Heinrich J.G., Aldinger F. (Eds.) Ceramic Materials and Components for Engines
Подождите немного. Документ загружается.


4.4
Conclusion
The material and processing approach presented offers
thin walled corrosion resistant structures of complex
shapes that give vital support to the system development
of thin steel slab casting. The considerably improved
thermal shock resistance allows preheating techniques
presently used in the steel industry. In order to combine
the achievements with proven materials the development
of hybrid designs and corresponding joining techniques
are of outstanding importance. The principle results can
be transferred to other applications in steel or non steel
industry by carefidly computer aided matching of
application temperature profiles with thermal expansion
hysteresis adjustments.
5.
Started Activities and Outlook
Each of the given examples of functional cavity
applications shows ist
own
behaviour concerning
material and processing selection, level of porosity,
proportion of closed and open pores, size distribution of
pores that needs to be grasped
as
an interlinked system in
terms of computer aided modelling and simulation /7/.
New activities have been started in the field of
immobilization of microorganisms and bone
replacement. Fig. 15 shows microorganism of about 1
pm settled onto ceramic cavities. They are used to
generate a preproduct of vitamin C.
At a porosity level of about
50
%
as
suitable pore size in
the range of 150 pm has been estimated by analytical
simulation of the immobilization process.
Fig. 15: Immobilization of
coryne bacterium glutamicum
Fig. 16 illustrates a bone replacement structure made of
oecopore
-
Ti02
-
ceramics copying the gradual pore
size distribution of natural bone. At the ingrowth
interface a pore size range of 150 to 400 pm at a porosity
level
of
about 35
%
seems to be favourable.
Fig 16: Dummy structure of bone replacement
The spectrum of running and future activities is
summerized in Fig. 17.
It
only presents the tip of the
iceberg of the fascinating innovative potential of
hctional cavities with ceramics.
0.1
1
10
100
1000
PoresizeI)lml
Fig. 17: Spectrum of running and future activities for
functional cavities with ceramics
6.
Acknowledgements
Thanks are expressed for the fruitful cooperation with
the industrial partners, Thomas Joseph Heimbach
GmbH, Arwin Chesswick and SMS-Demag
as
well
as
with the Institut
fiir
Luft- und Raumfahrt, RWTH
Aachen. Respect is payed to the public fundings of the
MWMT Nordrhein-Westfalen and the Deutsche
Bundesstihg Umwelt.
7.
References
/1/ Maier, H.R. et al.
"Method for Producing porous Molded Body."
United State Patent
No.:
6,017,473;
Jan25,2000
/2/ Maier, H.R.; Best, W.; Schuhmacher, U.;Schafer,W.
"Charakteristics and Design of Diesel Soot Filters
Based on Direct Electrical Regeneration"
in: CFV Ber. DKG (1998), Nr.
5,
S.
25-29
"Verfahren
zur
Herstellung poroser keramischer
Strukturen", Offenlegungsschrift DE 19605 149A 1
Datum: 14.08.97
/3/ Pfaff, E;Aneziris, C.; Maier, R.M.
/4/ Drobietz, R.
;
Krusch, C.
;
Neuwerth,
G.
;
Aneziris,C.; Jacob,
D.
;
Maier, H.R.:
"Keramische
Schalldtimpferstrukturen
fiir
Pkw- und
Flugmotoren". In: Werkstoff und Automobilantrieb.
Diisseldorf 1999,
S.
155-172.
(VDI
Bericht 1472)
ISBN 3-18-091472-6
151
Maier, H.R.; Aneziris, C.; Pfaff, E.
"Verfahren
zur
Herstellung eines Keramikwerkstoffs
auf Basis Zirkonoxid"
Patentanmeldung DE 19938752.4, Datum: 16.08.99
"MgO partial stabilisized zirconia materials with
titanium, alumina or spinel additives for the near net
shape processing"
Journal of the European Ceramic Society, accepted
and to be printed, Sept. 2000
"Porose Keramik, der Natur abgeschaute
Funktionshohlraume
als
Anreiz
fiir
neue
Anwendungen"
In:
Horizonte: die RWTH auf dem
Wege ins 21. Jahrhundert
/
Roland Walter (Hrsg.).
