Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

630 15. Buckyballs, Fullerenes, and Carbon Nanotubes
Protocol
1. In a fume hood, prepare a solution of 100 mg of C
60
dissolved in 100 ml of toluene.
2. Add to the fullerene solution with mixing, 25 mg of N-methyl glycine (sarcosine) and
20 mg of paraformaldehyde.
3. React by refl uxing for 2 hours with mixing.
4. Remove solvent under vacuum and purify the N-methyl-3,4-fulleropyrrolidine ( Figure
15.4 ) by fl ash chromatography using toluene as eluent.
A similar preparative procedure can be used to form the N-(triphenylmethyl)-3,4-fulleropyr-
rolidine, which is a trityl-protected amine derivative that may be deprotected using trifl uorometh-
anesulfonic acid (TFMSA) to create a free secondary amine for conjugation. Kurz et al. (1998)
used this C
60
derivative to react with 3-maleimidopropionyl chloride to form a sulfhydryl-reactive
fullerene. Although this derivative was not soluble in aqueous buffers, it was reactive enough as a
micro-precipitate to be conjugated to thiol-containing proteins using the following procedure.
Protocol
1. The thiol-containing protein is dissolved as a 60 M solution in 20 mM HEPES, pH 7.
2. Add the maleimide-C
60
derivative to the protein solution at 100 mole excess with stir-
ring. The addition of detergent to the solution may increase the solubility of the fullerene
compound.
3. React with mixing at 4°C for 72 hours.
4. Purify the labeled protein by size exclusion chromatography using a column with an
exclusion limit of molecular weight 5,000.
The nitrogen group in fulleropyrrolidines can be used for conjugation with crosslinking
agents or hydrophilic biotinylation compounds for subsequent use in bioconjugation reactions.
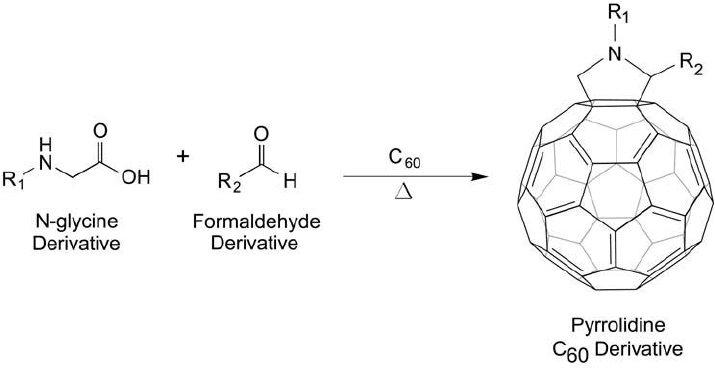
Figure 15.3 The reaction of a glycine derivative and a formaldehyde compound with a C
60
molecule leads to
the formation of a fulleropyrrolidine.
