Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

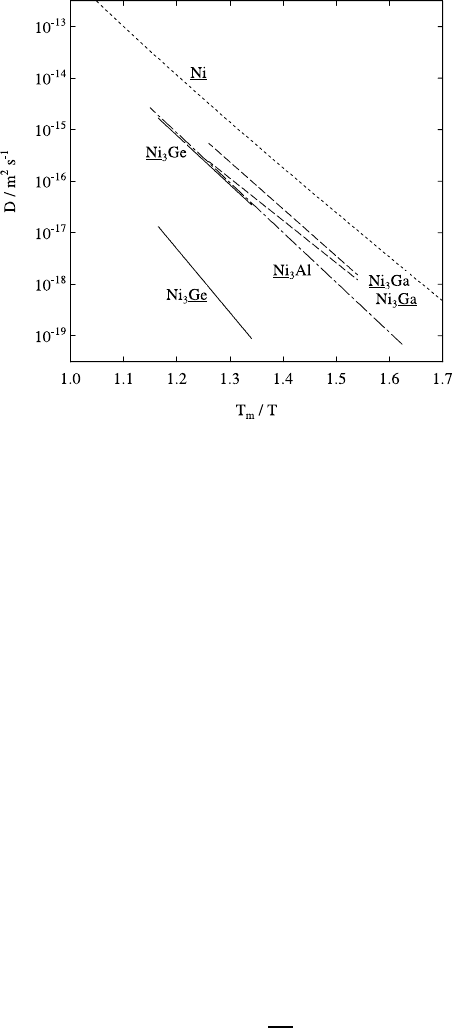
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 49
Fig. 1.25. Self-diffusion in three L1
2
structure intermetallics (Ni
3
Ge, Ni
3
Ga, Ni
3
Al)
and in Ni normalized to the melting temperatures (1405 K, 1383 K, 1635 K, and
1726 K).
1.11 Interdiffusion in Substitutional Binary Alloys
1.11.1 Boltzmann-Matano Method
A diffusion coefficient in a binary alloy measured in a chemical composition
gradient is denoted as chemical or interdiffusion coefficient
˜
D (see also Sect.
1.3 and Chap. 5). The experimental procedure of an interdiffusion study is
schematically illustrated in Fig. 1.26. A diffusion couple consisting of two
homogeneous, homo-phase alloys with different compositions (A
X
B
1−X
and
A
Y
B
1−Y
), but within the same phase field of the phase diagram, is formed.
Usually the thicknesses of the couple members are chosen very large as com-
pared to the average diffusion length. Then each couple member can be con-
sidered to be semi-infinite. The interdiffusion profile is measured after the
diffusion anneal, e.g., by electron microprobe analysis.
Boltzmann-Matano analysis, which is based on Boltzmann’s transforma-
tion of Fick’s second law [83] and a procedure suggested by Matano [84], is
employed to evaluate – in general concentration-dependent – interdiffusion
coefficients from an experimental profile according to
˜
D(c
∗
)=
2 t
∂c
∂x
x
∗
−1
c
∗
c
−
(x
M
− x)dc. (1.82)
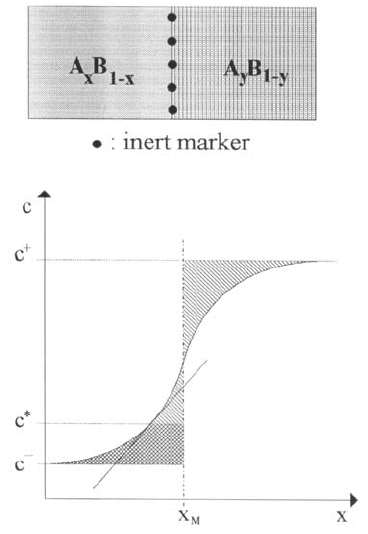
50 Helmut Mehrer
Fig. 1.26. Schematic illustration
of a single-phase interdiffusion
experiment. The original inter-
face (Kirkendall plane) is marked
by inert markers. The position of
the Matano plane (x
M
)isalsoin-
dicated.