Berlin 1999
:
Springer,
S.
127-136.
/6/
Maier,
H.R.;
Aneziris,
C.;
Pfaff,
E.
/7/ Maier, H.R.
ISBN 3-540-66373-8
542

FEASIBILITY STUDIES ON APPLYING IN-SITU SINGLE
CRYSTAL OXIDE CERAMIC EUTECTIC COMPOSITES IN NON-
COOLED HIGH EFFICIENCY TURBINE SYSTEM
K.
Hirano, T.
Suzuki,
A. Sasamoto
Mechanical Engineering Laboratory, Agency
of
Industrial Science and Technology,
MITI
Namiki
1-2
Tsukuba-shi, Ibaraki-ken
305-8564,
Japan
ABSTRACT
MGC materials, which are in-situ single crystal
oxide ceramic eutectic composites have many
potentialities, such
as
high strength, high creep
resistance, and high oxidation resistance
at
ultra-high
temperature. In the New Sunshine Program of the
Agency of Industrial Science and Technology, a leading
research and development has started since FY1998 for
three years to apply MGC materials
as
ultra-high
temperature structural materials for gas turbine systems.
In this paper, an outline of the leading research and
development is given, and the results established by the
Mechanical Engineering Laboratory are introduced.
INTRODUCTION
Recently, MGC(Melt Growth Composite) materials
have newly been researched and developed[l]. MGC
materials are in-situ single crystal oxide ceramic eutectic
composites such
as
A1,O3N,A1,O,,(YAG),
Al,O,/GdAIO,
(GAP)
and A1,03/Er,AI,0,,(EAG). MGC
materials are expected to be one of the most interesting
and attractive ultra-high temperature structural materials
in
the field of power generator industry, aeronautics, and
aerospace, because they have many potentialities that
they keep high strength, high creep resistance, and high
oxidation resistance at ultra-high temperature. In
addition they can deform at ultra-high temperature and
they have possibility to fabricate complex shape of
components. However, they do not have enough fracture
toughness and thermal shock resistance for structural
materials, because they are made of oxideloxide
ceramics. Also there have been few researches of fatigue
strength and fatigue crack
growth
resistance. Then in
order to apply MGC materials to ultra-high temperature
structural materials, it is necessary to improve MGC
materials in these points.
The New Sunshine Program of Agency
of
Industrial
Science and Technology has started a leading research
and development for “MGC Ultra High-Eficiency
Turbine System” aiming to utilize MGC materials for
ultra-high temperature structural materials
for
a gas
turbine system. In this paper, an outline of the leading
research and development is briefly given and the
current research activities by the Mechanical
Engineering Laboratory are introduced.
OUTLINE OF LEADING
RE-
SEARCH AND DEVELOPMENT
The leading research and develcpment for “MGC
Ultra High-Eficiency Turbine System” is one of the
New Sunshine Programs(Energy Saving Technology and
Development Programs) under the Agency of Industrial
Science and Technology. The period qf the leading
research and development is for ,hree years from
FY1998 and the following items are inves-
tigated.
(1)
Fracture mechanism and improvements
For
materials
performance in severe environments
(2) Possibility of low-cost process technology for large
complex, near-net shaped components
(3)
Aero-mech. design methodology for turbine
components based on computational fluid dynamics
(4)
Turbine cycle analysis and system integrated
technologies
Under the Agency
of
Industrial Science and
Technology, the leading research and development is
directly administered by NEDO(New Energy and
Industrial Technology Development Organization) with
Technology Research Association
of
Gas
Turbine for
Practical Ability Progress, Ishikawajima-Harima Heavy
Industries
Co.
Ltd., Kawasaki-Heavy Industries, Ltd.,
UBE Industries, Ltd., and Japan Ultra-high Temperature
Materials Research Institute.
