Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 39
are small relative to the vacancy formation enthalpies, which are in the order
of 1 eV [40]. Using ∆H = H
F
+ H
M
we get for the difference of the activation
enthalpies between solute- and self-diffusion
∆Q = ∆H
2
− ∆H = −H
B
+(H
M
2
− H
M
) − C. (1.73)
A useful theoretical approach associates ∆Q with the charge difference be-
tween solute and vacancy. Positive solutes, generally those of higher nominal
valence than the solvent, tend to have a net attraction with the vacancies.
Such solutes diffuse more rapidly and with lower activation energies than
self-diffusion. Calculations of ∆Q from a theory based on the free electron
model using the Thomas-Fermi approximation for the interaction potential
have been made, e. g., by LeClaire [52]. Good agreement was found with ex-
periments for solute diffusion in noble metals and zinc. ∆Q is negative and
its absolute value increases with the difference in valence between the solvent
and the solute. For transition-metal solutes in noble metals and for other sol-
vents such as the alkali metals, divalent magnesium, and trivalent aluminium
the calculated values of ∆Q do not agree with the experiment.
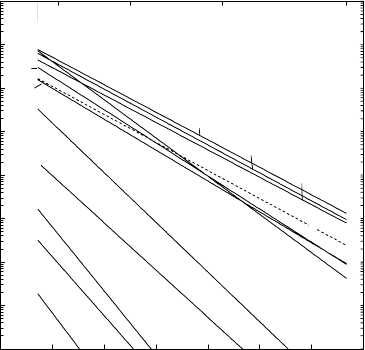
1.9.2 Slow Diffusion of Transition-Metal Solutes in Aluminium
Fig. 1.20 shows an Arrhenius diagram of various solutes in aluminium to-
gether with aluminium self-diffusion according to [53]. The transition ele-
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
10
-10
10 11 12
13 14 15
16 17
900 800 700 600
Cu
T
m
=933
K
Z
n
Al
V
Ti
Cr
Mn
F
e
Co
Ni
Ga
Ge
T
-1
/ 10
-4
K
-1
D / m
2
s
-1
T / K
Fig. 1.20. Diffusion of several solutes and self-diffusion (dashed line) in aluminium.

40 Helmut Mehrer
ments are diffusers with high activation energies and high pre-exponential fac-
tors. Most of them have extremely low diffusivities as compared to aluminium
self-diffusion. In contrast, non-transition elements have diffusion rates similar
or slightly higher than self-diffusion and show only small diffusivity disper-
sion.
The pressure dependence of the solute diffusion coefficients in aluminium
has been studied in [35,54]. The activation volumes of non-transition element
solutediffusersareclosetooneatomicvolume(Ω) and not much different
from the activation volume of self-diffusion. However, the transition elements
are diffusers with high activation volumes between 1.67 and 2.7 Ω.These
findings can be attributed to differences in vacancy-solute interaction in alu-
minium between transition and non-transition element solutes [54]. The large
∆H
2
values of transition element solutes according to (1.67) indicate a strong
repulsion between solute and vacancy (H
B
< 0) and/or a large activation en-
thalpy H
M
2
for the solute-vacancy exchange jump.
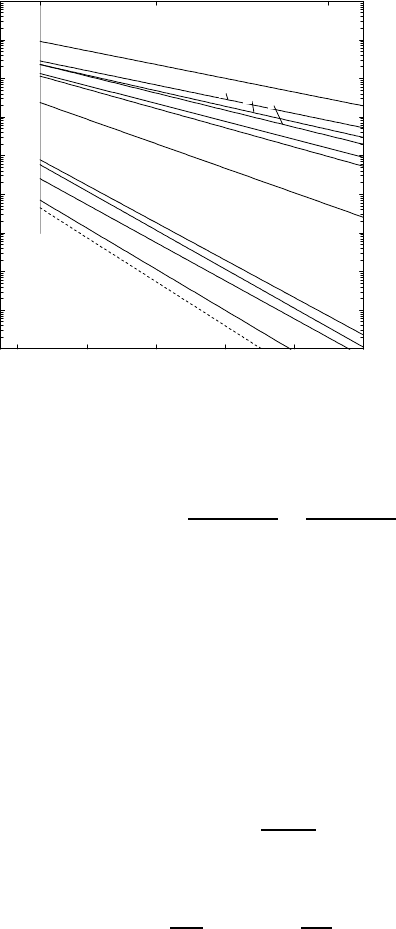
1.9.3 Fast Solute Diffusion in ‘Open’ Metals
Fast solute diffusion is observed in some polyvalent metals, which are some-
times also denoted as ‘open’ metals [55]. ’Open’ refers to the large ratio
between atomic and ionic radius of the solvent. This solvent property leads
for solutes with relatively small radii to the occurrence of fast solute diffusion.
