tail of many detergents will strongly adsorb to the nano-
tube’s outer graphene cylinder leaving the hydrophilic portions pointing outward and available
for conjugation, if they contain an appropriate functional group. The result is a hydrophilic
surface that is completely masked to prevent nonspecifi c protein binding. This approach to
nanotube functionalization also leaves the chemical structure of the graphene cylinder unaf-
fected, thus avoiding defects that could alter its electronic properties.
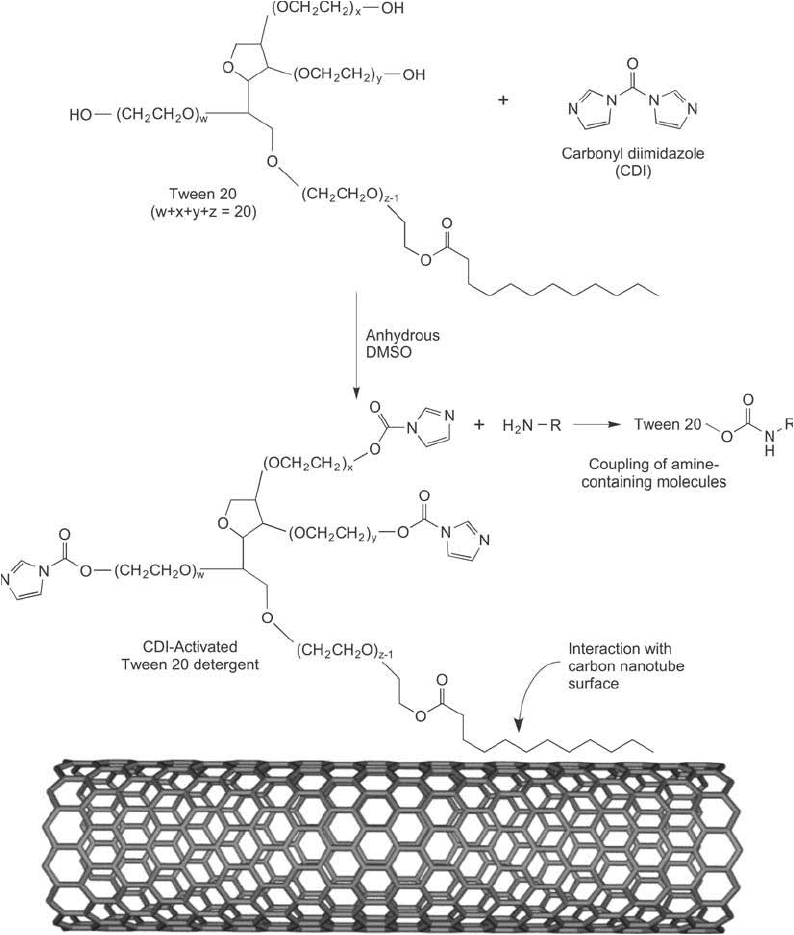
As an example of this strategy, Chen et al. (2003) used an activated Tween 20 detergent to
coat SWNTs for subsequent conjugation to biotin, protein A, and U1A antigen. Tween 20 is
a poly-oxyethylene sorbitan monolaurate compound with 20 ethylene oxide units, 1 sorbitol
unit, and 1 lauric acid group esterifi ed as the hydrophobic tail. The fatty acid group avidly
adsorbs to the carbon nanotube surface, leaving the three PEG arms, each containing terminal
2. Carbon Nanotubes 641
Figure 15.12 Detergent molecules can be used to solubilize carbon nanotubes by adsorption onto the surface
through hydrophobic interactions and create half-micelle structures with the hydrophilic head groups facing
outward into the aqueous environment.
642 15. Buckyballs, Fullerenes, and Carbon Nanotubes
Figure 15.13 Tween 20 can be activated with CDI using its hydroxyl groups to create an amine-reactive imida-
zole carbamate intermediate that then can be used to coat a carbon nanotube. The result is an activated nano-
tube that can be used to couple proteins and other amine-containing molecules.
hydroxyl groups, sticking out from the coating to create an extremely biocompatible construct
(Figure 15.13 ). The hydroxyl groups on the Tween molecules can be activated for coupling to
biomolecules using many of the methods discussed for hydroxylic particles in Chapter 14. For
instance, activation of Tween 20 with carbonyldiimidazole (CDI) or disuccinimidyl carbonate
(DSC) in a nonaqueous solution provides a reactive derivative suitable for coupling to amine-
containing molecules, such as proteins.