As a national laboratory in the Agency of Industrial
Science and Technology, the Mechanical Engineering
Laboratory joins the leading research and development,
and leads and
supports
it for its
own
knowledge base.
The Mechanical Engineering Laboratory also performs
the research especially in the field of evaluation of
mechanical properties to improve higher performance
MGC materials[2-4] and in the field of component
design to optimize MGC gas turbine blades by
computational fluid dynamics
CURRENT RESEARCH ACTIV-
ITIES IN MECHANICAL ENGI-
NEERING LABORATORY
In this paper, the current research activities in the
field of evaluation of mechanical properties of MGC
materials are indicated. Evaluation
of
fracture toughness
and fatigue crack
growth
resistance is discussed and
543
development of a materials testing system in simulated
gas turbine environments is shown.
MATERIALS AND EXPERIMENTAL PROCE
DURE
The MGC materials used in this study
are
binary in-
situ single crystal oxide ceramic eutectic composites
Al,03N,A15012 (YAG) and ternary in-situ ceramic
eutectic composites AI,O3N,AISO,, (YAG)/ZrO,. The
composition of the binary eutectic composites is
Alz0,iYAG=67/33wt%. They were fabricated by the
unidirectional solidification method at the melting
temperature
of
1900°C by lowering a crucible at a speed
of
5mm/h.
The composition of the ternary eutectic
composites is
A1,03NAG/Zr02=54/28/18wtO/o.
They
were also fabricated by the unidirectional solidification
method at the melting temperature of 1900
"C
by
lowering a crucible at a speed of 3Omm/h.
The microstructure for the AI,O,/YAG binary
eutectic composites is shown in Fig.1. Three-
dimensional networks of single crystal Al,O, and single
crystal YAG are observed.
Fracture toughness tests were conducted by the
indentation fracture method at a
room
temperature. In
the binary eutectic composites, in order to evaluate each
phase, the indentation load of
75gf was kept for 10
seconds. In the ternary eutectic composites, the
indentation load of 200gf was kept for 10 seconds. The