Here t is the time of the diffusion anneal and x the position. In (1.82) x
M
denotes the position of the so-called Matano plane, which is defined by
c
+
c
−
(x
M
− x) dc =0 . (1.83)
c
−
and c
+
refer to the initial compositions of the two starting alloys (see
Fig. 1.26). To deduce
˜
D one determines the Matano plane first. Afterwards,
the area corresponding to the integral in (1.82) and the slope of the tangent
to the c(x) curve at the concentration c
∗
must be calculated (see Fig. 1.26).
This procedure is repeated for various choices of c
∗
.Inthisway
˜
D values are
obtained for various compositions.
In (1.82) and in the rest of this chapter volume changes have been neglected
for reasons of simplicity. There are systems in which the volume will change
in the course of interdiffusion. The generalisation of (1.82) for non-constant
volume was given by Sauer and Freise [85] and can be found in textbooks [2,
3, 5].
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 51
1.11.2 Darken’s Equations
As already mentioned in Sect. 1.3 the intrinsic diffusion coefficients D
A
and
D
B
are related to the interdiffusion coefficient
˜
D and to the velocity of the
marker movement v
K
(Kirkendall velocity) in a Kirkendall experiment (see
Fig. 1.27). The relations deduced by Darken [14] are as follows:
˜
D = X
B
D
A
+ X
A
D
B
, (1.84)
where X
A
and X
B
denote the mole fractions of the elements A and B respec-
tively. The Kirkendall velocity is given by
v
K
=(D
A
− D
B
)
∂X
A
∂x
(1.85)
with ∂X
A
/∂x denoting the concentration gradient at the Kirkendall plane.
Using (1.84) and (1.85) the intrinsic diffusion coefficients D
A
and D
B
can be
deduced if the interdiffusion coefficient and the Kirkendall velocity are known
from the experiment.
The reason why diffusion occurs is always a decrease in the Gibbs free
energy of the system. This implies that the atoms are diffusing from regions
where their chemical potential is high to regions where it is low, i.e., the
driving force for diffusion is the gradient of the chemical potential (see also
Sect. 1.3.2). As a consequence the interdiffusion coefficient
˜
D is related to the
tracer self-diffusion coefficients of the components in a homogeneous alloy via
˜
D =(X
A
D
B
∗
AB
+ X
B
D
A
∗
AB
) Φ. (1.86)
Here X
A
and X
B
denote the mole fractions of the components and Φ is the
thermodynamic factor of the alloy. Using a classical result from thermody-
namics of binary systems, the thermodynamic factor can be written as [2]
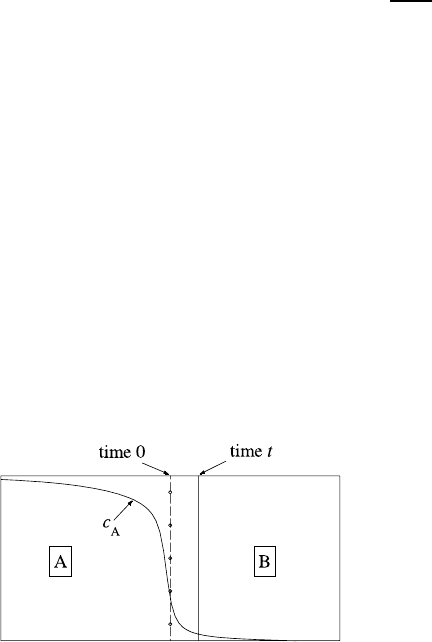
Fig. 1.27. Schematic illustration of the Kirkendall effect in a diffusion couple
composed of two pure metals A and B. The circles represent inert markers inserted
at the original interface. The line at time t represent the position of the markers
after the diffusion anneal. The solid line is a graph of the concentration of A atoms
in the couple after diffusion time t. As drawn, the B atoms diffuse into A faster than
those of A diffuse into B. As a result the interface moves to the right (D
B
>D
A
).

52 Helmut Mehrer
Φ =
X
A
X
B
RT
d
2
G
dX
2
A
=
∂ln a
i
∂ln X
i
=1+
∂ln γ
i
∂ln X
i
, (1.87)
where G denotes Gibbs free energy, a
i
the (chemical) activity, and γ
i
the
coefficient of activity of species i ( = A or B). According to the Gibbs-Duhem
relation for binary systems, the thermodynamic factor is identical for both
species. Equations (1.84), (1.85), and (1.86) are denoted as the equations of
Darken.