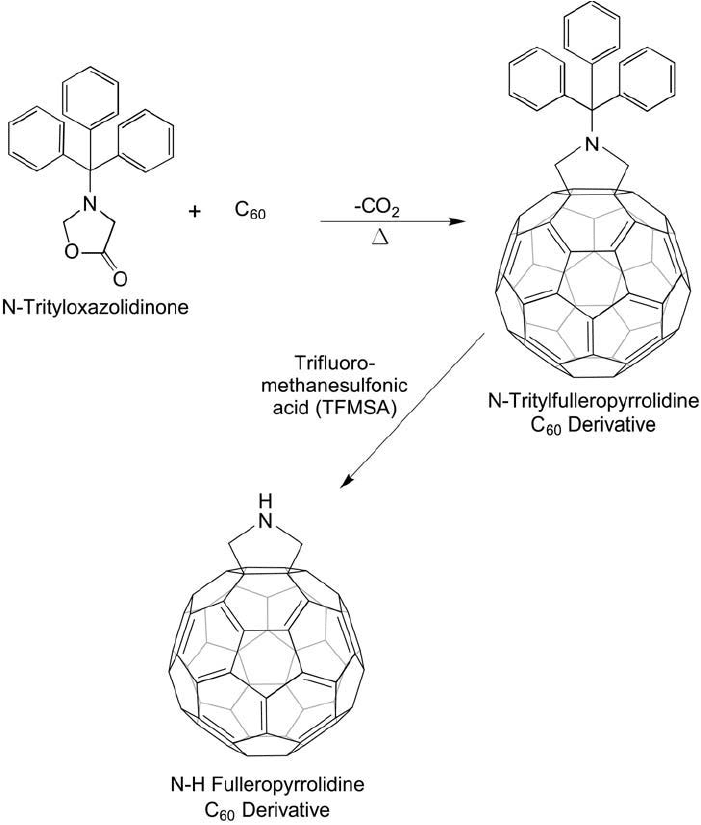
To create the free secondary amine group (N-H) fulleropyrrolidine, an amine-protected starting
material can be used in the reaction (Cai et al., 2006). For instance, a trityl-oxazolidinone
(using either triphenylmethyl- or better, 4-methoxytriphenylmethyl-protecting groups) can be
reacted with C
60
to yield the trityl-protected pyrrolidine ( Figure 15.5 ).
The following procedure for creating the N-H fulleropyrrolidine is adapted from Maggini
et al. (1994), Prato and Maggini (1998), and Prato et al. (1996). Extreme care should be taken
when using TFMSA, as this acid is 30 times stronger than concentrated sulfuric acid.
Protocol
1. Dissolve 100 mg of C
60
in 130 ml of chlorobenzene in a fume hood.
2. Add with mixing to the fullerene solution 54 mg of either N -(triphenylmethyl)-5-
oxazolidinone or the same amount of the methoxytrityl oxazolidinone.
3. Refl ux overnight in a fume hood with constant mixing.
4. Remove the solvent under vacuum and purify the residue using fl ash chromatography
with an 8:2 mixture of petroleum ether/toluene as the eluent.
5. Remove the trityl-protecting group by dissolving the derivatized fulleropyrrolidine in 5 ml of
dichloromethane in a fume hood and then adding 50 l of TFMSA (caution!) with stirring.
6. React at room temperature for 1 hour to remove the trityl-protecting group, yielding the
N-H pyrrolidine derivative of C
60
.
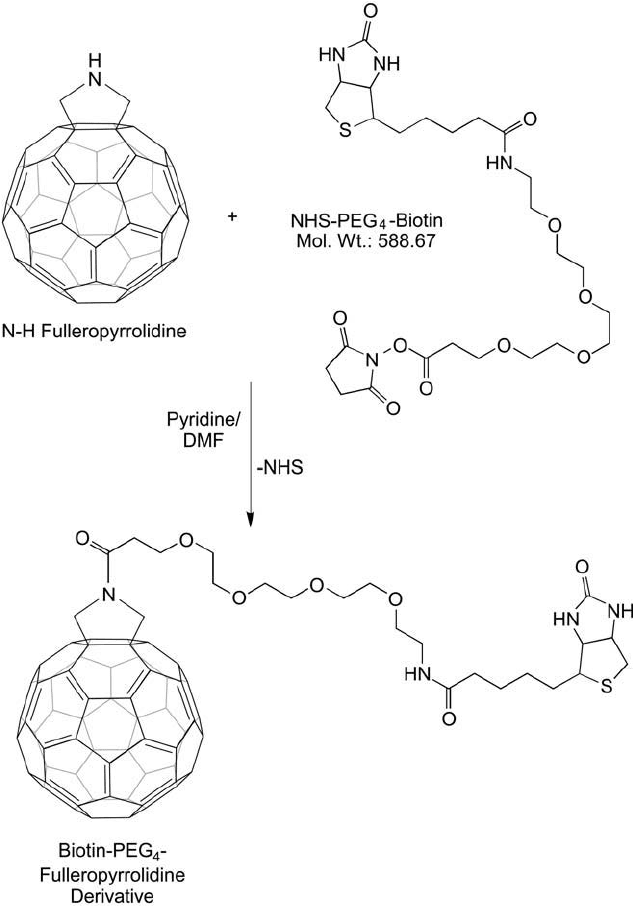
Capaccio et al. (2005) used the N-H fulleropyrrolidine derivative to couple long-chain biotin