As an example, Fig. 1.21 shows an Arrhenius diagram of solutes in lead to-
gether with lead self-diffusion (for references see Chap. 3 in [6]). Some solutes
(thallium, tin) in lead show ‘normal’ behaviour. However, noble metals, nickel
group solutes, and zinc have diffusivities which are three or more orders of
magnitude faster than self-diffusion.
Noble metal solutes are also fast diffusers in the group IVB metal tin
and in the group IIIB metals indium and thallium. The late transition el-
ements Fe, Co, and Ni in group IVA metals (α-titanium, α-zirconium, and
α-hafnium), and Co in niobium are very fast diffusers as well [55,56].
Fast solute diffusion in metals has been attributed to the dissociative
mechanism (see, e.g., [57]). This mechanism operates for solutes which are
incorporated not only on substitutional sites but also to some extend in
interstitial sites of the solvent metal (see Sect. 1.6). According to (1.47) the
dissociative reaction involves vacancies. Provided that local equilibrium is
established, the concentrations of the three involved species must fulfill the
law of mass action
C
i
C
V
C
s
= K(T )=
C
eq
i
C
eq
V
C
eq
s
, (1.74)
where C
i
, C
s
,andC
V
denote molar fractions of interstitial solute, substitu-
tional solute, and vacancies. K(T ) is a constant which depends on tempera-
ture and the superscript eq denotes thermal equilibrium.
A metal crystal with a normal density of dislocations has a sufficient
abundance of vacancy sources or sinks to keep the vacancies everywhere in
1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 41
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
10
-10
10
-9
10
-8
16 18 20
22 24
26
600 500
400
T
m
= 601
K
Pt
Au
Pd
Cu
N
i
Zn
Ag
Cd
Hg
Sn
Tl
Pb
T
-1
/ 10
-4
K
-1
D / m
2
s
-1
T / K
Fig. 1.21. Diffusion
of several solutes and
self-diffusion (dashed
line) in lead.
equilibrium. Then the effective diffusivity of solutes is given by [41, 57]
D
eff
=
D
s
C
eq
s
C
eq
i
+ C
eq
s
+
D
i
C
eq
i
C
eq
i
+ C
eq
s
, (1.75)
where D
i
denotes the diffusivity of the solute in its interstitial state and D
s
its vacancy-mediated diffusivity on substitutional sites.
For solutes with dominating interstitial solubility and diffusivity (C
eq
i
C
eq
s
and D
i
D
s
), (1.75) reduces to the trivial case of interstitial diffusion
D
eff
≈ D
i
. (1.76)
For so-called hybrid solutes the substitutional solubility dominates (C
eq
s
C
eq
i
) but the interstitial diffusivity is much faster than the substitutional one
(D
i
> D
s
). Then the effective diffusivity (1.75) approaches
D
eff
≈
D
i
C
eq
i
C
eq
s
. (1.77)
This relation contains the factor
C
eq
i
C
eq
s
=exp
−
G
is
RT
, (1.78)
where G
is
denotes the Gibbs free energy difference between the interstitial and
substitutional positions of the solute. The rather wide ‘diffusivity dispersion’
of fast solute diffusers can be largely attributed to this factor.
