Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

620 14. Microparticles and Nanoparticles
alkaline conditions above pH 8.0. Surface treatment with organosilane derivatives can stabilize
particles to hydrolysis, but long-term exposure to highly alkaline environments still should be
avoided. It is also recommended that at least 0.05 M NaCl be maintained to increase stability
of the particles in aqueous environments. The best stability for silica is obtained at neutral or
acidic pH conditions containing a low concentration of salt.
5.1. Fluorescent Silica Particles
Silica particles have been exploited in virtually every assay or detection strategy that polymer
particles have been used in for bioapplication purposes. Recently, fl uorescent dye-doped sil-
ica nanoparticles have been developed by a number of groups that have similar fl uorescence
characteristics to quantum dot nanocrystals (Chapter 9, Section 10). Fluorescent silica nano-
particles can be synthesized less expensively than quantum dots due to the fact that the silica
particles incorporate standard organic dyes (Ow et al., 2005; Wang et al., 2006) and are not
dependent on making reproducible populations of semiconductor particles with precise diam-
eters to tune emission wavelengths.
The preparation of fl uorescent silica particles can be done using a number of strate-
gies. Santra et al. (2001) describe a water-in-oil emulsion using detergent-mediated reverse
micelle formation and controlled hydrolysis of TEOS to create mono-disperse silica nanopar-
ticles (see also Arriagada and Osseo-Asare, 1995). Adding the water-soluble fl uorescent dye,
tris(2,2-bipyridyl) dichlororuthenium (II) hexahydrate (Ru(II)bpy
3
2
) to this emulsion
resulted in dye molecules being entrapped within the silica particle structure as it formed
(Figure 14.23 ) (also see Chapter 28, Section 4.2 for additional properties of Ru(II)bpy
3
2
).
Subsequent functionalization of the surface with silane derivatives can be done to facilitate lig-
and immobilization.
These Ru(II)bpy
3
2
fl uorescent silica nanoparticles were used to detect single bacterial
cells using antibodies conjugated to the surface after functionalization with trimethoxysilyl-
propyldiethylenetriamine followed by succinylation to create carboxylates. Specifi c antibody
molecules against E. coli O157 then were coupled to this modifi ed fl uorescent particle using
