Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 29
1.7.2 Hydrogen Diffusion
Hydrogen in metals provides itself an important and fascinating topic of ma-
terials science. It has also attracted considerable interest from the viewpoint
of applications. For example, the idea of hydrogen storage in metals is based
on the high solubility and fast diffusion of hydrogen in some metal-hydrogen
systems. Permeation of hydrogen through palladium membranes for hydro-
gen purification is an old and well-known application based on the very fast
diffusion. Nowadays, diffusion of hydrogen plays a key role for fuel cells which
use hydrogen or hydrocarbons as fuels.
Hydrogen forms interstitial solid solutions with most metallic elements.
Some metals have a negative enthalpy of solution and a high solubility for
hydrogen (e.g. group IV transition metals, group V transitions metals, and
palladium) and form hydrides at higher hydrogen concentrations. Other met-
als have a positive enthalpy of solution and a relatively low solubility (e.g.
group VI metals, group VII metals, and iron).
From a scientific point of view diffusion of hydrogen is significantly dif-
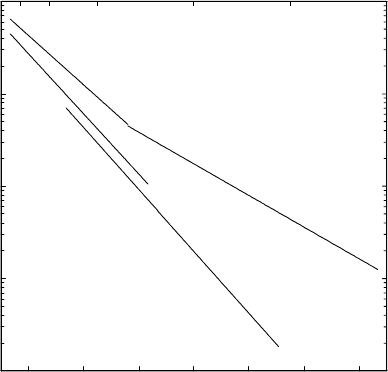
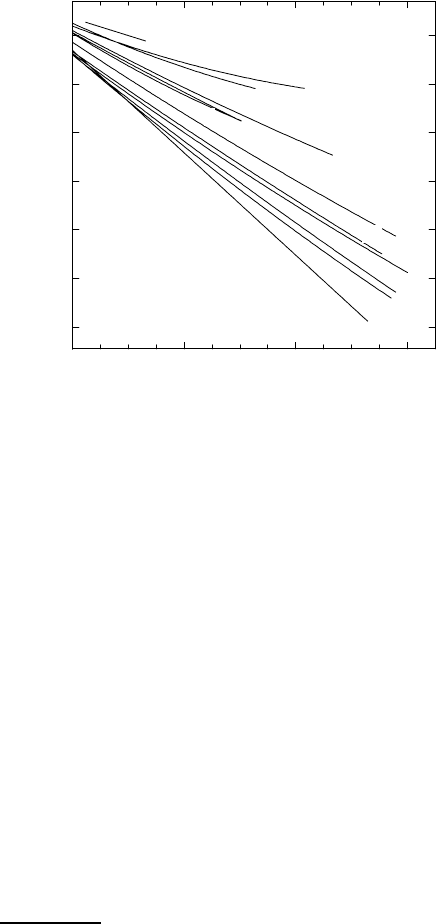
ferent from diffusion of other interstitial solutes. Fig. 1.14 shows an Arrhe-
nius plot for diffusion of hydrogen and its isotopes deuterium and tritium in
the bcc transition metal niobium (for references see Chap. 9 in [6]). We use
Fig. 1.14 to illustrate some characteristic features of hydrogen diffusion:
10
-12
10
-11
10
-10
10
-9
10
-8
2
3 4 5 6 7 8
500 400 300
200 150
H
T
D
T
-1
/ 10
-3
K
-1
D / m
2
s
-1
T / K
Fig. 1.14. Diffusion of the hydrogen isotopes H, D, and T in niobium.
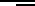
30 Helmut Mehrer
– Diffusion of hydrogen and its isotopes is extremely rapid: Diffusivities
exceed that of heavier interstitials by many orders of magnitude as can
be seen from a comparison of Figs. 1.13 and 1.14. From Fig. 1.14 we get
D
H
=8× 10
−9
m
2
s
−1
near 300
0
C. According to (1.36) the reciprocal
mean residence time τ
−1
of H atoms is about 10
12
s
−1
.Thisextremely
high value is of the order of the Debye frequency of niobium.
– Hydrogen has three isotopes (H, D, T): These isotopes differ consider-
ably in their isotopic masses, which offers the possibility to study large
isotope effects. Fig. 1.14 shows that normal hydrogen (H) diffuses more
rapidly than deuterium (D), and deuterium diffuses more rapidly than
tritium (T). In addition the activation enthalpies of hydrogen isotopes
are different and obey the inequalities
∆H
H
<∆H
D
<∆H
T
. (1.53)
In the classical regime the activation enthalpy of a diffuser is exclusively
determined by the chemistry of the system and not by the isotopic mass.