42 Helmut Mehrer
Fast diffusion of solutes is well known for the semiconducting elements sil-
icon and germanium [41,58,59] (see also Chap. 4). It has been also attributed
to interstitial-substitutional exchange mechanisms. The kick-out mechanism
is dominating diffusion of Au, Pt, and Zn in silicon [41,42], whereas the disso-
ciative mechanism is operating, e. g., for Cu in germanium [60]. From a chem-
ical viewpoint these similarities are not surprising. Silicon and germanium are
group IV elements such as the ‘open’ metals lead and tin. Fast diffusion is
also observed for compound semiconductors (e. g. Zn in GaAs [61]). Actually,
the concepts growing out from studies of fast diffusion in semiconductors
(see Chap. 4) have strongly influenced the interpretation of fast diffusion in
metals.
1.10 Self-Diffusion in Binary Intermetallics
Some intermetallics (intermetallic compounds or ordered alloys) have at-
tracted much attention as technological materials for high-temperature ap-
plications. A knowledge of their diffusion behaviour is of interest for the
production of these materials and for their use in technological applications.
Whereas diffusion in many pure metals and dilute alloys is thoroughly in-
vestigated and reasonably well understood, systematic diffusion studies for
intermetallics are still relatively scarce although considerable progress has
been achieved in recent years [62–67].
An atomistic understanding of diffusion in intermetallics in terms of de-
fect structure and diffusion mechanisms is obviously more complex than for
metallic elements:
Intermetallics crystallize in a variety of structures with ordered atom dis-
tributions. Examples are the B2-, D0
3
-, L1
2
-, L1
0
-, D0
19
-, B20-, and Laves-
phase structures. Some intermetallics are ordered up to the melting temper-
ature, others undergo order-disorder transitions because entropy favours a
less ordered or even a random arrangement of atoms at high temperatures.
We know intermetallic phases with wide phase fields and others which exist
as line compounds. Some intermetallics occur for certain stoichiometric com-
positions, others are observed for off-stoichiometric compositions only. Some
phases compensate off-stoichiometry by vacancies others by antisite atoms.
Let us concentrate on the more common cubic intermetallics. Their struc-
tures are the following (see Fig. 1.22):
B2 (or CsCl) Structure: The approximate composition is AB. The
B2 structure can be derived from the bcc lattice, if the two primitive cubic
sublattices are occupied by different kinds of atoms. Examples are FeAl,
CoAl, NiAl, CoGa, PdIn, CuZn, AuZn, and AuCd.
D0
3
(or Fe
3
Si) Structure: The approximate composition is A
3
B. The
D0
3
-structure can also be considered as an ordered structure derived from
the bcc lattice. In Fig. 1.22 (middle) A-atoms occupy white and grey sites,

1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 43
Fig. 1.22. Some frequent structures of intermetallics: B2 (left), D0
3
(middle), L1
2
(right).
B-atoms occupy black sites. D0
3
order is observed for example in Fe
3
Si, in
Fe
3
Al below about 825 K, and for some high-temperature phases.
L1
2
(or Cu
3
Au) Structure: The approximate composition is A
3
B. The
L1
2
-structure is an ordered structure in the fcc lattice. In Fig. 1.22 (right)
A-atoms occupy white sites, B-atoms occupy black sites . Examples are the
Ni-based compounds Ni
3
Al, Ni
3
Ga, and Ni
3
Ge.
Self-diffusion is the most basic diffusion process also in alloys and com-
pounds. Like in the case of pure metals studies of self-diffusion utilize such
tiny amounts of tracer atoms (see Sect. 1.4) of the diffusing species that the
chemical composition of the sample does practically not change due to dif-
fusion. In a binary system two tracer self-diffusion coefficients – one for A
atoms and another one for B atoms – can be determined.
Table 1.4 compiles those binary intermetallics with B2, D0
3
,andL1
2
structures for which self-diffusion data are available. In some cases self-
diffusion of both components has indeed been studied. For NiAl and FeAl
impurity diffusion of some solutes has been studied, since no appropriate Al
tracer is available. Also interdiffusion studies were used to deduce the Al
diffusivity via the Darken-Manning equation (see Sect. 1.11) from the in-
terdiffusion coefficient, the tracer diffusivity of the other alloy constituent,
and the thermodynamic factor (see, e. g., [68]). In what follows it may suffice
to illustrate some characteristic features of self-diffusion in intermetallics. A
comprehensive discussion of all aspects of self-diffusion is beyond the scope
of this chapter. For further information the reader is referred to several
overviews [62–66,69].