value of fracture toughness
K,
was evaluated by the
following equation.
K,c=O.O1
1E04P 6a4
'(
l/a)d
Where; E: Young modulus 21: Crack length
P: Indentation load 2a:Indentation size
Cyclic fatigue crack
growth
tests were conducted at a
room temperature in a load-controlled mode at a stress
ratio R=O.l and at frequency
f=l
-
lOHz
with a
sinusoidal wave using a closed-loop electro-hydraulic
materials testing machine.
I
I-
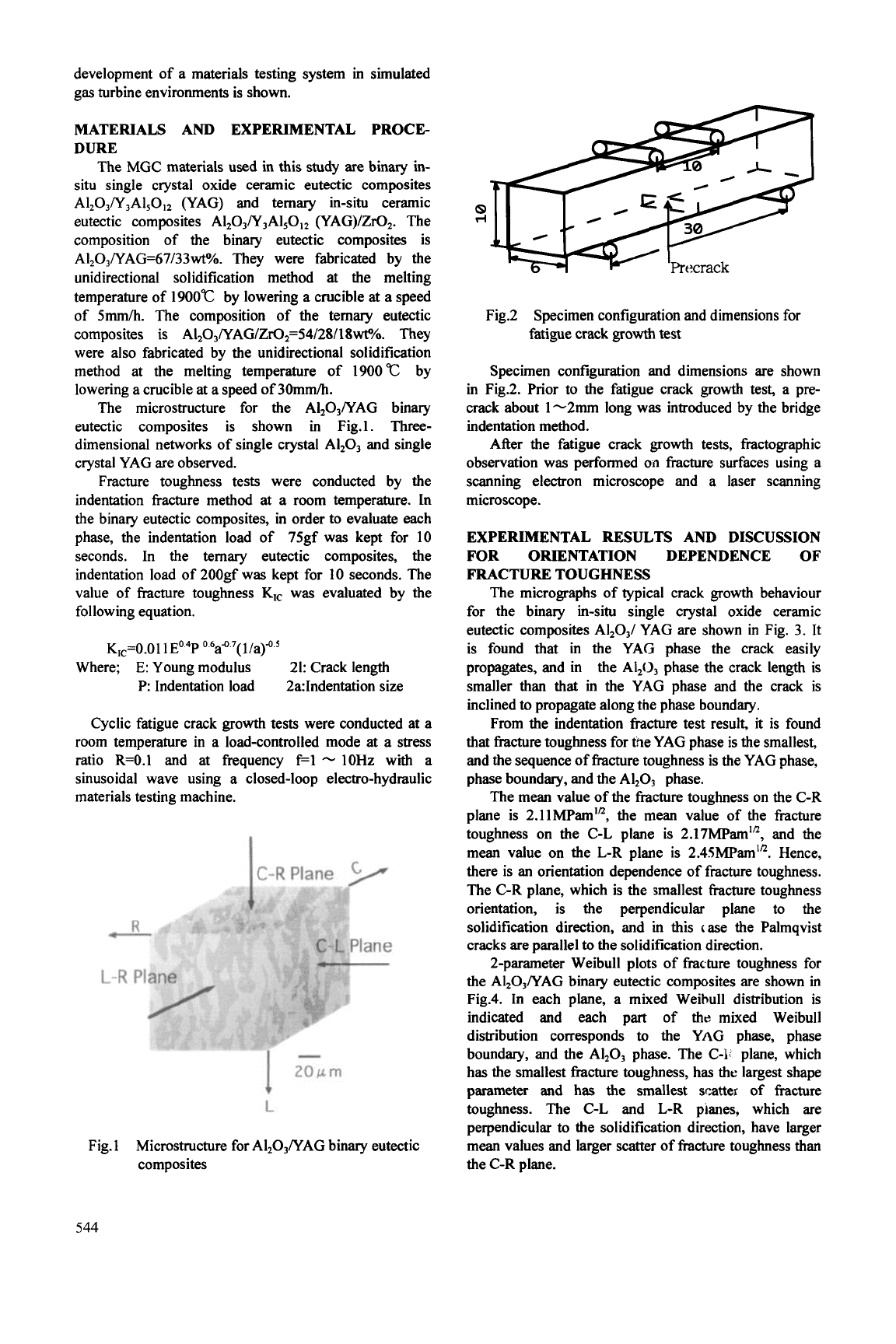
Fig2
Specimen configuration and dimensions for
fatigue crack
growth
test
Specimen configuration and dimensions are shown
in Fig.2.
hior
to
the fatigue crack
growth
test, a pre-
crack about 1-2mm long was introduced by the bridge
indentation method.
After the fatigue crack
growth
tests, fractographic
observation was performed
on
fracture surfaces using a
scanning electron microscope and a laser scanning
microscope.
EXPERIMENTAL RESULTS AND DISCUSSION
FOR
ORIENTATION DEPENDENCE
OF
FRACTURE TOUGHNESS
The micrographs of typical crack
growth
behaviour
for the binary in-situ single crystal oxide ceramic
eutectic composites Al,03/ YAG are shown in Fig. 3. It
is found that in the YAG phase the crack easily
propagates, and in
the A1203 phase the crack length is
smaller than that in the YAG phase and the crack is
inclined
to
propagate along the phase boundary.
From the indentation hcture test result, it is found
that fracture toughness for tne YAG phase is the smallest,
and the sequence of fracture toughness is the YAG phase,
phase boundary, and the A1,03
phase.
The mean value of the fracture toughness on the C-R
plane is 2.11~Pam", the mean value of the fracture
toughness on the C-L plane is 2.17MPamIR, and the
mean value on the L-R plane is 2.4SMPam". Hence,
there is
an
orientation dependence of
fracture
toughness.
The C-R plane, which is the smallest fracture toughness
orientation,
is
the perpendicular plane to the
solidification direction, and in this case the Palmqvist
cracks
are
parallel to the solidification direction.