The following protocol for the activation of Tween 20 with CDI and its subsequent use
in modifying a carbon nanotube and coupling an affi nity ligand is based on the method of
Chen et al . (2003).
Protocol
Activation of Tween 20
1. Dissolve 5 mg of Tween 20 in 25 ml of dry DMSO with stirring.
2. Add 4 g of CDI with mixing and react for 1 hour at room temperature.
3. Precipitate the CDI-activated Tween 20 by the addition of ethyl ether, and then isolate
the precipitate using centrifugation or fi ltration. Redissolve the precipitated product in
DMSO and repeat the precipitation process two more times to insure removal of excess
reactants and reaction by-products. After the fi nal precipitation, dry the isolated precipi-
tate overnight in vacuo to remove remaining solvent.
Coating Nanotubes with CDI-Activated Tween 20
4. Suspend the SWNTs in 1 percent (w/w) CDI-activated Tween 20 solution in water using
sonication and allow the detergent molecules to bind for 30 minutes at room temperature.
5. Quickly remove excess detergent by fi ltration on a 0.2 m fi lter and washing the modi-
fi ed nanotubes with water.
Coupling of Activated Tween 20 to an Amine-Containing Ligand
6. Dissolve an amine-containing ligand in 0.1 M sodium carbonate, pH 9.5. If coupling a
small ligand, such as a biotin-PEG-amine compound (Chapter 18), then use a concentra-
tion of about 5–10 mM in the carbonate buffer. For proteins, concentrations of 10 nM
to 1 M can be used with success.
7. Resuspend the activated nanotubes in the ligand-containing carbonate buffer and react
with mixing overnight at room temperature or 4 ° C (e.g., for sensitive proteins).
8. Wash the coupled nanotubes with water or a suitable buffer using fi ltration and fi nally
store them in buffer containing a preservative at 4 ° C.
In a similar approach to the noncovalent modifi cation of carbon nanotubes with detergent
molecules, Kam et al. (2005) used phospholipid derivatives to coat SWNTs for photo-
therapeutic agents against tumor cells in vivo. A phospholipid containing a PEG-NH
2
group
was used to couple folic acid as an affi nity ligand (using EDC to form an amide bond), which
preferentially can be taken up by cancer cells. SWNTs were modifi ed by the lipid derivative in
aqueous solution using sonication for 1 hour and centrifuged to remove insoluble material. The
aqueous fraction contained modifi ed nanotubes, which contained the surface adsorbed lipid
derivative. After the modifi ed SWNTs were incubated with cells, the solution was irradiated
2. Carbon Nanotubes 643
644 15. Buckyballs, Fullerenes, and Carbon Nanotubes
using an 808 nm laser. The nanotubes absorb light in this region of the spectrum and heat up
to the point of causing cell death.