the carbodiimide method with EDC and NHS (Zhao et al. , 2004).
Another type of fl uorescent silica particle was formed from silica bubbles created on the
surface of gold nanoparticles (Liz-Marzan et al., 1996; Makarova et al., 1999). Fluorescein
isothiocyanate (FITC) was adsorbed onto the gold nanoparticle surface and then reacted with
3-aminopropyltrimethoxy silane. The isothiocyanate groups on the adsorbed dye molecules
coupled to the amine groups on the silane as it polymerized on the gold surface, effectively
forming a silica shell around the gold particle. The gold core then was dissolved by reaction
with cyanide ions to leave behind the silica nanobubbles fi lled with water, which also left the
fl uorescent molecules attached on the inner surface ( Figure 14.24 ).
Fluorescent silica nanoparticles, called FloDots, were created by Yao et al. (2006) by two
synthetic routes. Hydrophilic particles were produced using a reverse micro-emulsion process,
wherein detergent micelles formed in a water-in-oil system form discrete nanodroplets in which
the silica particles are formed. The addition of water-soluble fl uorescent dyes resulted in the
entrapment of dye molecules in the silica nanoparticle. In an alternative method, dye molecules
were entrapped in silica using the Stöber process, which typically results in hydrophobic par-
ticles. Either process resulted in luminescent particles that then can be surface modifi ed with
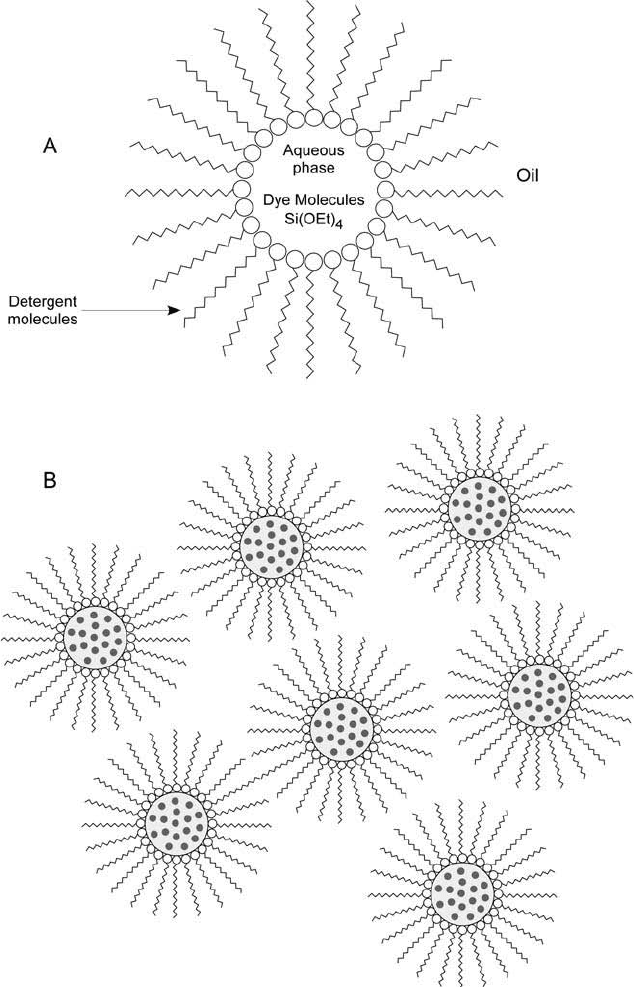
Figure 14.23 Silica nanoparticles containing fl uorescent dye molecules can be prepared using a reverse micelle
suspension process: (a) The water-in-oil emulsion is formed with the aqueous phase droplets containing TEOS
and dye molecules in detergent. (b) The fi nal particles contain entrapped dye within the silica particle matrix,
creating highly fl uorescent particles.
5. Silica Particles 621
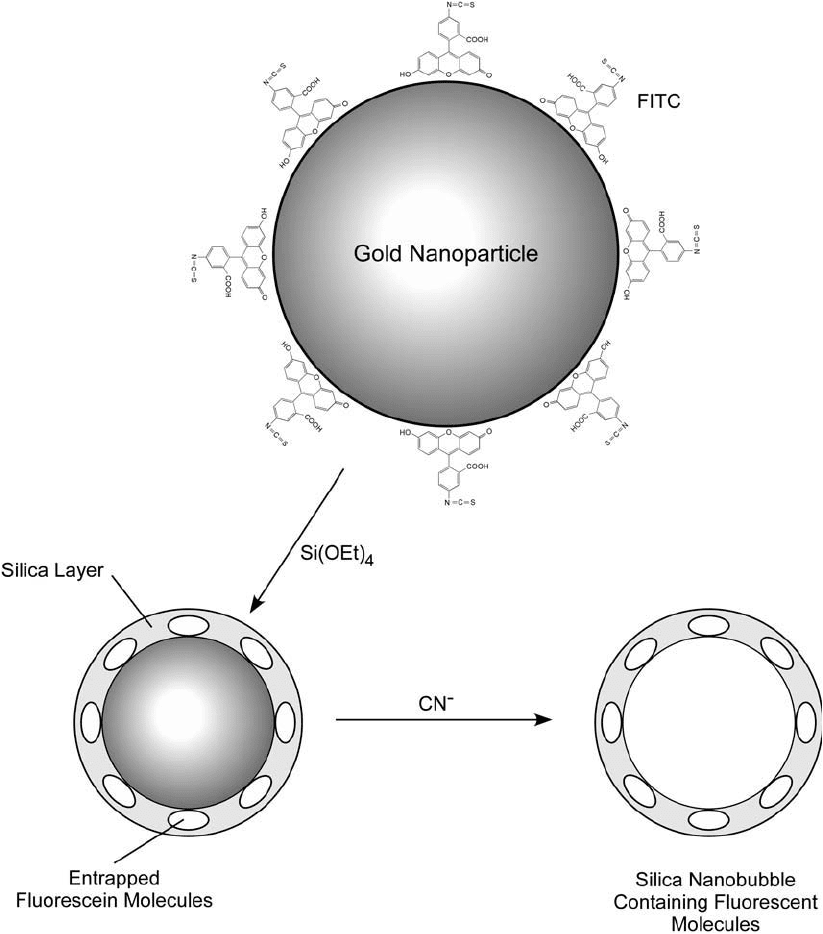
622 14. Microparticles and Nanoparticles
Figure 14.24 Fluorescent silica nanobubbles have been created using gold nanoparticle seeds that initially are
coated by adsorption with a fl uorescent dye. The particles then are capped by a layer of silica by polymerizing
TEOS and entrapping the dye molecules within it. Finally, the gold core is dissolved by reaction with cyanide,
leaving behind hollow fl uorescent silica nanobubbles.