2-parameter Weibull plots of fracture toughness for
the A1,03/YAG binary eutectic composites are shown in
Fig.4. In each plane, a mixed Weihull distribution is
indicated and each part of the mixed Weibull
distribution corresponds
to
the YAG phase, phase
boundary, and the Al,03 phase. The C-l.
plane, which
1
20bm
has the smallest fracture toughness, has thc largest shape
parameter and has the smallest scatter of fiacture
L
toughness. The C-L and L-R planes, which are
perpendicular
to
the solidification direction, have larger
mean values and larger scatter of fracture toughness than
Fig. 1
Microstructure for AI,O,/YAG binary eutectic
composites the C-R plane.
544
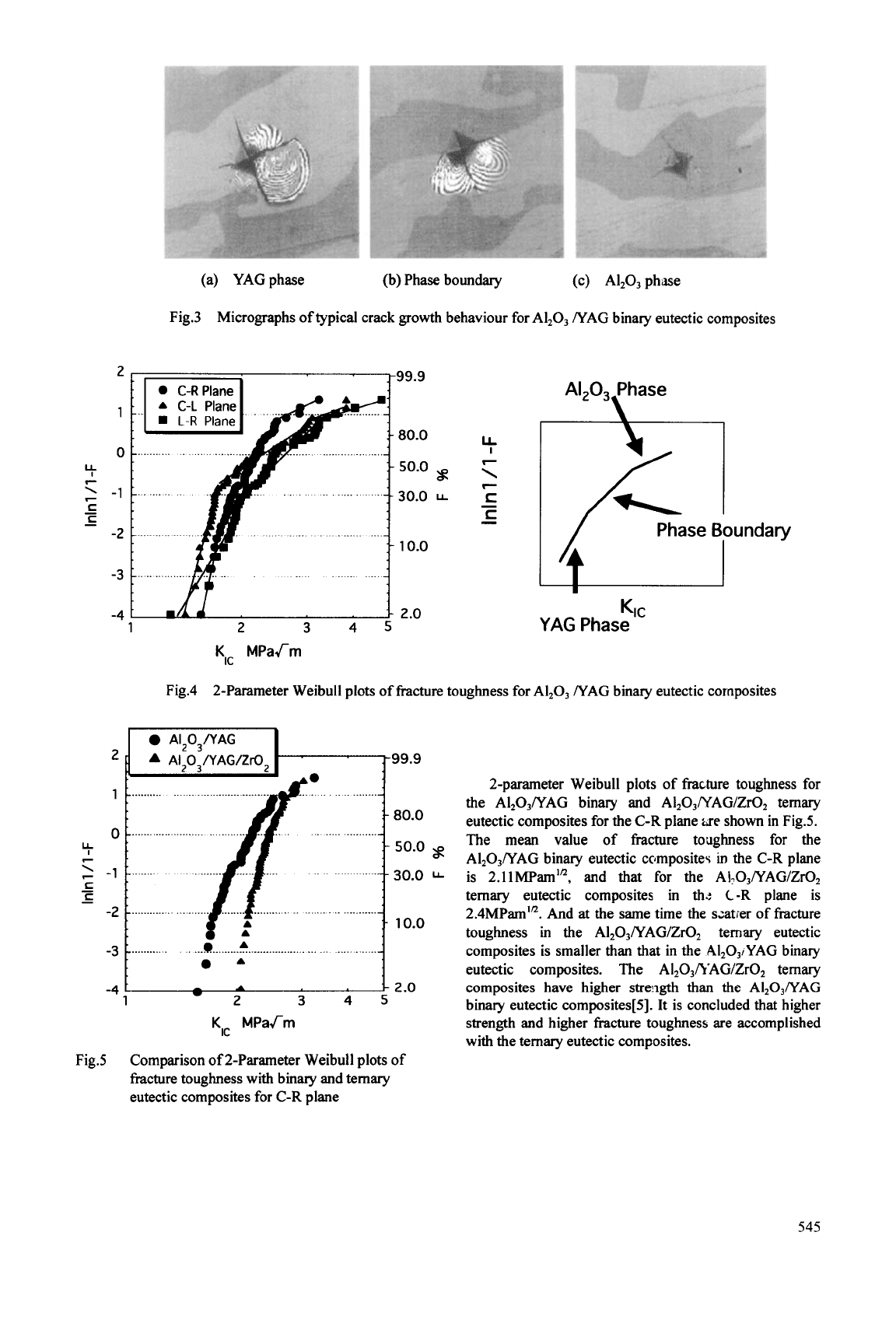
(a) YAGphase (b) Phase boundary (c) A1203 phase
Fig.3
Micrographs
of
typical crack growth behaviour
for
A1203 NAG binary eutectic composites
0
A120,/YAG
A
Al20,/YAG/Zr0,
.-99.9
7
C
C
-
-
1
0'-
'
-1
Lt
-2
-3
7
7
C
C
-
-
-4
......................