2.4. Pyrene Modifi cation of Carbon Nanotubes
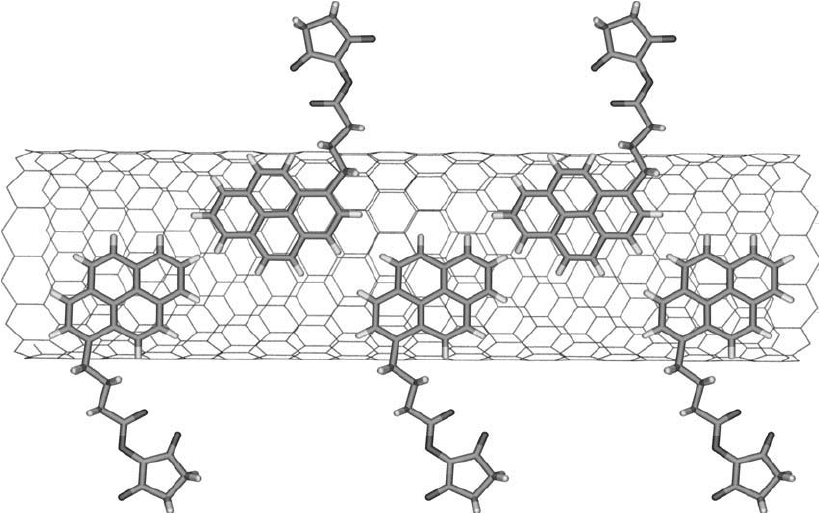
Another method for the noncovalent modifi cation of carbon nanotubes involves the interaction
of pyrene derivatives with the graphene sidewalls, presumably due to -stacking (Nakashima
et al., 2002) ( Figure 15.14 ). This interaction forms tight complexes that completely coat the
nanotube surface, and if the pyrene contains a hydrophilic portion, it can impart water solubil-
ity to the SWNT, as well. The additional presence of reactive groups or functional groups on a
pyrene side chain permits conjugation to affi nity ligands for biological applications.
Nakashima et al. (2002) found that a pyrene derivative containing a single side chain termi-
nating in a positively charged quaternary amine imparted water solubility to SWNTs treated
with the compound. The application of other pyrene derivatives can be done to contribute
both water solubility and an appropriate functionality for bioconjugation. Examples of pyrene
derivatives that are suitable for the noncovalent modifi cation of carbon nanotubes include
those that have a single modifi cation to the pyrene ring structure. Derivatives that contain mul-
tiple modifi cations off the pyrene group may affect its interaction potential for the SWNTs
and should be avoided. For instance, water-soluble poly-sulfonated derivatives of pyrene, such
Figure 15.14 The NHS ester of a pyrene butyric acid derivative can be used to modify a carbon nanotube by
adsorption of its rings onto the surface of the tube. The NHS ester groups then can be used to couple amine-
containing molecules to form amide bonds.
as the Cascade Blue fl uorescent dyes described in Chapter 9, Section 5, should not be used,
because the pyrene structure is too hydrophilic to associate with the nanotube surface due to
the three negative charges contributed by the sulfonic acids.
Some commercially available pyrene compounds that may be used to functionalize a car-
bon nanotube by this method include 1-pyrenebutyric acid and 1-aminopyrene (from Acros or
Aldrich) as well as N-(1-pyrenyl)maleimide, 2-(1-pyrenyl)ethyl chloroformate, 1-pyrenebutyric
acid N-hydroxysuccinimide ester, 1-pyrenecarboxaldehyde, and 1-pyreneacetic acid (from
Aldrich). Each of these compounds provides a single site of derivatization off the basic pyrene
rings to contain either a functional group or reactive group for coupling ligands. Molecules
modifi ed with these pyrene derivatives may be used to treat a carbon nanotube to form a stable
noncovalent complex.
The pyrene derivatives containing a carboxylate group, chloroformate, aldehyde, or an NHS
ester can be used to couple to amine-containing ligands, including proteins. The maleimide-
pyrene derivative may be used to couple with thiol-containing ligands, while the amine–pyrene
compound may be used to conjugate with carboxylate-containing ligands. Also available are
(1-pyrenyl)butyric acid hydrazide and pyrene-1-isothiocyanate from Molecular BioSciences,