functional silanes to contain appropriate functionalities for coupling to affi nity ligands or bio-
molecules. Ru(II)bpy
3
2
or standard organic fl uorescent dyes can be incorporated into such sil-
ica particles with high effi ciency. In one example, a 70 nm particle containing Ru(II)bpy
3
2
was
found to be equal in fl uorescence intensity to 39 quantum dots having an emission at 605 nm
(Yao et al., 2006). Another silica nanoparticle prepared using a rhodamine dye contained about
1,290 molecules of the dye entrapped within each particle, which produced intensely fl uores-
cent labels.
In a different method of producing dye-doped silica particles, van Blaaderen and Vrij (1992)
and Verhaegh and van Blaaderen (1994) developed a method to covalently link amine-reactive
dyes to APTS and then polymerize the resultant conjugate with TEOS in mixtures of ammonia,
water, and ethanol to form fl uorescent “ organosilica ” spheres. The dye molecules could be dis-
persed throughout the entire particle, contained in the core, or within discrete spherical regions
within the particles. Either hydrophilic or hydrophobic particles could be created, depending
on the surface treatment used subsequent to particle formation. Fluorescent particles consisting
of either fl uorescein or rhodamine dyes made by this method were shown to be susceptible to
photobleaching similar to the organic dyes in solution.
The following protocol is based on the creation of fl uorescent silica core/shell particles using
the method of van Blaaderen and Vrij (1992).
Protocol
Formation of dye-silica core particles:
1. React APTS (11.5 mg) with FITC (10.6 mg) in anhydrous ethanol (1 ml) with mixing for
12 hours (protect from light). The use of anhydrous conditions will prevent the hydroly-
sis of the APTS alkoxy groups, which would cause premature condensation.
2. Prepare a solution consisting of 75 ml of ethanol containing 8.5 ml of ammonia.
3. To the stirring ethanol/ammonia solution, add 3.3 ml of TEOS along with the completed
reaction solution from step 1, which is now the conjugate of fl uorescein and APTS linked
through the amino group on the alkyl silane.
4. The reaction is allowed to continue for 24 hours with slow stirring.
5. Wash the resultant core particles twice with the ethanol/ammonia solution using
centrifugation.
Formation of the silica shell:
1. Dilute the particles to 1.2 l using a 10:1 mixture of ethanol:ammonia. Add 28 ml of
TEOS to the particle suspension and mix to dissolve.
2. React for 24 hours with slow mixing.
3. Wash the particles with the ethanol/ammonia solution several times using centrifuga-
tion. Finally, wash the particles into the desired solution (ethanolic or aqueous) without
ammonia present to prevent silica hydrolysis upon storage.