to the C
60
using the carbodiimide dicyclohexyl carbodiimide (DCC) in a pyridine/DMF/CH
2
Cl
2
solvent mixture and reacting overnight at room temperature. This derivative was prepared
using a hydrophobic, aliphatic long-chain biotin, but similar derivatives could be prepared
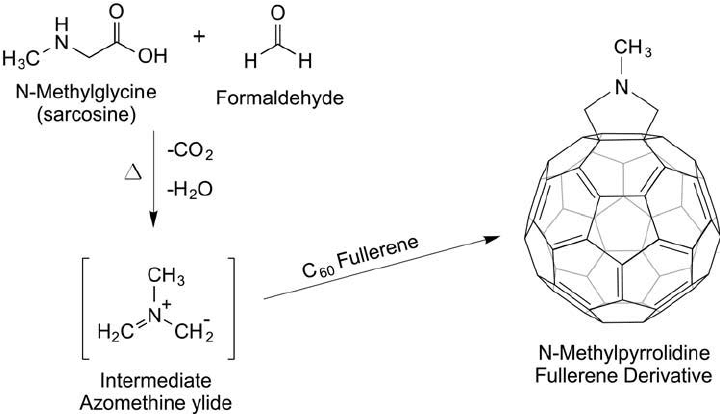
Figure 15.4 The synthesis of N -methylpyrrolidine-C
60
proceeds through an intermediate azomethine ylide by
the reaction of N -methylglycine plus formaldehyde upon heating.
1. Buckyballs and Fullerenes 631
632 15. Buckyballs, Fullerenes, and Carbon Nanotubes
using a biotin-PEG-carboxylate compound (e.g., NHS–PEG
4
–biotin) to create a hydrophilic
fullerene modifi cation ( Figure 15.6 ). The biotinylated fullerene could be used with streptavidin
and another biotinylated enzyme to create a bioconjugate.
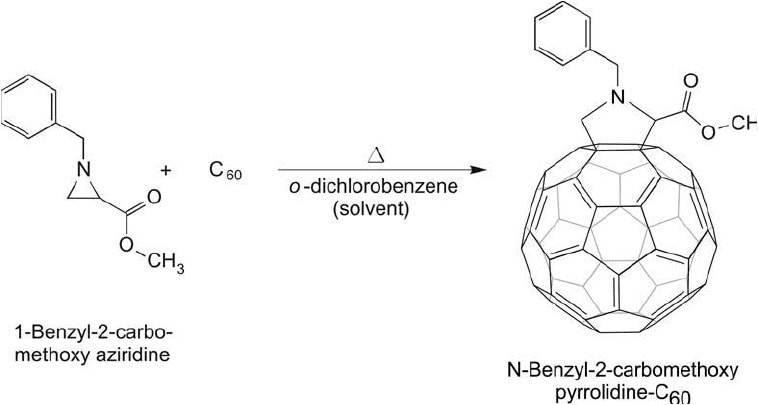
Similar to the fullerene modifi cations using either glycine/formaldehyde derivatives or
oxazolidinone compounds, Maggini and Scorrano (1993) found that aziridines could yield simi-
lar pyrrolidine derivatives. Heating aziridine compounds in toluene was found to result in ring
Figure 15.5 A trityl-protected pyrrolidine derivative of C
60
can be prepared by the reaction of N -trityl-
oxazolidinone with a fullerene. Deprotection of the trityl group using methanesulfonic acid gives the secondary
amine, which can be used in further conjugation reactions.
opening of the aziridine with subsequent covalent linking to the C
60
ring system ( Figure 15.7 ).