The latter enters the diffusion coefficient only via the mass dependence of
the attempt frequency. If many-body and quantum effects are negligible
the attempt frequency ν
0
is related to the mass m of the diffuser simply
by
ν
0
∝
1
√
m
. (1.54)
This shows that (1.53) represents a non-classical effect. Similar non-
classical effects have been observed for hydrogen diffusion in the metals
vanadium and tantalum.
– Hydrogen is the lightest atom. As a consequence, quantum effects in dif-
fusion can be observed which are hardly detectable for heavier diffusers.
The deviation of hydrogen diffusivity from an Arrhenius law below room
temperature (see Fig. 1.14) has been attributed to incoherent tunneling
(e.g. [33]).
– Positive muons can be considered in several respects as light isotopes of
hydrogen [43]. The muon mass is by a factor of nine smaller than the
proton mass. Like the hydrogen nuclei, the muons may be considered as
very heavy compared with the electrons but nevertheless light compared
with most metal atoms. A prefered topic studied with positive muons in
metals is quantum diffusion (see, e. g., [44]).
The very fast diffusion and the often high solubility of hydrogen have con-
sequences for the experimental techniques used in hydrogen diffusion stud-
ies. Concentration-profile methods, permeation methods based on Fick’s first
law, absorption and desorption methods, electrochemical methods, and re-
laxation methods (Gorsky effect, magnetic after effect, etc.) are in use. Due
to the favourable gyromagnetic ratio of the proton and due its large incoher-
ent scattering cross section for neutrons, NMR and QENS, respectively, (see
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 31
Chaps. 2 and 3 and [33]) are well suited for hydrogen diffusion studies. For a
more thorough discussion of hydrogen diffusion the reader is referred to text-
books [2,3], to reviews [30,32] and to Chap. 9 of the data collection [6]. Muon
diffusion can be studied by the muon spin resonance (µSR) technique [45],
which has some similarities with β-NMR (see Chap. 9).
1.8 Self-Diffusion in Metals
Self-diffusion is most conveniently studied by the tracer method described in
Sect. 1.4. As an example D values from
63
Ni tracer measurements in nickel are
displayed in Fig. 1.15 according to [46, 47]. An enormous diffusivity range of
about 9 orders of magnitude has been covered by the combination of mechan-
ical sectioning [46] and sputter sectioning [47]. Most of the more important
metallic elements have been studied by tracer methods over similarly wide
ranges. In some cases the tracer data have been supplemented by additional
techniques. For example, NMR proved to be very useful in the cases of alu-
minium and lithium (cf. Chap. 9) where no radioisotopes appropriate for
tracer diffusion studies are available. For a collection of data and information
about the method(s) used for each metal the reader is referred to Chap. 2
in [6].
10
-22
10
-21
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
5 6 7 8
9 10
11 12 13
1600 1200 1000 800
grinding
sputtering
T
-1
/ 10
-4
K
-1
D / m
2
s
-1
T / K
Fig. 1.15. Self-diffusion of
63
Ni in nickel. Open circles: data from mechanical sec-
tioning; full circles: data from sputter sectioning.

32 Helmut Mehrer
The dominating mechanism of self-diffusion
12
in metallic elements is the
vacancy mechanism illustrated in Fig. 1.9. Using (1.37) and (1.43) the diffu-
sion coefficient of tracer atoms can be written as
D = fa
2
ωC
eq
V
= fa
2
ν
0
exp
S
F
+ S
M
R
exp
−
H
F
+ H
M
RT
. (1.55)
The Arrhenius parameters of (1.20) have the following meaning for vacancy
mediated self-diffusion:
∆H = H
F
+ H
M
(1.56)
and
D
0
= fa
2
ν
0
exp
S
F
+ S
M
R
. (1.57)
As already mentioned in Sect. 1.6 the correlation factor accounts for the
fact that for vacancy-mediated diffusion the tracer atom experiences some
‘backward correlation’ whereas the vacancy itself performs a random walk.
For monovacancies in cubic lattices f is a temperature-independent quantity,
which is approximately given by
f ≈ 1 −
2
Z
(1.58)
where Z is the coordination number. Vacancy jumps into one of the Z di-
rections occur with the probability 1/Z and an immediate backward jump
effectively ‘cancels’ two tracer jumps. (1.58) is a ‘rule of thumb’. Exact values
according to [2] are listed in Table 1.2.
Table 1.2. Correlation factors of vacancy-mediated self-diffusion in cubic lattices.