The thermodynamic factor is unity for ideal solutions. It is larger than
unity for phases with negative deviations from ideality and smaller than unity
in the opposite case. Negative deviations are expected for systems with or-
der. Therefore thermodynamic factors of intermetallic compounds are often
larger, sometimes even considerably larger than unity due to the attractive
interaction between the constituents of the intermetallic phase. As a con-
sequence interdiffusion coefficients are often larger than the bracket term in
(1.86). The latter corresponds to a weighted average of the tracer diffusivities.
Activation enthalpies of interdiffusion are often smaller than those for tracer
diffusion due to the temperature variation of the thermodynamic factor.
1.11.3 Darken-Manning Relations
The Darken relation between tracer diffusivities and the chemical diffusion
coefficient (1.86) is widely used in practice. Although it remains a very useful
equation it was recognized soon that from a theoretical viewpoint (1.86) is
incomplete. Manning [15, 86] developed equations for vacancy-mediated dif-
fusion in a so-called ’random alloy’ (vacancies and A and B atoms distributed
at random on the same lattice). In general, the results are like those obtained
by Darken but with ’vacancy-wind’ corrections
18
. The intrinsic diffusion co-
efficients and the interdiffusion coefficient, respectively, are given by
D
A
= D
A
∗
AB
Φ
1+
1 − f
f
X
A
(D
A
∗
AB
− D
B
∗
AB
)
(X
A
D
A
∗
AB
+ X
B
D
B
∗
AB
)
(1.88)
and an analogous equation for D
B
,and
˜
D =(X
A
D
B
∗
AB
+ X
B
D
A
∗
AB
) Φ
×
1+
1 − f
f
X
A
X
B
(D
A
∗
AB
− D
B
∗
AB
)
2
(X
A
D
A
∗
AB
+ X
B
D
B
∗
AB
)(X
A
D
B
∗
AB
+ X
B
D
A
∗
AB
)
. (1.89)
The factors in square brackets are Manning’s vacancy-wind corrections to
Darken’s equations. One also finds that the Kirkendall velocity is increased
by the addition of a factor f
−1
to (1.85). As usual f denotes the correla-
tion factor of self-diffusion. In substitutional alloys the Manning corrections,
18
A transparent derivation can be found in [4].
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 53
while significant, will never be very large even when one of the tracer self-
diffusivities is much greater than the other. For instance the Kirkendall ve-
locity is increased by about 30 % in an fcc alloy.
It is a debated question whether the Darken-Manning equations can be
applied to ordered intermetallics. The answer depends on the structure, the
type of disorder, and the diffusion mechanism. For example, according to
Belova and Murch [87] (1.89) can be used for B2 compounds with antisite
disorder.
1.12 Multiphase Diffusion in Binary Systems
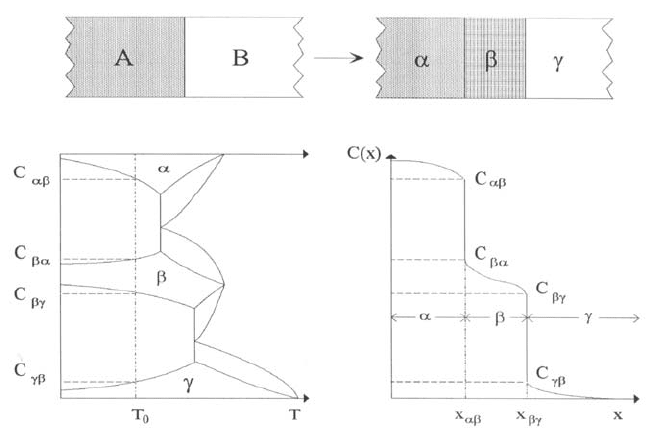
The experimental procedure in a multiphase diffusion experiment is schemat-
ically illustrated in the upper part of Fig. 1.28. A diffusion couple is formed
from the two elements A and B or from two compounds A
X
B
1−X
and
A
Y
B
1−Y
. Let us suppose that the phase diagram of the system contains inter-
metallics. Then interdiffusion will give rise to the formation of intermetallic
layers. A hypothetical phase diagram (containing only one intermetallic com-
pound) and a concentration profile resulting from interdiffusion is illustrated
in the lower part of Fig. 1.28.