1.10.1 Influence of Order-Disorder Transition
An order-disorder transition occurs, for example, between the β-andβ
-brass
phases of the Cu-Zn system. Below the order-disorder transition (at about
741 K) the compound shows B2 order (β
brass). At high temperatures the

44 Helmut Mehrer
Table 1.4. Self-diffusion in B2, D0
3
,andL1
2
structure intermetallics. For the
underlined components tracer self-diffusion data or data of suitable substitutional
substitutes (in brackets) are available
16
.
Structure Intermetallic
B2 CuZn,AuCd,AuZn,CoGa,PdIn,FeCo,NiAl(Ga),
Fe
Al(Zn,In,Cr,Mn,Ni,Co)AgMg,NiGa
L1
2
Ni
3
Al, Ni
3
Ge,Ni
3
Ga,Co
3
Ti, Pt
3
Mn,Cu
3
Au (disordered)
D0
3
Fe
3
Si(Ge), Cu
3
Sn,Cu
3
Sb, Ni
3
Sb, Fe
3
Al
disordered A2 structure (β brass) is formed. The beautiful pioneering work
of Kuper et al. [70] on self-diffusion of
64
Cu and
65
Zn in CuZn is displayed
in Fig. 1.23. The influence of the order-disorder transition on the diffusion
behaviour of both components is visible as a change in slope of the Arrhenius
plot. The activation energies obey the inequality
Q
B2
>Q
A2
. (1.79)
The occurrence of order impedes the diffusion of both components in a similar
way. Similar effects of the B2-A2 transition have been observed for diffusion
in FeCo.
Recently Fe diffusion in Fe
3
Al has been studied over a wide temperature
range [21]. Fe
3
Al undergoes two order-disorder transitions from D0
3
order
at low temperatures to B2 order at elevated temperatures to the completely
disordered A2 structure at high temperatures. At the critical temperatures
the slope of the Arrhenius diagram changes and the activation energies obey
the following sequence
Q
D0
3
>Q
B2
>Q
A2
. (1.80)
The activation energy is highest for the structure with the highest degree
of order and lowest for the disordered structure. The effect is, however, less
pronounced in Fe
3
Al than in CuZn.
1.10.2 Coupled Diffusion in B2 Intermetallics
Fig. 1.23 reveals another remarkable feature of self-diffusion in B2 inter-
metallics. We recognize that Zn in β-brass diffuses only slightly faster than
Cu and that the ratio D
Zn
/D
Cu
never exceeds 2.3 [70]. For equiatomic FeCo
16
For references see [63–65].

1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 45
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
10
-10
10 11 12 13 14
15 16
17 18
1000
900 800 700 600
468°
C
B2
CsCl
A2
bcc
CuZn
CuZ
n
T
-1
/ 10
-4
K
-1
D / m
2
s
-1
T / K
Fig. 1.23. Self-
diffusion of
64
Cu and
65
Zn in CuZn.
the ratio D
Fe
/D
Co
is always close to unity [71]. This type of ‘coupling’ be-
tween the diffusivities of the components seems to be typical of B2 phases.
It can be observed in Fig. 1.24 for practically all B2 compounds, for which
both constituents have been investigated. In some cases (e.g. NiGa, CoGa)
the bounds for D
A
∗
AB
/D
B
∗
AB
are somewhat wider than in the cases of CuZn and
FeCo. However, the difference between the diffusivities is less than one or-
der of magnitude. This ‘coupling’ between the diffusivities of the components
indicates that the diffusion of both atomic species is likely mediated by the
same defect.
As already mentioned the B2 structure consists of two primitive cubic
sublattices. In the completely ordered state of a stoichiometric B2 compound,
A atoms occupy one sublattice and B atoms the other. This implies that
each A atom is surrounded by 8 B atoms on nearest-neighbour sites and vice
versa. If the A and B atoms are distributed at random, a body-centered cubic
(bcc) structure (A2 structure) is obtained. When atomic diffusion in a highly
B2 ordered compound would take place by a random interchange between
vacancies and atoms via nearest-neighbour jumps, migrating vacancies would
leave traces of antisite defects (A
B
and B
A
) behind. In order to maintain order
such disordered regions must either be avoided or compensated during the
diffusion process.