Ow et al. (2005) developed an improved method of incorporating fl uorescent molecules into
silica particles using a modifi ed Stöber synthesis, which resulted in both enhanced fl uorescence
and photostability of the encapsulated dyes. In this two-stage procedure, reactive organic dyes
5. Silica Particles 623
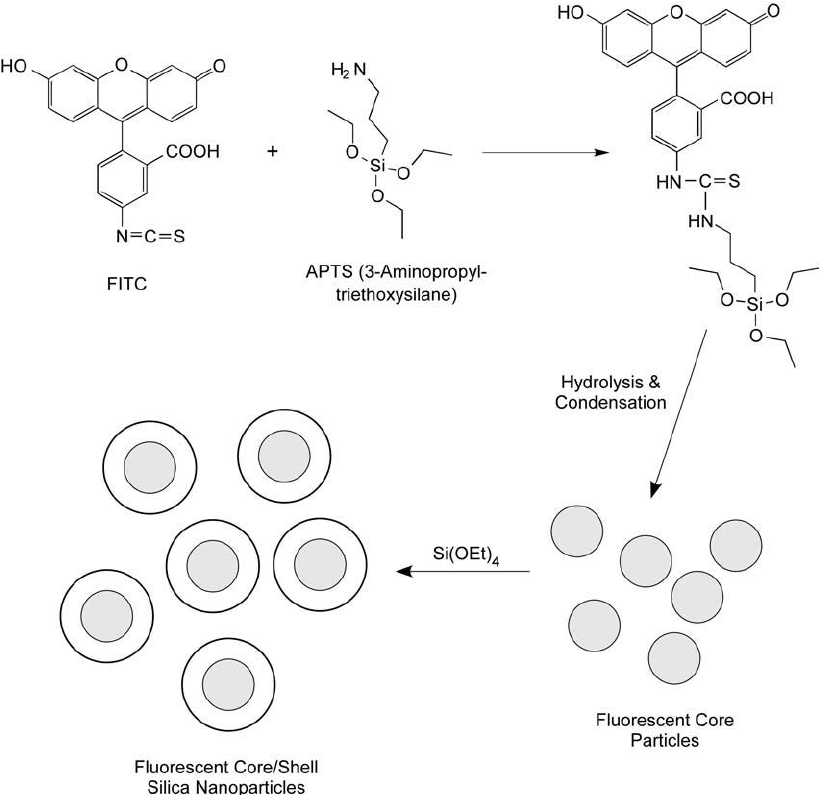
624 14. Microparticles and Nanoparticles
fi rst are conjugated to a silane derivative and condensed to form a polysiloxane-dye-rich nano-
particle core structure. No TEOS is added at this point. These dyed core nanoparticles then are
used as seed for the addition of silica sol–gel monomers to condense around the core and cre-
ate a silica network (shell) around the dye-rich core ( Figure 14.25 ). Nyffenegger et al. (1993)
used a similar process to form fl uorescein particles by fi rst coupling FITC to 3-aminopropylt-
rimethoxy silane to form a thiourea derivative, and then condensing the dye silane derivative
with tetramethoxy silane to form the dyed silica particles.
Figure 14.25 The preparation of highly controlled fl uorescent silica nanoparticles can be done by fi rst polymer-
izing APTS that has been covalently modifi ed with an amine-reactive dye to form fl uorescent core particles. The
core then is capped by a shell of silica by polymerization of TEOS. The shell layer can be further derivatized
with silane coupling agents to provide functional groups for conjugation.
The method developed by Ow et al. permits control over the size of the particles and allows
the incorporation of virtually any organic dye into silica, provided it can be fi rst conjugated
to a silane derivative to form the core. Fluorescent particles made by this procedure may be
made as monodisperse populations having diameters from less than 10 nm to over 1 m sized
spheres, with a high degree of precision. The core size also can be varied by changing the con-
centration of the dye-silane derivative during the condensation process. The procedure for
making these particles is similar to that described above using the method of van Blaaderen
and Vrij (1992), but without the addition of TEOS for creation of the dye-silane core. Exact
procedures are given in Wiesner et al. (2006).
In the preparation of 15 nm core–shell fl uorescent silica particles, Ow et al. (2004) reported
that the naked core (2.2 nm) alone produced a fl uorescence intensity of less than the free dye
in solution, presumably due to dye quenching. However, upon addition of the outer silica shell
around the core, the brightness of the particles increased to 30 times that of the free dye (using
tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC)). They speculate that shell may pro-
tect the core from solvent effects, as evidenced by a lack of spectral shift upon changing the
solvent in which the particles are suspended.
The enhanced photophysical properties of these fl uorescent core–shell silica nanoparticles
make them potentially as useful as semiconductor quantum dots for bioconjugation purposes.
In this case, standard, commercially available, organic dyes can be incorporated into the sil-
ica nanoparticles to provide a range of emission properties as diverse as those available using
the dyes alone. In addition, once encapsulated in the particles, the dyes display much better
photostability and increased fl uorescence (brightness) compared to the free dyes in solution.
A major advantage of silica-based particles is that they are known to have greater biocom-
patibility than quantum dots in that they are nontoxic, hydrophilic, and can be conjugated
to proteins and other targeting molecules with relative ease. The ability to add any desired
fl uorescence characteristic to such particles simply by choosing the appropriate organic dye or
metal chelate luminescent molecule makes dye-doped silica nanoparticles especially useful for
bioapplications.