Structure fcc bcc sc diamond
f 0.7815 0.727 0.653 0.5
For self-diffusion in non-cubic crystals correlation factors are orientation
and temperature dependent. For example, the two correlation factors in hcp
crystals depend on the ratio of the jump rates within and oblique to the basal
plane (see, e.g., [2]).
1.8.1 Face-Centered Cubic Metals
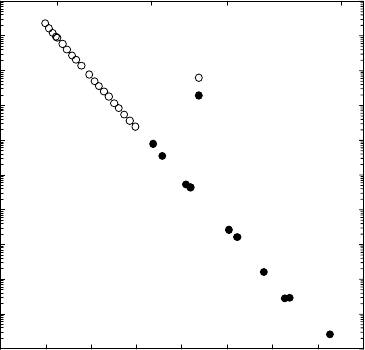
Self-diffusion coefficients of several face-centered cubic (fcc) metals are shown
in the Arrhenius diagram of Fig. 1.16. For each metal the temperature scale is
12
For fcc metals at temperatures above about 2/3 of the melting temperature an
additional contribution of divacancies (see Fig. 1.10) has been proposed (see [22]
and the textbooks [2, 3]), which for reasons of simplicity will be not considered
here.
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 33
10
-23
10
-22
10
-21
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
1.00 1.25
1.50 1.75
2.00 2.25 2.50
Pt
Pd
Ag
C
u
Ni
A
u
T
m
/ T
D / m
2
s
-1
Fig. 1.16. Self-diffusion of several fcc metals versus reciprocal temperature nor-
malized to their melting temperatures T
m
.
normalized by its melting temperature T
m
(homologous temperature scale).
The following empirical rules for fcc metals are evident:
– Diffusivities near the melting temperature are similar for all fcc metals
and lie between about 10
−12
m
2
s
−1
and 10
−13
m
2
s
−1
.
– The Arrhenius lines on homologous scale have approximately the same
slope. Since the slope equals −∆H/RT
m
an empirical correlation between
the activation enthalpy ∆H and the melting temperature T
m
exists. This
can be stated as follows:
∆H ≈ (17 to 18)RT
m
. (1.59)
Equation (1.59) is sometimes denoted as the rule of van Liempt. The
melting temperature in (1.59) is expressed in Kelvin.
– The pre-exponential factors lie typically within the following range:
5 × 10
−6
m
2
s
−1
<D
0
< 10
−3
m
2
s
−1
. (1.60)
For the explanation of the empirical rules the reader is referred to [48]. Ac-
tivation parameters for self-diffusion of some fcc metals are listed in Table 1.3
(for references see Chap. 2 in [6]). According to (1.57) the pre-exponential
factors of Table 1.3 correspond to self-diffusion entropies

34 Helmut Mehrer
Table 1.3. Activation parameters for self-diffusion in some fcc metals.
Metal Cu Ag Au Ni Pd Pt
∆H [kJ mol
−1
] 204 170 165 285 266 257
D
0
[10
−4
m
2
s
−1
] 0.35 0.046 0.027 1.77 0.205 0.05
∆S = S
F
+ S
M
(1.61)
of several R. According to (1.56) the activation enthalpy Q equals the sum of
formation and migration enthalpies of the vacancy. For fcc metals indepen-
dent experimental determinations of the formation and migration properties
show that
H
M
H
F
≤ 1 (1.62)
is fulfilled. For a review of point defect properties in metals the reader is
referred to [40].
1.8.2 Body-Centered Cubic Metals
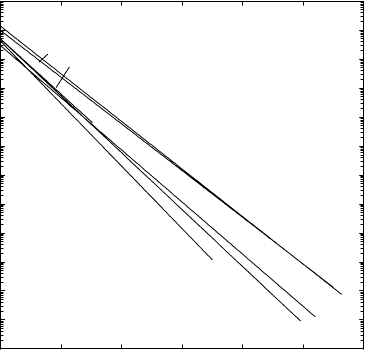
Self-diffusion coefficients of several body-centered cubic (bcc) metals are
shown in the Arrhenius diagram of Fig. 1.17 on a homologous temperature
scale. A comparison of Figs. 1.17 and 1.16 reveals the following differences
between fcc and bcc metals:
– Diffusivities at homologous temperatures are usually higher for bcc metals
than for fcc metals. At the melting temperature T
m
the difference is about
one order of magnitude. Diffusivities for bcc metals near T
m
lie between
about 10
−11
m
2
s
−1
and 10
−12
m
2
s
−1
.
– The ‘spectrum’ of self-diffusivities is much wider for bcc metals than
for fcc metals. Self-diffusion is slowest for group VI metals and fastest
for group IV metals. It is interesting to note that the latter undergo
a structural phase transition from a hexagonal close-packed (hcp) low-
temperature to a bcc high-temperature phase.