Thus, synthesizing pyrrolidine derivatives of fullerenes can be done by several routes with success.
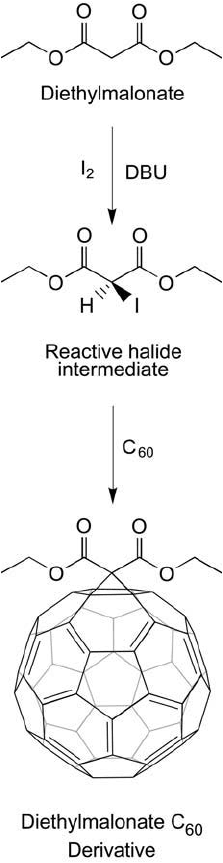
Another route to the preparation of C
60
derivatives involves the reaction of a halogen deriv-
ative of diethylmalonate in the presence of a strong base with fullerenes, which results in a
Figure 15.6 Reaction of an N-H fulleropyrrolidine with NHS–PEG
4
–biotin creates the biotinylated C
60
deriva-
tive via an amide bond.
1. Buckyballs and Fullerenes 633
634 15. Buckyballs, Fullerenes, and Carbon Nanotubes
cyclopropanation product (Bingel, 1993; Isaacs and Diederich, 1993; Isaacs et al., 1993). Using
this reaction, various malonate derivatives of C
60
can be made to facilitate production of bio-
conjugates (Zakharian et al., 2005). This reaction often is called the Bingel reaction (or Bingel–
Hirsch addition) after the publication by the author in 1993 (see also Hirsch et al., 1994). The
reaction can yield carboxylate derivatives that then can be used to couple biomolecules through
carbodiimide conjugation ( Figure 15.8 ).
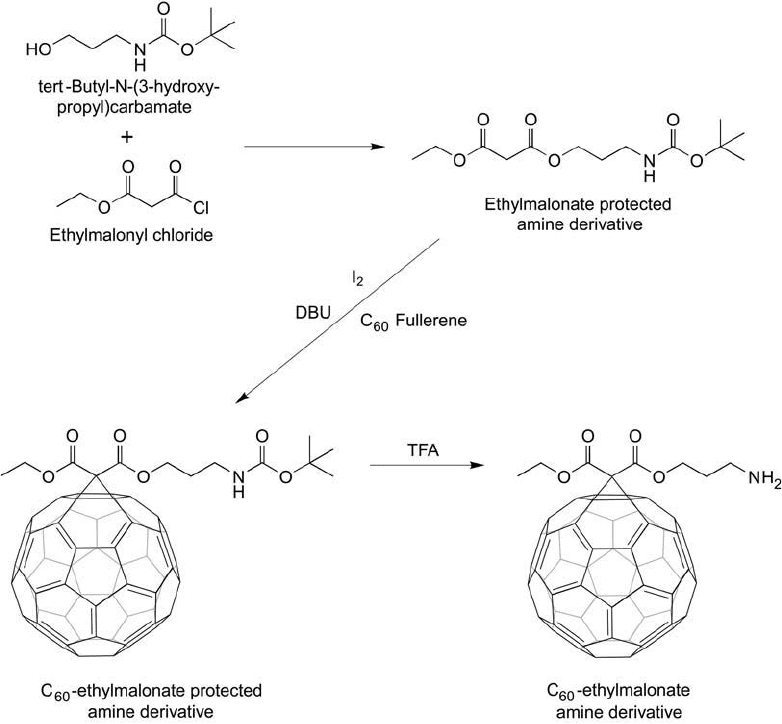
The following protocol represents a C
60
modifi cation with an ethylmalonate derivative using
the Bingel-type reaction, which creates an amine functional group on the fullerene surface,
and is based on the method of Zakharian et al., 2005 ( Figure 15.9 ). Ashcroft et al. (2006)
used this method to create C
60
immunoconjugates with the murine anti-gp240 melanoma anti-
body, which was made water-soluble through the additional modifi cation of the fullerene with
hydrophilic malonodiserinolamide groups.