....................
.........
................
..................
..........................
\
0
..................................................
.-
f
80.0
........................................
-
50.0
g
-
30.0
.............................
...................
1
t
..................................................................
10.0
A
................
.................................................
.A
k
2.0
w
-
P
i-
e
OA
80.0
50.0
%
30.0
LL
10.0
-4
LL
2.0
1
2
3
45
Kc
MPaJm
KIC
YAG
Phase
Fig.4
2-Parameter Weibull plots
of
fracture toughness
for
A1,0, NAG binary eutectic composites
2-parameter Weibull plots
of
fracture toughness
for
the AI,O,NAG binary and AI,O,NAG/ZrO, ternary
eutectic composites
for
the C-R plane
iJe
shown in Fig.5.
The mean value
of
fracture toughness
for
the
AI,O,/YAG binary eutectic composite$
in
the C-R plane
is 2.11MPam1', and that
for
the AI-0,NAG/Zr02
ternary eutectic composites in th.:
C-R
plane is
2.4MPam''. And at the same time the szatier
of fracture
toughness in the AI,03NAG/Zr02 ternary eutectic
composites is smaller than that in the AI20,/YAG binary
eutectic composites. The AI2O3/k'AG/ZrO2 ternary
composites have higher straigth
than
the
AI,O,/YAG
binary eutectic composites[5]. It is concluded that higher
strength and higher fracture toughness are accomplished
with the ternary eutectic composites.