– The Arrhenius diagram of self-diffusion for some bcc high-temperature
phases (β-titanium, β-zirconium) shows considerable upward curvature.
A common feature of fcc and bcc metals is that within one group of the
periodic table self-diffusion at homologous temperatures is slowest for the
lightest and fastest for the heaviest element of the group.
The very fast self-diffusion of bcc high-temperature phases and the wide
spectrum of diffusivities of bcc metals has been attributed to special fea-
tures of the lattice dynamics in bcc structures. A nearest-neighbour jump of
a self-atom in the bcc lattice is a jump in [111] direction. For bcc metals the
longitudinal phonon branch shows a minimum for 2/3[111] phonons, which is
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 35
10
-23
10
-21
10
-19
10
-17
10
-15
10
-13
10
-11
1.0 1.4 1.8 2.2
C
r
Mo
W
V
Nb
Ta
K
Li
N
a
β-Ti
β-Zr
β
-Hf
T
m
/ T
D / m
2
s
-1
Fig. 1.17. Self-diffusion of several bcc metals versus reciprocal temperature nor-
malized to their melting temperatures.
most pronounced for group IV metals. The associated low-phonon frequen-
cies indicate a small activation barrier for nearest-neighbour exchange jumps
between atom and vacancy [49, 50].
1.9 Impurity Diffusion in Metals
Let us now consider a very dilute substitutional binary alloy of metals A and
B with the mole fraction of B atoms much smaller than that of A atoms.
Then A is denoted as solvent (or matrix) and B is denoted as solute. Dif-
fusion in a dilute alloy has always two aspects: solute diffusion and solvent
diffusion. In this section we consider only solute diffusion in very dilute fcc
alloys
13
. This is often denoted as impurity diffusion. It implies that the solute
is isolated from other solutes in the matrix. In what follows we consider first
the ‘normal’ behaviour of substitutional impurities. There are, however, some
remarkable exceptions from ‘normal’ impurity diffusion, which will be men-
tioned afterwards.
13
For solvent diffusion in dilute alloys the reader may consult [2,3].
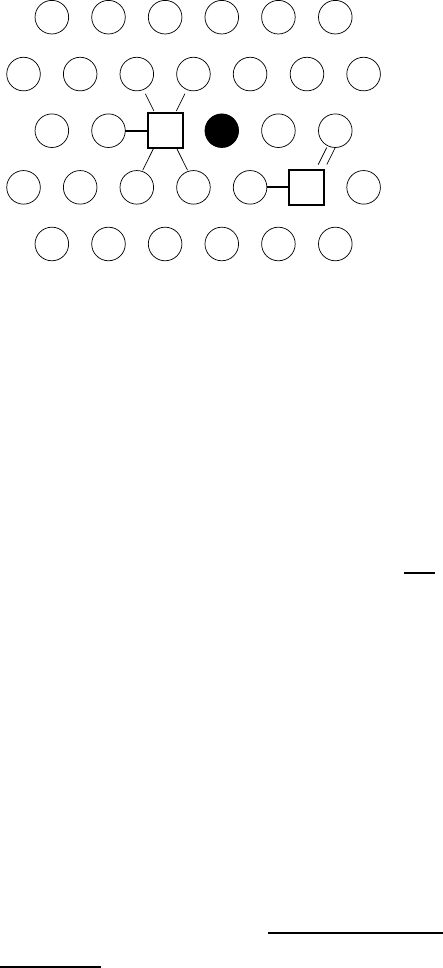
36 Helmut Mehrer
⇒
K
U
ω
4
ωω
ω
3
ω
3
ω
3
ω
1
ω
2
ω
1
Fig. 1.18. Substitutional impurity diffusion in an fcc lattice: ‘five-frequency model’
for vacancy jumps in the presence of an impurity atom. Impurity (full circle), solvent
atoms (open circles), vacancy (squares).
1.9.1 ‘Normal’ Impurity Diffusion in fcc Metals
From the atomistic expression (1.41) derived in Sect. 1.6.1 and (1.44) we get
for the impurity diffusion coefficient D
B
∗
A
(denoted in the following by D
2
)of
vacancy-mediated diffusion in cubic Bravais lattices
D
2
= f
2
a
2
ω
2
C
eq
V
exp
G
B
RT
. (1.63)
To be specific we consider in the following fcc solvents
14
.Ifonlynearest-
neighbour interactions between vacancy and impurity occur, four vacancy
jump rates apart from the jump rate ω in the pure solvent must be considered.