Fig. 1.28. Schematic illustration of a multi-phase diffusion experiment. Upper
part: Diffusion couple before and after a diffusion anneal. Lower part: Interrelation
between a hypothetical phase diagram and an interdiffusion profile after a diffusion
anneal at temperature T
0
.

54 Helmut Mehrer
The name multi-phase diffusion emphasizes the diffusional aspect of the
whole process. However, there is also the aspect of a chemical reaction be-
tween the atomic species at the phase boundaries. The overall kinetics of
phase formation and growth can be either governed by diffusion across the
growing product phase(s) or by reaction(s) occurring at the interfaces. In the
first case the process is denoted to be diffusion controlled; in the second case
it is called to be reaction controlled. In general the kinetics of formation and
growth of intermetallic layers will be the result of both processes. For this
phenomenon the name reaction (reactive) diffusion has been suggested. In
very thin layers gradients are very large and diffusion processes proceed very
fast. Then the process is in the interface-reaction controlled regime. However,
during progress of reaction diffusion the layer thickness increases. Then dif-
fusion of the reacting species needs more and more time. Finally, diffusion
processes determine the overall kinetics [88,89].
When the whole process is diffusion controlled, the growth of the inter-
metallic layers in a binary system obeys a parabolic growth law
∆x
2
β
=2k
β
t, (1.90)
where ∆x
β
denotes the thickness of the layer. Kidson has shown that growth
constants have the following meaning [90]:
k
β
=2
˜
D
γβ
K
γβ
−
˜
D
βγ
K
βγ
C
βγ
− C
γβ
−
˜
D
βα
K
βα
−
˜
D
αβ
K
αβ
C
αβ
− C
βα
2
. (1.91)
C
αβ
denotes the equilibrium composition (in atomic fractions) on the α-side
of an α/β-interface,
˜
D
αβ
the interdiffusion coefficient in the α-phase near the
α/β -interface, and K
αβ
is the composition gradient in the α-phase near the
α/β-interface in a diagram of concentration versus x/
√
t. As already men-
tioned, in the derivation of (1.91) any explicit influence of interface processes
like phase nucleation, atomic transfer across the interface, and the creation
and/or annihilation of point defects at the interfaces has been disregarded.
This is only justified for long diffusion times. Then the growth process is
diffusion controlled [91, 92].
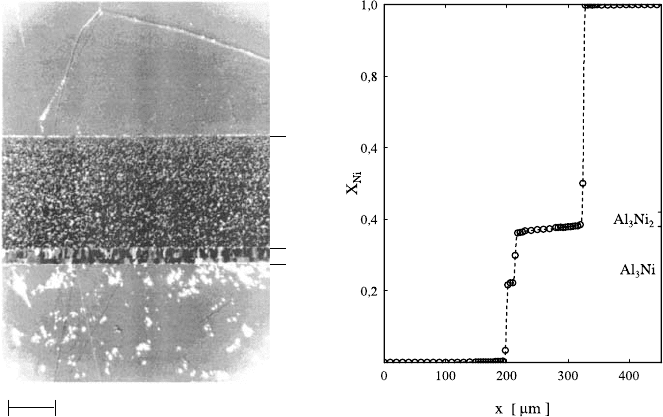
We consider as an example multiphase diffusion in the system Ni-Al [93].
Apart from the primary and terminal solid solutions five intermetallic com-
pounds are present in the phase diagram of Ni–Al: Al
3
Ni, Al
3
Ni
2
, AlNi,
Al
3
Ni
5
and Ni
3
Al [94]. Fig. 1.29 shows an optical micrograph of a diffusion
couple of pure Al and Ni after a diffusion anneal of 48 h at 823 K. Layers
of the Al-rich intermetallic compounds Al
3
Ni and Al
3
Ni
2
are clearly visible.
Their compositions can be identified in the profile across the diffusion zone
measured by electron microprobe analysis. The layer thicknesses of Ni-rich
compounds – at the relatively low temperature of the diffusion anneal – are
too small to be detectable in Fig. 1.29. As can be seen from Fig. 1.30 the
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 55
50 m
(Al)
(Ni)
Al Ni
32
Al Ni
3
Fig. 1.29. Optical micrograph of Al–Ni diffusion couple after a diffusion anneal of
48 h at 823 K (left). Composition profile obtained by electron microprobe analysis
(right).
compound Al
3
Ni
2
indeed shows a parabolic growth. The growth constant
obtained from these experiments is thermally activated with an enthalpy of
126 kJ mol
−1
.