5.2. Silane Functionalization of Silica Particles
Surface functionalization of silica particles or fl uorescent silica particles typically is done using
functional alkyl silanes. The process may be used to add a reactive group to the surface of the
particles for spontaneous coupling to biomolecules or it may be used to add the appropriate
nucleophilic group to the surface, such as an amine or a carboxylate. Silane modifi cation chem-
istry is discussed in more detail in Chapter 13.
The following protocol for modifi cation of silica nanoparticles is based on the method of
Zhao et al. (2004), which describes the addition of amine functionalities using trimethoxysilyl-
propyldiethylenetriamine. Other functional silane modifi cations may be done similarly.
Protocol
1. Add 32 mg of silica nanoparticles (fl uorescent or plain) to 20 ml of 1 mM acetic acid con-
taining 1 percent trimethoxysilyl-propyldiethylenetriamine with stirring. Other concen-
trations of silane derivatives used for particle modifi cation typically range from 1 to 5
5. Silica Particles 625
626 14. Microparticles and Nanoparticles
percent (w/w). Optimization of this concentration may have to be done for a particular
silica particle size and type.
2. React with mixing for 30 minutes at room temperature.
3. Wash the amine-derivatized particles at least 3 times with water using centrifugation to
remove excess reactants.
4. Store the particles in a suitable buffer at neutral or slightly acidic pH containing a preservative.
The amine-modifi ed silica particles may be used to couple with carboxylate-containing
ligands using a carbodiimide reaction. Similar coupling protocols may be used as that
previously described for amine-containing polymer particles (Section 4, this chapter). The
amine-particles also may be further derivatized by reaction with succinic anhydride to
create carboxylated particles for coupling to proteins or other amine-containing ligands.
5. To prepare the succinylated carboxylate derivative of the amine particles, wash the parti-
cles with water and then into DMF.
6. Suspend the amine-particles in DMF containing 10 percent succinic anhydride.
7. React for 6 hours under nitrogen gas with mixing.
8. Wash the carboxylated particles at least 3 times with DMF by centrifugation. Resuspend
in water and wash 3 times with water to remove DMF. Store the particles in water or a
suitable buffer at neutral or slightly acidic pH.
Carboxylated silica particles may be coupled with amine-containing ligands, such as proteins,
using a carbodiimide reaction with EDC. A similar protocol to that previously described for cou-
pling to carboxylate polymer particles may be used. The following protocol is based on the method
of Zhao et al. (2004), which was used for immobilizing monoclonal antibodies to E. coli O157.
Protocol
1. Suspend the carboxylated silica particles from step 8, above, in 10 ml of 0.1 M MES, pH 6.8.
2. With mixing, add 500 mg of EDC and 500 mg of NHS (or sulfo-NHS).
3. React for 25 minutes at room temperature with stirring.
4. Quickly wash the activated particles with water using centrifugation to remove excess
reactants. Resuspend the washed particles in 10 ml of 0.1 M sodium phosphate, pH 7.3.
5. Add a quantity of protein or antibody to the activated particles representing a 1–10 excess
of protein over the calculated monolayer for the type of particles used. For coupling a limit-
ing amount of antibody to the activated fl uorescent particles, something that may be desir-
able when using expensive monoclonals, the reaction should be carried out in very dilute
particle suspension (i.e., 0.1 mg/ml) to prevent aggregation of particles due to the possibility
of one antibody reacting with more than one particle. Zhao et al. (2004) used an antibody
concentration of just 5 g/ml to create successfully anti-bacterial fl uorescent particles.
6. React for 2–4 hours at room temperature.
7.
Add a blocking agent, such as a non-relevant protein (e.g., BSA) to a fi nal concentration of
1 percent to mask any nonspecifi c binding sites and to couple with any remaining reactive
groups on the silica particle surface. This is important especially if a limiting amount of
antibody was initially reacted with the particles in step 5. React for 30 minutes to 1 hour
at room temperature.
8. Wash the particles several times with PBS, pH 7.2, to remove excess protein and reaction
by-products. Store products in neutral or slightly acidic buffer containing a preservative.