This so-called five-frequency model introduced by Lidiard [51] is illustrated
in Fig. 1.18.
15
The jump rates of the vacancy are denoted as follows:
ω
1
: jump rate for rotation of vacancy-solute complex,
ω
2
: jump rate of vacancy-solute exchange,
ω
3
: dissociation jump rate of vacancy-solute complex,
ω
4
: association jump rate of vacancy-solute complex,
ω: vacancy jump rate in the pure solvent.
As shown by Manning [15], the correlation factor of impurity diffusion
can be written as
f
2
=
ω
1
+(7/2)Fω
3
ω
2
+ ω
1
+(7/2)Fω
3
, (1.64)
14
For impurity diffusion in bcc or hcp solvents see [2, 3].
15
We adopt the name ‘five-frequency model’ commonly used in the literature. The
physical meaning of the quantities ω
i
is that of jump rates.
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 37
where F = F (ω/ω
4
) is a function of the rate ratio ω/ω
4
[15]. F approaches
unity for small ω
4
/ω. F =0.7357 for ω
4
= ω and F =2/7forlargeω
4
/ω.In
the case of self-diffusion all jump rates are equal. Then f
2
= f =0.7815 (see
Table 1.2). It is interesting to note that because of the condition of detailed
thermal equilibrium the relationship
ω
4
ω
3
=exp
G
B
RT
(1.65)
must hold. This equation relates the dissociation and association rates of the
vacancy-solute complex to its Gibbs free energy of binding G
B
.
The impurity diffusion coefficient (1.63) can be recast to give
D
2
= f
2
a
2
ν
0
exp
S
F
− S
B
+ S
M
2
R
exp
−
H
F
− H
B
+ H
M
2
RT
, (1.66)
where H
B
and S
B
denote binding enthalpy and entropy of the vacancy-solute
complex and H
M
2
and S
M
2
enthalpy and entropy of the vacancy-solute ex-
change jump. The activation enthalpy for impurity diffusion is then given
by
∆H
2
= H
F
− H
B
+ H
M
2
− C (1.67)
where the correlation term
C = R
∂ ln f
2
∂
1
T
(1.68)
results from the temperature dependence of the impurity diffusion correlation
factor.
It is evident from (1.55), (1.63), and (1.65) that the ratio of the diffusion
coefficients between impurity- and self-diffusion can be written as
D
2
D
=
ω
4
ω
3
ω
2
ω
f
2
f
. (1.69)
This expression shows that the diffusion coefficient D
2
of a substitutional
impurity differs from that for self-diffusion D in the pure solvent for three
reasons:
–Thefactorω
4
/ω
3
shows that the probability to find a vacancy on a
nearest-neighbour site of the impurity is different from the equilibrium
vacancy concentration in the pure matrix because of vacancy-solute in-
teraction (1.65).
–Thefactorω
2
/ω arises because the exchange rate between vacancy and
impurity and those between vacancy and host atoms are different.
–Thefactorf
2
/f arises because the correlation factor f
2
of impurity dif-
fusion is no longer a geometric factor as in the case of self-diffusion. It
depends on various jump rates and hence also on temperature.

38 Helmut Mehrer
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
8 9 10 11
12 13
1200 1100 1000
900 800
T
m
= 12
34
K
G
e
Sb
Al
Hg
Co
Cr
Ag
Fe
Au
Pd
Pt
T
-1
/ 10
-4
K
-1
D / m
2
s
-1
T / K
Fig. 1.19. Diffusion of several solutes and self-diffusion (dashed line) in silver.
Fig. 1.19 shows an Arrhenius diagram of various solutes in silver together
with silver self-diffusion (for references see Chap. 3 in [6]). It reveals charac-
teristic features of ‘normal’ diffusion of substitutional solute atoms:
–TheD
2
values of solutes lie in a relatively narrow band around self-
diffusion according to
1/100 D
2
/D 100. (1.70)
– The following empirical limits are found for pre-exponential factors
0.1
D
0
(solute)
D
0
(self)
10 (1.71)
and activation enthalpies
0.75
∆H
2
(solute)
∆H(self)
1.25. (1.72)
Substitutional solutes in other fcc solvents like Cu, Au, and Ni also confirm
these limits (see Chap. 3 in [6] for references).
Since solute and solvent atoms are located on the same lattice and since
their diffusion is mediated by vacancies this rather small diffusivity disper-
sion is not surprising. It reflects the high efficiency of screening of point
charges in some metals (e.g. noble metals) which normally limits the vacancy-
impurity interaction enthalpy to values between 0.1 and 0.3 eV. Such values