We emphasize that according to (1.91) growth constants have a complex
meaning. They depend on diffusivities in the bordering layers as well as on
the diffusivity of the growing phase, on the concentration gradients on both
sides of the interfaces, and on the (in general temperature dependent) solubil-
ity limits of the phases. If one deduces an activation enthalpy for the growth
process it has a complex meaning as well. Its value is usually not identical
with the activation enthalpy of interdiffusion in the growing layer. The ac-
tivation enthalpy assigned to the growth of an intermetallic layer is at most
only an average of several more fundamental activation enthalpies. If a linear
Arrhenius plot is observed for the growth constant, this is only empirically
useful. It indicates either that one of the terms dominates over the others or
that the activation enthalpies are similar to each other. In addition, growth
constants may be influenced by mass transport along diffusion short circuits
like grain boundaries in the growing layer.
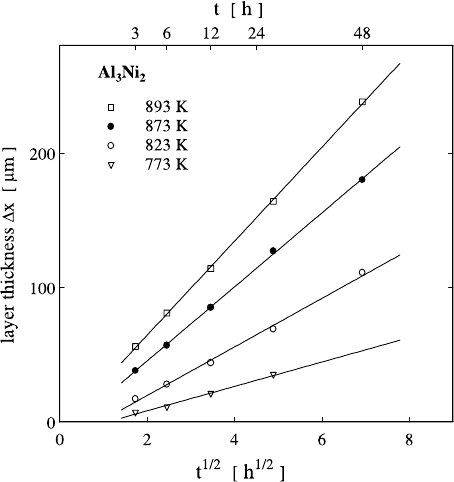
56 Helmut Mehrer
Fig. 1.30. Parabolic
growth of the inter-
metallic compound
Al
3
Ni
2
in Al–Ni diffu-
sion couples.
1.13 Conclusion
Diffusion is an important topic of materials science since the phenomenon of
diffusion is of both practical importance and of fundamental interest. In this
chapter we have outlined the continuum description and the basic atomic
mechanisms of bulk diffusion in solids. The various diffusion coefficients,
which are relevant for diffusion in solid elements and binary alloys, have been
introduced: the tracer diffusion coefficient(s) of self-diffusion, tracer diffusion
coefficients of foreign elements, the chemical (or interdiffusion) coefficient in
a composition gradient, and the intrinsic diffusion coefficients. The most im-
portant experimental method for diffusion studies is the tracer technique in
combination with mechanical and/or sputter sectioning for depth profiling.
Various other techniques for the measurement of diffusion profiles have been
also considered. Indirect methods including mechanical and magnetic relax-
ation and nuclear methods such as nuclear magnetic relaxation, M¨oßbauer
spectroscopy and quasielastic neutron scattering have been mentioned only
briefly since they are the main topics of Chaps. 2, 3 and 9.
Self-diffusion is the most basic diffusion process in a solid. It is well studied
for most metallic elements. In close-packed metals self-diffusion occurs pre-
dominantly by a vacancy mechanism with some small contributions of diva-
cancies at temperatures above 2/3 of the melting temperature. Self-diffusion
in bcc metals is also vacancy-mediated. In a homologous temperature scale
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 57
it is faster and the range of diffusivities is much wider than for close-packed
metals.
Diffusion of small foreign atoms proceeds much faster than self-diffusion
of the host metal. Small foreign atoms such as H, C, N, and O are usually
incorporated in octahedral or tetrahedral interstitial sites of the host lattice
and diffuse via a direct interstitial mechanism. Diffusion of hydrogen is a
particularly interesting case for scientific and application-oriented reasons. Its
diffusion is extremely rapid, non-classical isotope effects occur, and quantum
effects may cause strong deviations from a linear Arrhenius-like temperature
dependence of H diffusion.