which react with aldehydes and amines, respectively. Once a ligand is conjugated to a pyrene
derivative, the complex may be incubated with a carbon nanotube to produce the fi nal non-
covalent complex. Since the initial modifi cation is done on a water-insoluble nanotube, it is
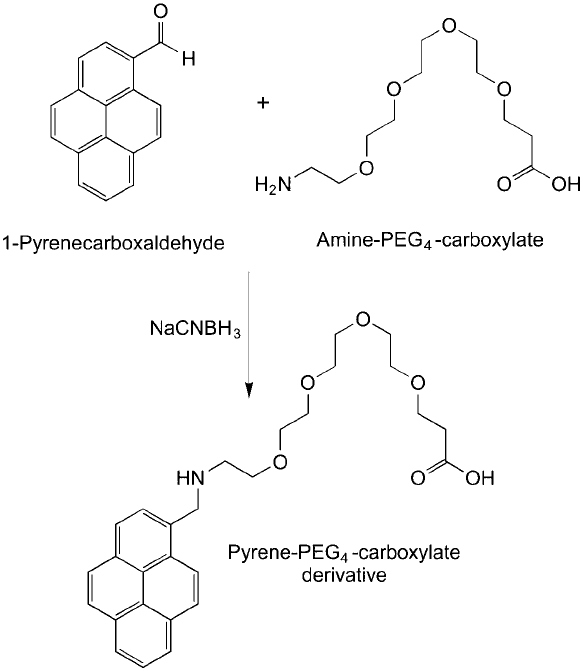
best to do the primary coating of the pyrene derivative in an organic solvent, such as DMF. It
then is desirable to make the nanotube water-soluble by linking a hydrophilic spacer arm to
the pyrene–nanotube complex. If a hydrophilic spacer is built into the resultant pyrene conju-
gate, such as the use of a short-chain PEG compound, then the resultant SWNT complex will
be completely water-soluble (for example, see Figure 15.15 ). The PEG spacer chosen for this
purpose should contain a terminal functional group for coupling to another molecule. At this
point, the water-soluble complex can be reacted with a protein or other affi nity ligand in aque-
ous buffer to make the desired bioconjugate. This multi-step process will result in a biocom-
patible carbon nanotube that retains its electronic properties, is water-soluble, and has added
fl uorescent properties due to the pyrene molecules coating its surface.
Maehashi et al. (2007) used pyrene adsorption to make carbon nanotubes labeled with
DNA aptamers and incorporated them into a fi eld effect transistor constructed to produce a
label-free biosensor. The biosensor could measure the concentration of IgE in samples down
to 250 pM, as the antibody molecules bound to the aptamers on the nanotubes. Felekis and
Tagmatarchis (2005) used a positively charged pyrene compound to prepare water-soluble
SWNTs and then electrostatically adsorb porphyrin rings to study electron transfer interac-
tions. Pyrene derivatives also have been used successfully to add a chromophore to carbon
nanotubes using covalent coupling to an oxidized SWNT (Álvaro et al., 2004). In this case, the
pyrene ring structure was not used to adsorb directly to the nanotube surface, but a side-chain
functional group was used to link it covalently to modifi ed SWNTs.
2.5. Modifi cation of Carbon Nanotubes by Cycloaddition
The covalent methods previously discussed for fullerene modifi cation using cycloaddition
reactions also can be applied to carbon nanotubes. This strategy results in chemically link-
ing molecules to the graphene rings on the outer surface of the cylinder, resulting in stable
2. Carbon Nanotubes 645
646 15. Buckyballs, Fullerenes, and Carbon Nanotubes
conjugates that can be designed to include hydrophilic groups for water solubilization.
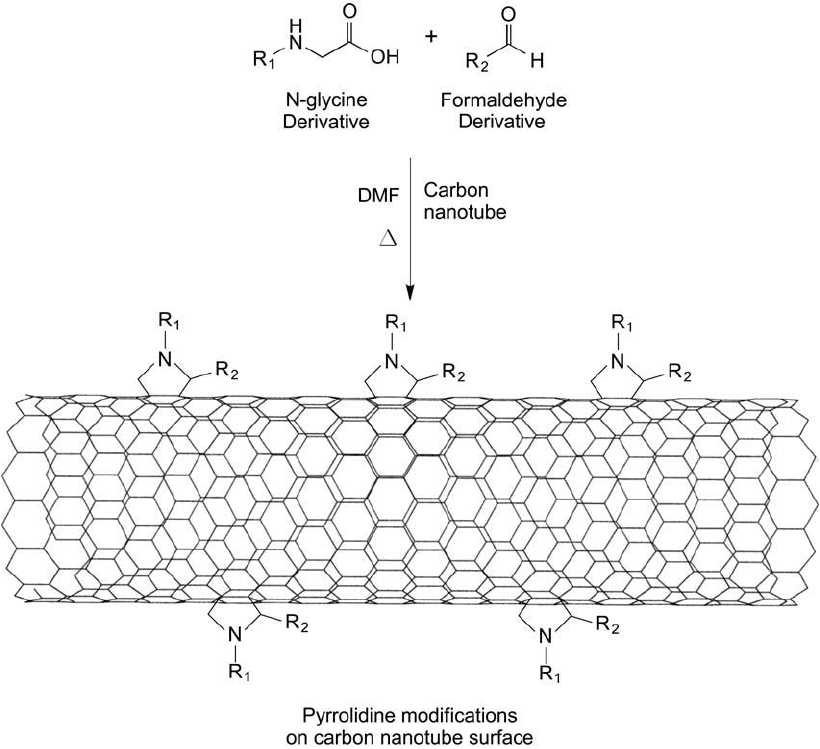
Georgakilas et al. (2002) describe the use of a 1,3-dipolar cycloaddition process to carbon nan-
otubes with azomethine ylides, generated by condensation of an amino acid derivative and an
aldehyde. The reaction occurs in organic solvent at high temperature over a time period of sev-
eral days.