627
15
1. Buckyballs and Fullerenes
1.1. Properties of Fullerenes
Carbon is an incredible element that is able to form structures having highly diverse properties
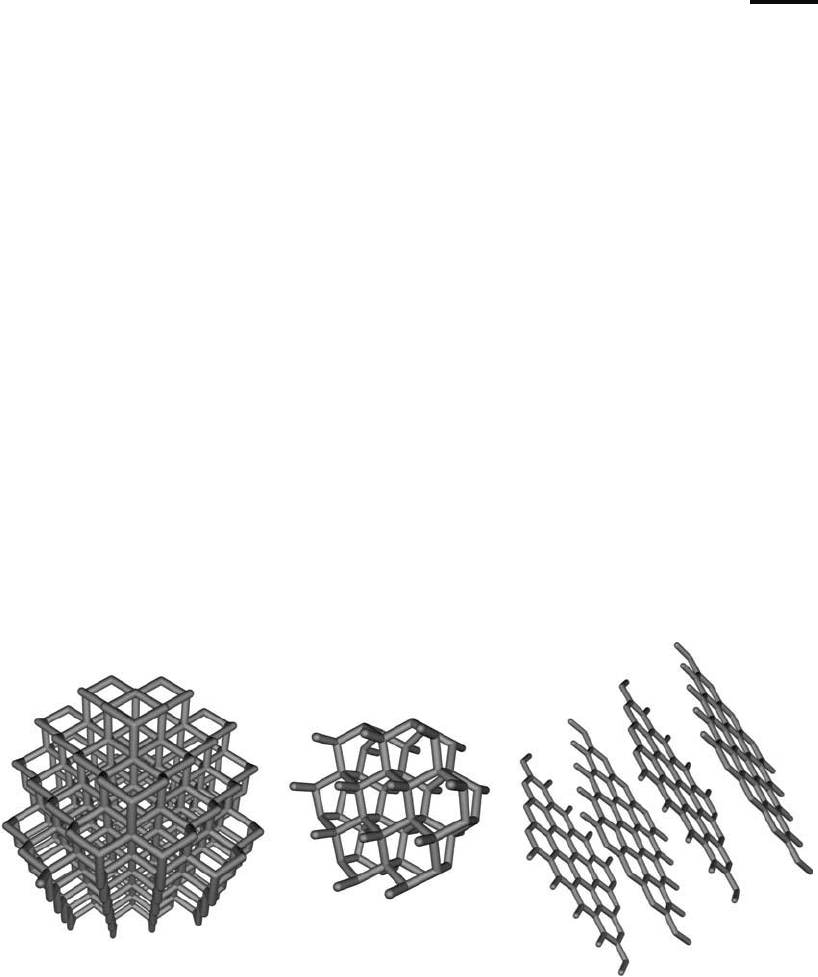
depending on its bonding patterns and three-dimensional organization. Natural allotropes of
carbon include diamond, graphite, amorphous carbon, and several other known forms ( Figure
15.1 ). Depending on the bond structure and atomic orientation that carbon takes on within the
structure of an allotrope, the resultant characteristics can range from the hardest known abra-
sive mineral, diamond, to the extremely soft, graphite, which is used as a lubricant.
In 1985, the story of carbon allotropes took a dramatic turn with the discovery of C
60
,
which resulted in a new type of carbon structure, called the fullerenes (Kroto et al., 1985).
This discovery earned the 1996 Nobel Prize in chemistry for Harold Kroto, Robert Curl, and
Buckyballs, Fullerenes, and Carbon Nanotubes
Figure 15.1 Three major allotropes of carbon (l to r): diamond, lonsdaleite, and graphite.
628 15. Buckyballs, Fullerenes, and Carbon Nanotubes
Richard Smalley. Buckminsterfullerene (named after Buckminster Fuller for his geodesic dome
architectural design) is a spherical cage of carbon having 60 atoms forming a truncated icosa-
hedron of average diameter 0.72 nm, which contains 12 pentagons and 20 hexagons of bonded
carbon ( Figure 15.2 ). The shape is exactly the same as a modern soccer ball, with the pentagon
and hexagon confi gurations clearly outlined on its surface.
There now are known to be a whole family of caged carbon structures having various num-
bers of carbon atoms, including C
30
,C
50
,C
70
,C
72
,C
76
,C
84
, and the huge C
540
. The name
“ fullerene” has replaced the unwieldy, “Buckminsterfullerene” used to describe this general
spheroid structure of carbon, although they still are referred to as “Buckyballs”.