Protocol
1. In a fume hood, prepare a solution of 1.16 g tert -butyl- N -(3-hydroxypropyl)carbamate
(Aldrich) in 100 ml of dry CH
2
Cl
2
and add 1 ml of pyridine with stirring.
2. Cool the solution to 0 °C and slowly add 1 g of ethylmalonyl chloride (Aldrich) under
nitrogen with stirring.
3. React for 12 hours with stirring at room temperature.
4. The resultant ethylmalonate-protected-amine compound is concentrated in vacuo and
then purifi ed using silica gel chromatography with a hexane/ethyl acetate (1:1) eluent.
5. In a fume hood, dissolve 400 mg of C
60
in 700 ml of toluene with stirring.
6. Add to the C
60
solution 100 mg of the purifi ed ethylmalonate-protected-amine com-
pound from step 4 along with 88 mg of I
2
and 105 mg of DBU (Aldrich).
Figure 15.7 The reaction of aziridine derivatives with fullerenes also can give pyrrolidine derivatives useful for
bioconjugation.
7. React for 30 minutes at room temperature with mixing.
8. Remove the solvent in vacuo and purify the resultant mixture using silica gel chromatog-
raphy. The initial eluent is toluene, which will remove the unreacted C
60
, and then fol-
lowed by a 10:1 mixture of toluene/ethyl acetate to elute the desired C
60
-protected-amine
derivative.
Figure 15.8 The Bingel reaction for the modifi cation of fullerenes involves the in situ formation of a reactive
halogen species in the presence of the strong base DBU. The cyclopropanation product can be used to create
many bioconjugates.
1. Buckyballs and Fullerenes 635
636 15. Buckyballs, Fullerenes, and Carbon Nanotubes
9. To remove the tert-butyl-protecting group on the amine, in a fume hood add 190 mg
of the C
60
-protected-amine derivative from step 8 to 50 ml CH
2
Cl
2
with stirring. Add
to this solution with stirring 50 ml of TFA and mix for 30 minutes. Remove the solvent
in vacuo to yield the fi nal amine–C
60
compound.
The amine group on the C
60
fullerene may be used to couple carboxylate-containing spac-
ers or affi nity ligands using a carbodiimide conjugation reaction to form an amide linkage. The
reaction may be done in organic solvent or aqueous buffer, depending on the hydrophilicity
of the fullerene derivative. A mixture of organic and aqueous solution also may be done if the
ligand is more soluble in an aqueous environment.
Figure 15.9 The reaction of the amine-blocked derivative of 3-hydroxypropylamine with ethylmalonyl chloride
gives an ethylmalonate-protected-amine compound, which can be used in the Bingel reaction to create an amine
group on a fullerene surface. Reaction with C
60
in the presence of I
2
and DBU gives the cyclopropanation prod-
uct that can be deprotected with TFA to yield the free amine.