Typically, SWNTs are suspended in DMF using sonication and the aldehyde and glycine
derivatives are added to the mixture with stirring. The -amine derivative of glycine can include
hydrophilic spacers to make the resultant nanotube water-soluble as well as include protected
functional groups to couple affi nity ligands after deprotection (Kurz et al., 1998). The aldehyde
also can include R groups that add water solubility or functionality to the nanotube. A combi-
nation of a glycine derivative with an aldehyde derivative can result in both hydrophilicity and
a functional group to conjugate ligands ( Figure 15.16 ).
Figure 15.15 An aldehyde derivative of pyrene can be used to couple a hydrophilic amino-PEG-carboxylate
spacer by reductive amination. The resultant derivative then can be used to coat a carbon nanotube through
pyrene ring adsorption and result in a water-soluble derivative containing terminal carboxylates for coupling
amine-containing ligands.
Felekis and Tagmatarchis (2005) used this cycloaddition process to prepare SWNT deriva-
tives possessing photoactive components, such as the addition of ferrocene groups. They used a
short PEG-type spacer on the glycine to impart water solubility at the same time.
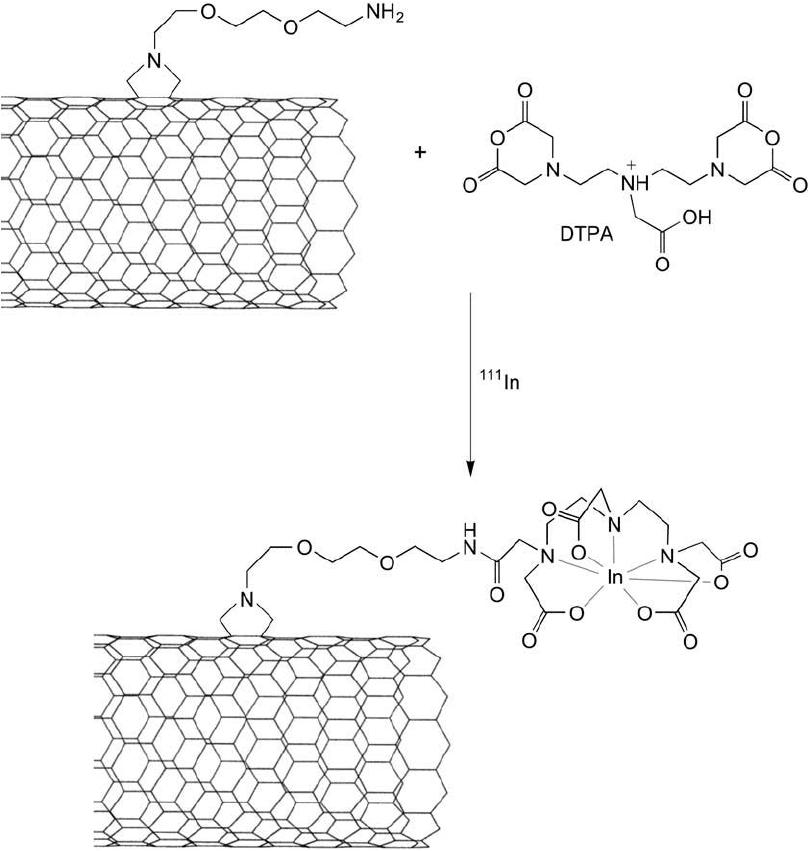
Singh et al. (2006) also used cycloaddition to prepare carbon nanotubes containing indium
labeled diethylenetriamine pentaacetic acid (DTPA) derivatives ( Figure 15.17 ). In the initial
modifi cation, a SWNT was derivatized to contain a primary amine at the end of a short PEG
spacer. The resultant water-soluble nanotube then was reacted with DTPA to create a metal
chelating group at the end of the chain. Subsequent loading of the chelate with
111
In created a
radionuclide–SWNT complex for in vivo biodistribution studies.