Of all the fullerene forms, the nearly spherical properties of C
60
have attracted the greatest
attention, especially in the fi eld of bioconjugation. In addition to its physical properties, C
60
fullerenes have unique photo-optical and electro-chemical properties, which make them useful
as carriers for biomedical research applications. For instance, upon exposure to light C
60
will
generate singlet oxygen, which can be used in vivo to cleave biological molecules, particularly
DNA and RNA. Studies indicate that irradiation of C
60
in solution can be used to destroy virus
contamination (Kasermann and Kempf, 1997). Solutions of Buckminsterfullerene are a deep
purple color, whereas other sizes of fullerenes display a variety of other colors.
Figure 15.2 The structure of a C
60
fullerene, also called a Buckyball.
Fullerene C
60
also functions effi ciently as an antioxidant, actually being better than other
lipid-soluble antioxidants at scavenging reactive oxygen species (ROS) (Wang et al., 1999).
Water-soluble derivatives of C
60
, such as a poly-hydroxyl form, are able to function in the same
respect in aqueous environments.
Pure fullerenes are insoluble in aqueous environments and only sparingly soluble in many
organic solvents. The greatest solubility is found in 1,2,4-trichlorobenzene (20 mg/ml), carbon
disulfi de (12 mg/ml), toluene (3.2 mg/ml), and benzene (1.8 mg/ml) (Wikipedia.org). Solubility
calculations have been performed on C
60
in 75 different organic solvents (Sivaraman et al., 2001).
1.2. Modifi cation of Fullerenes
Many chemical derivatization methods have been developed to afford fullerene solubility in
particular environments and to provide functional handles for bioconjugation (Bosi et al .,
2003). The combination of adding polar groups and reactive functionalities to fullerenes, such
as COOH, NH
2
, and OH groups, provides water solubility and bioconjugation targets.
Examples of these modifi cations include the method of Brettreich and Hirsch (1998) to add
multiple carboxylates in a dendritic fashion and Wang et al. (1999) who added multiple pairs
of carboxylates to the surface carbons. In addition, Cusan et al. (2002) developed a C
60
–PEG
dendrimer-based diamine derivative using a substituted fulleropyrrolidine modifi cation linked
to the surface. Polymer carriers also have been used to provide water solubility and sites of
attachment. Cyclodextrins have been found to be excellent carriers of C
60
by holding the fuller-
ene within its hydrophobic core (Andersson et al., 1992; Braun, 1997; Samal and Geckeler,
2000; Filippone et al ., 2002).
In many methods for derivatization of C
60
, the initial modifi cation is based on the reac-
tion at a 6,6 ring junction on the fullerene with an azomethine ylide to form the 1,3-dipolar
cycloaddition product, a fulleropyrrolidine (Prato et al., 1996). The reaction is done overnight
with heating to refl ux in organic solvent. Typical reactants that combine with C
60
in this reac-
tion include an N-glycine derivative (with a constituent off the -amino group) and an alde-
hyde derivative, which gives the fulleropyrrolidine compound according to Figure 15.3 . By
judicious choice of the right starting materials, the process provides a range of derivative pos-
sibilities to employ fullerenes in various bioconjugate applications. If the reactants are added
in large excess over the concentration of the fullerene, then up to 9 such pyrrolidine groups
can be introduced per C
60
molecule. The number of modifi cations actually ends up being a bell
shaped curve from 5 to 9 pyrrolidine derivatives, with a peak at 7 modifi cations (Prato and
Maggini, 1998). By controlling the length of the reaction, a mono-substituted product can be
obtained in 40–50 percent yields.
In addition, the use of appropriate hydrophilic constituents on the aldehyde or glycine reac-
tants can result in excellent water solubility of the C
60
derivative. Two such modifi cation arms
can be added simultaneously to the pyrrolidine ring, thus providing a functional group for fur-
ther conjugation and a hydrophilic arm for increased water solubility. PEG derivatives have
been formed in this manner, which create highly soluble fullerene derivatives.
The following procedure adapted from Prato et al. (1996) is an example of how glycine and
formaldehyde derivatives may be used to create fullerene modifi cations for subsequent biocon-
jugation purposes.
1. Buckyballs and Fullerenes 629