545
EXPERIMENTAL RESULTS AND DISCUSSION
ON FATIGUE CRACK
GROWTH
RESISTANCE
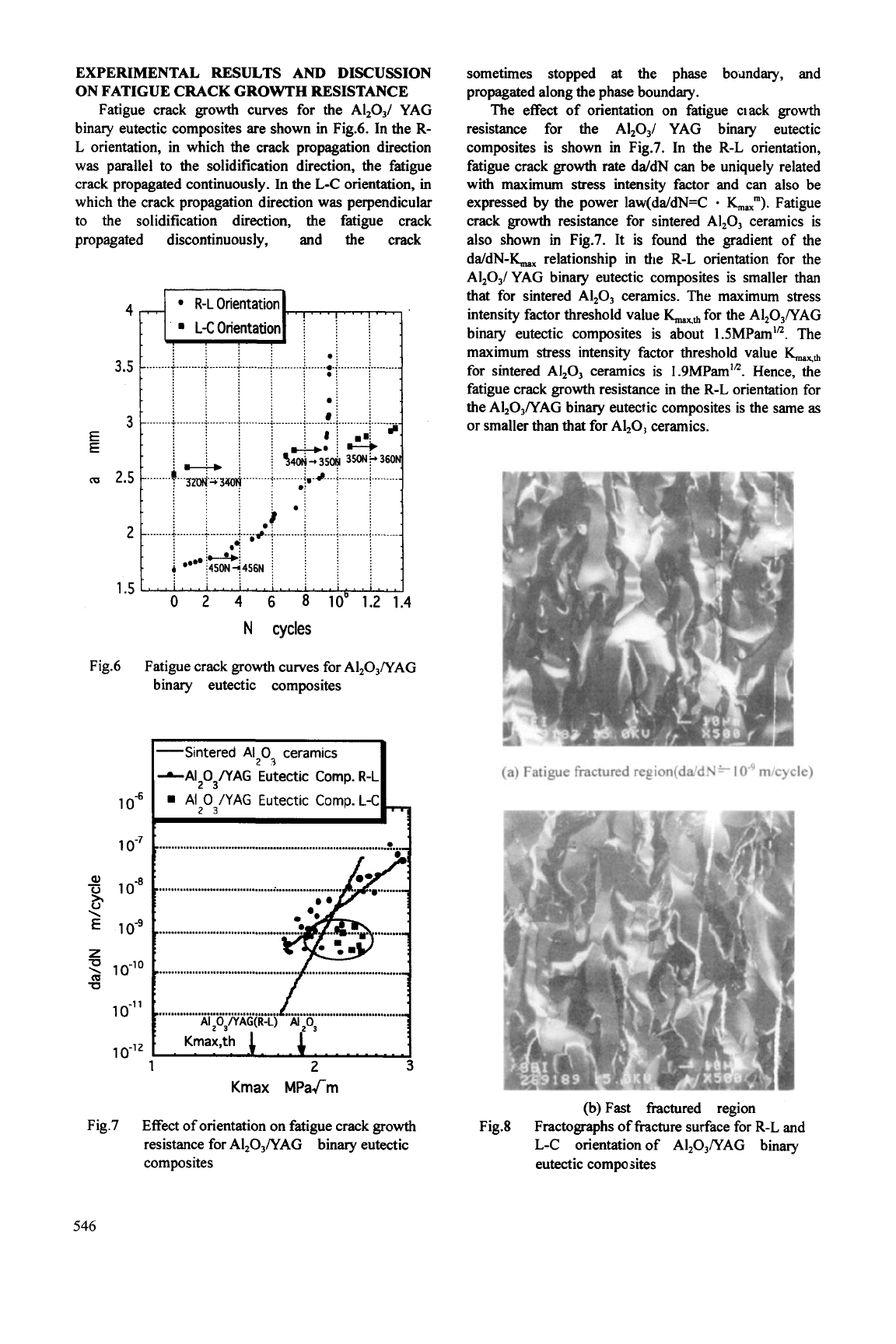
Fatigue crack growth curves for the Al,O,/ YAG
binary eutectic composites are shown in Fig.6. In the R-
L orientation, in which the crack propagation direction
was parallel to the solidification direction, the fatigue
crack propagated continuously. In the L-C orientation,
in
which the crack propagation direction was perpendicular
to the solidification direction, the fatigue crack
propagated discontinuously, and the crack
i .i
:
_
........
;
.........
:
..........
:
..........
:
..........
:
.......
.+
.........
:
........._
!
0;
i
L
0;
!
8:
j
_
........
;
.........
4
..........
:
..........
4
..........
:
..........
4
..........
:
.........-
:
*.
i
8
i
.mi
i
+*;w
.
i
540~~354
350N+360N
I
.
fi
,*
.
"'"'i""tT,
L-C
Orientation
3.5
3
E
E
m
2.5
Fig.6
Fatigue crack growth curves for AI,O,/YAG
binary eutectic composites
I
-Sintered
Al2O3
ceramics
-A1203/YAG
Eutectic
Cornp.
R-L
I
loa
I
Al
0
/YAG
Eutectic
Comp.
L-CL
23
1
o-~
10-l1
1
0-l2
............................................................................
2
...-
1
2
3
Kmax MPaJm
Fig.7
Effect of orientation on fatigue crack growth
resistance for Al,O,/YAG binary eutectic
composites
sometimes stopped
at
the phase bomdary, and
propagated along the phase boundary.