Figure 15.16 Some modifi cation methods that are useful for fullerenes also can be used with carbon nanotubes.
The reaction of an N-glycine compound with an aldehyde derivative can result in cycloaddition products, which
create pyrrolidine modifi cations on the nanotube surface.
2. Carbon Nanotubes 647
648 15. Buckyballs, Fullerenes, and Carbon Nanotubes
Figure 15.17 An amino-PEG-pyrrolidine derivative of carbon nanotubes can be used to couple metal chelating
groups, such as DTPA. Subsequent coordination of
111
In results in an indium chelate that can be used for imag-
ing applications.
The demonstration that the 1,3-dipolar cycloaddition process with azomethine ylides works
with nanotubes implies that similar reactions developed for use with fullerenes also may
be successful with carbon nanotubes. In particular, the cyclopropanation reactions discussed
previously for the modifi cation of C
60
, likely will work for derivatization of SWNTs and
MWNTs (Zakharian et al ., 2005).

649
16
Mass spectrometry has become one of the most important tools for analyzing proteins in com-
plex biological samples. The ability to separate proteins and peptides in high resolution has made
possible the simultaneous identifi cation of hundreds of proteins within samples (for reviews, see
Gingras et al., 2005; Hamdan and Righetti, 2005; Siuzdak, 2006). Proteins can be analyzed for
their presence or compared between samples for their relative expression level. One cell popula-
tion treated with a drug candidate, for instance, can be compared by mass spec to another sample
as control to assess the affect of the drug on expression levels of certain proteins.
There are several ways that proteins can be analyzed using mass spec. Whole proteins can
be separated using electrospray ionization (ESI) technique or by using matrix-assisted laser
desorption/ionization (MALDI). Both of these methods inject intact proteins into the mass
spectrometer, ionize them, and separate the resultant charged components by their individual
mass/charge ratios. These methods work well for small and medium sized proteins in sam-
ples of low complexity, but analysis of larger proteins or highly complex samples is diffi cult.
Alternatively, proteins fi rst can be proteolytically digested using an enzyme such as trypsin and
then the peptides analyzed by mass spec. This proteolysis method is more universally appli-
cable, because analysis of peptide fragments allows mass spec separation to be done for all
proteins regardless of their original intact mass prior to digestion. The peptides typically are
subjected next to a higher energy secondary mass spec separation that fragments them into
their component amino acids, which then can be identifi ed by their masses. Samples then are
analyzed by correlation of the peptide sequences to online databases of mass spec information,
which can identify the protein that each peptide came from.
However, the interpretation of mass spec data on whole samples can be daunting, especially
when analyzing proteolytically digested samples, which results in many times more species to
analyze per protein than intact proteins. In order to reduce the complexity of sample analy-
sis, a number of techniques have been developed to fractionate the proteome prior to mass
spec separation. For instance, two-dimensional electrophoresis can separate proteins both by
charge and by molecular weight and allow picking of only certain spots for subsequent mass
spec analysis. However, two-dimensional electrophoresis is severely limited in its sensitivity for
picking up low or medium copy proteins (Gygi et al., 2000). Alternatively, affi nity separations
on resins or surfaces can be done to capture only those proteins having certain epitopes or
chemical characteristics, such as post-translational modifi cations. In addition, nanoliter HPLC
Mass Tags and Isotope Tags