If the order energy is high and the degree of order close to unity sublattice
diffusion of each component via second nearest-neighbour jumps is conceiv-
able. It is well known that diffusion of the components in ionic crystals and in
simple oxides occurs indeed by sublattice diffusion (see Chap. 5 and, e. g., [2]).

46 Helmut Mehrer
10
-21
10
-20
10
-19
10
-18
10
-17
10
-16
10
-15
10
-14
10
-13
10
-12
10
-11
10
-10
6
7 8
9 10
11 12 13 14 15 16
17 18 19
1300 1100 900
800 700 600
FeC
o
FeCo
NiAl
AgMg
F
eAl
Ni
Ga
NiGa
AgMg
PdIn C
uZn
C
uZ
n
CoG
a
CoGa
PdIn
Au
Cd
A
u
Cd
A
uZn
A
u
Zn
T
-1
/ 10
-4
K
-1
D / m
2
s
-1
T / K
Fig. 1.24. Self-
diffusion in B2 struc-
ture intermetallics.
However, sublattice diffusion cannot be the dominating mechanism in B2 in-
termetallics since sublattice diffusion does not lead to a coupled diffusion of
the components.
Ingenious order-retaining mechanisms, for which diffusion of both com-
ponents is coupled, have been proposed for B2 intermetallics:
– Six-Jump-Cycle Mechanism: A vacancy trajectory of 6 consecutive
nearest-neighbour jumps displaces atoms in such a way that after the
cycle is completed the order is re-established [72]. The ratio of the diffu-
sivities for this mechanism for a highly ordered stochiometric compound
lies within the following narrow limits [69]: 0.5 <D
A
∗
AB
/D
B
∗
AB
< 2.
– Triple-Defect Mechanism: In a stoichiometric B2 compound vacancies
and antisite defects can associate to form triple defects A
B
+2V
A
,withV
A
denoting a vacancy on the A-sublattice and A
B
an A-antite atom on the B-
sublattice. Then the ratio of the diffusivities for this mechanism lies within
the following limits: 1/13.3 <D
A
∗
AB
/D
B
∗
AB
< 13.3 [73]. As the composition
deviates from stochiometry antisite atoms widen these limits [74]. Thus,
in a less ordered state, values of D
A
∗
AB
/D
B
∗
AB
beyond these limits can no
longer be considered as an indication that the six-jump-cycle mechanism
does not operate.
– Antistructure-Bridge Mechanism: For an ordered B2 phase with
some substitutional disorder antisite defects can act as ‘bridges’ to es-
tablish low-energy sequences for vacancy jumps [75]. Long-range diffusion

1 Diffusion: Introduction and Case Studies in Metals and Binary Alloys 47
via this mechanism requires a sufficient concentration of antisite defects
to reach the percolation threshold [76].
– Vacancy-Pair Mechanism: A bound pair of vacancies (on both sublat-
tices) can mediate diffusion of both components by successive correlated
next-nearest-neighbour jumps. Whereas this mechanism has some rele-
vance for CsCl-type ionic crystals it is unlikely for B2 intermetallics.
A detailed description of these mechanisms can be found in [63,67]. It seems
that in those B2 compounds, which are composed of a group VIIIB metal
(Co, Fe, Ni, Pd, etc.) and a group IIIA metal (Al, Ga, In, etc.), the triple
defect mechanism is important. By contrast, B2 phases composed of a noble
metal (Cu, Ag, Au) and a divalent metal (Mg, Zn, Cd) are considered as
candidates for the 6-jump cycle mechanism. Clearly, the antistructure bridge
mechanism becomes more important at deviations from stoichiometry.
Perhaps the most thoroughly studied B2 compound is NiAl, where Ni
diffusion has been measured for various compositions on both Al- and Ni-
rich sides and over wide temperature intervals [77, 78]. While the Ni tracer
diffusivity increases notably on the Ni-rich side of the stoichiometric compo-
sition it is practically not changed with the composition on the Al-rich side in
spite of the large amount of structural Ni-vacancies (several %). Calculations
of the atomic mechanism using embedded atom potentials showed that the
triple-defect mechanism dominates self-diffusion on the Al-rich side and for
stoichiometric NiAl. With increasing Ni-content after reaching the percola-
tion threshold the antistructure-bridge mechanism dominates on the Ni-rich
side [77]. These findings for NiAl agree with the pioneering work on the B2
phase CoGa by Stolwijk et al. [79] .