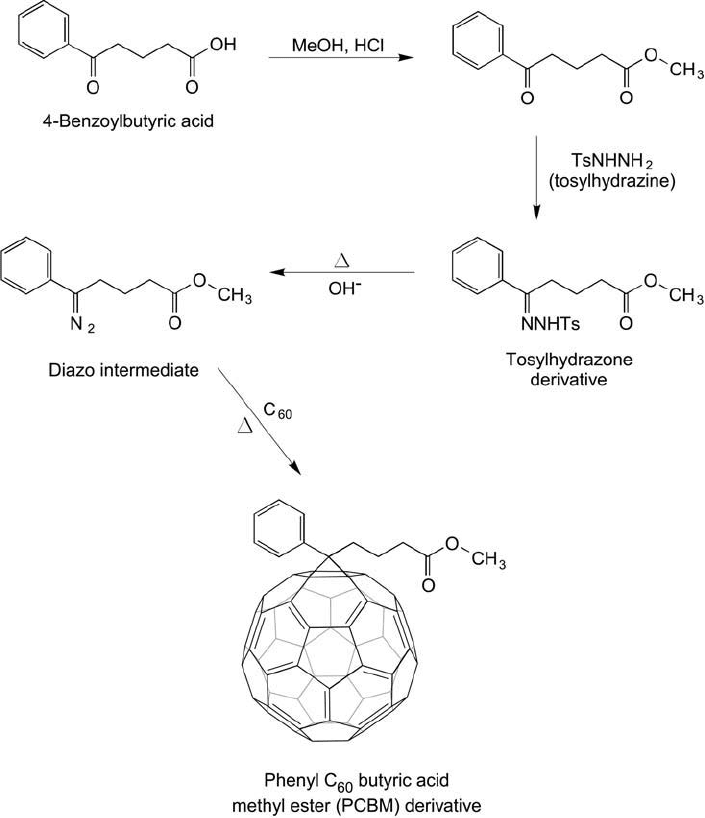
A similar fullerene modifi cation process developed by Hummelen et al. (1995) resulted in
a number of phenyl C
60
butyric acid methyl ester (PCBM) derivatives, which now are com-
mercially available (Nano-C, Solenne). The reaction proceeds through the creation of a diazo
derivative of methyl 4-benzoylbutyrate, which then is reacted with the aromatic ring system of
C
60
to give the cycloaddition product ( Figure 15.10 ). The conjugate forms through the forma-
tion of both a 5,6 ring addition product (fulleroid) or a 6,6 ring addition product (methanof-
ullerene). The fulleroid product results in breaking the 5,6 ring junction on the C
60
molecule
with dimeric bond formation to the aryl methyl group on PCBM.
1. Buckyballs and Fullerenes 637
Figure 15.10 Fullerene-PCBM derivatives can be prepared using reactive diazo intermediates, which yield a
cyclopropanation product similar to the Bingel reaction derivatives.
638 15. Buckyballs, Fullerenes, and Carbon Nanotubes
The PCBM methyl ester can be used for coupling amine-containing ligands after removal
of the methyl group and activation of the carboxylate using a number of different reaction
strategies. Hummelen et al. (1995) successfully coupled cholestanol and histamine to the fuller-
ene-PCBM derivative (after acid chloride formation) for use in fabrication of photodetectors
and biological studies, respectively. For specifi c applications of PCBM-fullerenes, see Shaheen
et al. (2001), Brabec et al. (2001), Yu et al. (1995), Mecher et al. (2002), Meijer et al. (2003),
van Duren et al . (2004), and Anthopoulos et al . (2004).
Various commercial suppliers now offer fullerene derivatives with functionalities avail-
able for bioconjugation, including carboxylic and poly-hydroxylic derivatives, which are very
hydrophilic and water-soluble (BuckyUSA, NanoLab, NanoNB, Nano-C, and Aldrich).
2. Carbon Nanotubes
2.1. Nanotube Properties
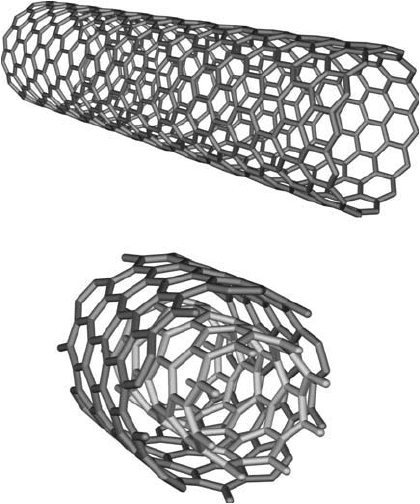
A cousin of the spheroidal fullerene molecules is carbon nanotubes. These are cylindrical fuller-
enes with either open or closed ends and consist of carbon atoms arranged in a hexagonal pat-
tern. There are two main families of carbon nanotubes that are distinguished by being either
single-walled nanotubes (SWNTs) or multi-walled nanotubes (MWNTs). SWNTs actually are
a graphene sheet that is seamlessly wound into a cylinder. MWNTs consist of multiple SWNTs
that are concentrically wound around each other and nested together to create a tubes-within-
tubes confi guration ( Figure 15.11 ). The type of nanotube also is determined by manner in
which the hexagonal pattern of carbon rings is arranged in the cylindrical structure.