Diffusion of solutes in substitutional alloys is vacancy-mediated. Solute
diffusion in very dilute alloys (impurity diffusion) is normally described by
diffusion coefficients which lie in a relatively narrow band around those of
self-diffusion of the host (solvent). This rather small diffusivity dispersion
is understandable since solute and solvent atoms are located on sites of the
same lattice and both use vacancies as diffusion vehicles. It reflects the high
efficiency for screening of electrical point charges of some metallic hosts such
as the noble metals and zinc. This efficient screening limits the solute-vacancy
interaction to relatively small values.
Remarkable exceptions from ‘normal’ behaviour of solute diffusion are
observed for polyvalent metals: Transition-metal solutes in aluminium are
extremely slow diffusers with high activation energies, high pre-exponential
factors and high activation volumes. Very likely this indicates a strong repul-
sion between vacancy and solute somewhere on the vacancy-solute exchange
path. Fast solute diffusion is observed, e.g., for noble metal solutes in lead
and tin and for late-transition-element solutes in group IVA solvents (zirco-
nium, titanium and hafnium). The fast diffusion has been attributed to the
dissociative mechanism. The latter operates for solutes which are dissolved
on substitutional and interstitial sites. Even when its interstitial fraction is
very small it can be responsible for the rapid diffusion. Fast solute diffusion
is also observed in the semiconductors silicon and germanium (see Chap. 4).
Systematic studies of diffusion in intermetallics (intermetallic compounds
and ordered alloys) have become available only recently. An understanding
of diffusion in terms of atomic mechanisms is more complex than for metal-
lic elements. The current knowledge about self-diffusion has been illustrated
for binary intermetallics with B2-, L1
2
-, and D0
3
-structures. Important fac-
tors which influence diffusion such as the crystal structure, the state of order
and disorder, the temperature and the composition have been illustrated. In
a broad sense diffusion is mediated by vacancy-type defects, which include
triple-defects and antistructure-bridge mechanisms. Relevant atomic mech-
anism must take into account that the degree of order in the material is
maintained during diffusion. Despite of the progress made in recent years,
diffusion in intermetallics is a field that deserves further attention.
58 Helmut Mehrer
The field of interdiffusion in binary alloys has been only touched in this
chapter. The Boltzmann-Matano method (or a related method) is necessary
to deduce interdiffusion coefficients from composition profiles. A detailed in-
terpretation of the interdiffusion coefficient requires also measurements of
the Kirkendall effect. The analysis given by Darken and its refinement by
Manning permits to deduce the intrinsic diffusion coefficients. The latter are
related to the tracer diffusion coefficients of the components in homogeneous
alloys via the thermodynamic factor of the alloy and the so-called vacancy-
wind corrections.
Multiphase diffusion or reaction diffusion occurs in a binary alloy system
with one or several intermediate phases. In an appropriate diffusion couple
layers of the intermediate phase(s) form and grow. Their growth is often diffu-
sion controlled. Then their growth constants are related to the interdiffusion
coefficients.
Notation
a cubic lattice parameter
a
i
activity (chemical) of species i
c volume concentration of diffusing species
C
V
(C
eq
V
) mole fraction of vacancies (in thermal equilibrium)
C
2V
(C
eq
2V
) mole fraction of divacancies (in thermal equilibrium)
C
i
(C
eq
i
) mole fraction of interstitial solutes (in equilibrium)
C
s
(C
eq
s
) mole fraction of substitutional solutes (in equilibrium)
D diffusion coefficient (second rank tensor)
D
I
,D
II
,D
III
diffusion coefficients in principal diffusion directions
D diffusion coefficient, also self-diffusion coefficient in a cubic
crystal
˜
D interdiffusion coefficient (also chemical diffusion coefficient)
D
2
diffusion coefficient of solute (impurity) in dilute alloy (also
D
C
∗
A
)
D
i
diffusion coefficient of a solute in interstitial sites
D
A
∗
A
, tracer self-diffusion coefficients of in pure metal A
D
A
∗
AB
, D
B
∗
AB
tracer diffusion coefficients of component A or B in a binary
material
D
C
∗
A
, D
C
∗
AB
, tracer diffusion coefficients of impurity C in pure metal A
or in a binary A-B material
D
0
pre-exponential factor of diffusion (including diffusion en-
tropy)
D
0
pre-exponential factor of diffusion (without diffusion en-
tropy)
f correlation factor, correlation factor of self-diffusion
f
2
correlation factor of solute diffusion
g geometry factor