1.10.3 The Cu
3
Au Rule
The Cu
3
Au rule (see, e.g., [2]) provides a logical tool to fathom the self-
diffusion behaviour in non-equiatomic intermetallics. It states that in some
compounds of type A
m
B
n
,wheretheratiom/n is equal to or greater than
2, the majority element diffuses faster than the minority element:
D
A
∗
AB
>D
B
∗
AB
. (1.81)
Here D
A
∗
AB
and D
B
∗
AB
denote the tracer diffusion coefficients of the components
A and B. For geometric reasons discussed below, A
3
B intermetallics with L1
2
or D0
3
structure are good candidates to test the validity of the ‘Cu
3
Au’ rule
17
.
17
According to [2] it should only be applied to intermetallics in which diffusion
occurs via vacancies. Phases such as, e.g., Fe
3
C, where one of the two elements
is sufficiently small to occupy interstitial sites in a matrix composed of the other
element, must be excluded. A nice essay about the Cu
3
Au rule can be found
in [80].
48 Helmut Mehrer
The A sublattice in D0
3
structure compounds is interconnected by nearest-
neighbour bonds, whereas this is not the case for the B sublattice (see
Fig. 1.22). Thus the A atoms can diffuse within their own sublattice via
nearest-neighbour (NN) jumps. If B atoms migrate within their own sublat-
tice, their jump vector corresponds to a third-nearest neighbour jump with
respect to the bcc unit cell. An alternative for the diffusion of B atoms are
nearest-neighbour jumps which, however, create B antisite defects. Both op-
tions are very likely associated with higher activation enthalpies for diffusion
of B atoms compared to A atoms. Those D0
3
compounds (Fe
3
Si, Cu
3
Sn, see
Table 1.4), for which reliable diffusion data for both constituents are available,
indeed fulfill this rule [63]. In L1
2
compounds each A atom is surrounded by
8 A atoms and 4 B atoms on nearest-neighbour sites (see Fig. 1.22). In con-
trast to this situation, a B atom faces only A atoms on surrounding nearest-
neighbour sites. This implies that similar to the D0
3
structure the sublattice
of the majority component A is interconnected by nearest-neighbour bonds,
whereas this is not the case for the sublattice of the minority component B.
Vacancy motion restricted to the majority sublattice can promote diffusion
of A atoms. However, diffusion of B atoms either requires jump lengths larger
than the nearest-neighbour distance or the formation of antisite defects, if it
is promoted by NN jumps of vacancies.
As can be seen from Fig. 1.25, diffusion of the majority component Ni
in Ni
3
Ge is indeed significantly faster than that of the minority component
Ge. Experiments on Ni
3
Ga revealed that the trend is similar to the case of
Ni
3
Ge, but the difference of the diffusivities is not so large [81].
For the technologically important compound Ni
3
Al no unquestioned data
of Al diffusion are available [62,63]. There are, however, indications that the
ratio of the two diffusion coefficients is not far from unity [64]. It is quite
natural that Ni diffusion in L1
2
compounds occurs by a sublattice vacancy
mechanism. On the other hand, it is not clear how the diffusion of the minor-
ity elements occurs in Ni based L1
2
compounds. Possible mechanisms have
been discussed in [64]. Most likely minority elements diffuse as antisite atoms
in the majority sublattice. Thus the Cu
3
Au rule should not be considered to
be universal. A compound which is a beautiful example for the validity of the
rule is MoSi
2
. Tracer diffusion studies of Si and Mo diffusion revealed a huge
asymmetry between the diffusion of the majority and the minority compo-
nent. Si diffusion is 6 to 7 orders of magnitude faster than Mo diffusion [82].
It is interesting to note that MoSi
2
is one of the compounds on which the
formulation of the rule was based to interpret silicide formation from thin
Mo layers on Si wafers [80].