In most publications, Iijima is given credit for the discovery in 1991 of the nanotube struc-
ture of carbon (Iijima, 1991; Bethune et al., 1993; Iijima and Ichihashi, 1993). However, it
has been said that Oberlin et al. (1976) also imaged carbon nanotubes, perhaps even SWNTs.
Incredibly, nearly a century earlier, there was a study on the thermal decomposition of methane
that resulted in the formation of long carbon strands, which were proposed at the time as a
candidate for fi laments in light bulbs (see Bacon and Bowman, 1957).
SWNTs are typically only a few nanometers in diameter (0.4 to 3 nm), but MWNTs can
be from about 1.4 nm to over 100 nm in diameter, depending on the number of concentric
nanotubes making up the bundle (Baughman et al., 2002). However, carbon nanotubes can be
from nanometers to millimeters or even microns in length, depending on how they are made.
The length-to-diameter ratio typically exceeds 10,000 in most preparations. This unique molec-
ular structure results in fascinating properties, which include extremely high tensile strength,
electrical conductivity (or even semiconductor properties, depending on how the graphene
sheet is wrapped), resistance to heat, and a great deal of chemical robustness. Nanotubes are
being explored for use in applications ranging from electronics, optics, material science, bio-
medicine, biosensors, hydrogen storage, and nanoelectromechanical systems (NEMS) fabrica-
tion. The rate of growth in nanotube related patents and publications have been nearly on an
exponential increase since the early 1990s, demonstrating the broad applications they can be
used in (Baughman et al ., 2002; Park et al ., 2003).
The tensile strength of carbon nanotubes has been determined to be over 50 times that of
high-carbon steel (Yu et al., 2000). The strength of the bond structure in carbon nanotubes
results from the fact that they are entirely sp
2
bonds, which are even stronger than the sp
3
carbon bonds in diamond. The addition of nanotubes to polymers, metals, and other struc-
tural materials has been used to add considerable strength to these materials. In fact, a carbon
nanotube tether into low Earth orbit has been proposed as the only way of creating an elevator
system into space.
The orbital bonding nature within carbon nanotubes creates unique electrical properties
within a non-metallic molecule, which is a result of the delocalization of the -electron donated
by each atom. Electrical conductivity can take place along the entire nanotube due to the free-
dom of -electron fl ow, making possible the design of circuits of extremely low nanometer
diameter.
Synthetic methods for the production of carbon nanotubes include arc discharge from
graphite electrodes (Iijima, 1991; Collins and Avouris, 2000), pulsed laser ablation of a graph-
ite substrate under high temperature (Guo et al., 1995a, b), chemical vapor deposition (Jose-
Yacaman et al., 1993; Ren et al., 1998), and high-pressure carbon monoxide (HiPco) method
developed by Carbon Nanotechnologies, Inc. Methods for nanotube purifi cation have been
developed that result in 99.9 percent pure single-wall nanotubes (Chiang et al ., 2001).
Unlike the smaller, spheroidal fullerenes discussed previously, carbon nanotubes are not eas-
ily solubilized, even in organic solution. The reality is that all SWNTs and MWNTs are insolu-
ble in all solvent systems. They also have a strong tendency to bind together and aggregate due
to van der Waals attractive forces along the length of the nanotube. Since the length-to-diameter
2. Carbon Nanotubes 639
Figure 15.11 An example of a single-walled carbon nanotube (a) and a multi-walled carbon nanotube (b).
Multi-walled varieties can consist of numerous tubes within tubes.