The effect of orientation on fatigue clack growth
resistance for the Al,03/ YAG binary eutectic
composites is shown in Fig.7. In the R-L orientation,
fatigue crack growth rate dddN can be uniquely related
with maximum stress intensity factor and can also be
expressed by the power law(da/dN=C
0
Lax"').
Fatigue
crack growth resistance
for
sintered
Al,03
ceramics is
also shown
in
Fig.7. It is found the gradient
of
the
da/dN-IC, relationship in the R-L orientation
for
the
Al,O,/ YAG binary eutectic composites is smaller
than
that for sintered A1,0, ceramics.
The
maximum stress
intensity factor threshold value
LKfi
for
the Al,03/YAG
binary eutectic composites is about
1
SMPam".
The
maximum stress intensity factor threshold value
for
sintered AI,03 ceramics is
1.9MPam".
Hence, the
fatigue crack growth resistance in the R-L orientation for
the Al,O,/YAG binary eutectic composites is the same
as
or
smaller than that for A1,0, ceramics.
(a) Fatigue fractured region(da/dN* m/cycle)
(b) Fast fractured region
Fractographs of fracture surface
for
R-L and
L-C orientation of Al,O,/YAG binary
eutectic composites
Fig.8
546

High Pressure Vessel+
Materals Testing Machine
Actuator
~-.n
Controller+
I
computer
I
Fig.9 Schematic representation of a materials testing system
in
simulated gas turbine environment
In the L-C orientation, in which discontinuous
fatigue crack growth behaviour was observed, it has not
been clarified whether the crack growth rate dddN is
uniquely expressed by the maximum stress intensity
factor
Lax.
However, fatigue crack growth rates in the
L-C
orientation for the AI,O,/YAG binary eutectic
composites are much smaller than those for sintered
AI,O, ceramics at any
Lax.
Hence, it is concluded that
fatigue crack growth resistance in the L-C orientation for
the AI,O,/YAG binary eutectic composites is larger
than that for sintered A1,03 ceramics.
Fig.8 shows fractographs of fracture surfaces
in
the
R-L and L-C orientations of the A1,03/YAG binary
eutectic composites. In both the fatigue fractured
region and the fast fractured region in the R-L and L-C
orientations, only intergranular fracture is observed. In
fatigue fracture of sintered Al,O, ceramics, it is well
known that transgranular fracture is usually observed.
Hence, the fatigue crack growth mechanism for the
AI,O,/YAG binary eutectic composites is quite different
from that for sintered AI,O, ceramics.
DEVELOPMENT
OF
A MATERIALS TESTING
SYSTEM IN SIMULATED GAS TURBINE
ENVIRONMENTS
Gas
turbine structural materials are actually used in
ultra-high temperature, high-pressure water vapor
environments. Then, it is necessary to develop a
materials testing system in simulated gas turbine
environments.
The schematic representation for the system
is
shown in Fig.9. The system consists of a testing
machine with a high-pressure vessel, an environ-
mental simulator, and a computer and
a contoller.
The materials testing machine is controlled by a
closed-loop electro-mechanical servomotor. The cross-
head speed is 0.0001-1.0mm/min and the maximum
load is 20kN.
The high-pressure vessel consists of
two
concentric
chambers. The environment is only coritrolled in the
inside chamber. The environmental sirnulator consists of
a water vapor circuit device and a gas supplier, and it
can control water vapor, N, gas, and
O2
gas
environments up to 0.98MPa. The endurance tests will
be conducted by the materials testing system.
FUTURE PROSPECT
In order to improve the thermal efficiency of gas
turbine systems, it is necessiuy to develop a new heat
resistant material to improve the turbine inlet
temperature without cooling the turbine structural
components. From the results
of
the leading research
and development[5], the thermal efficiency increases
about 15% if MGC materials are applied
to
the turbine
structural components such
as
turbine blade and
combustion liner. Now the data on the mechanical
properties and fabricating technique
for
MGC materials
are increasing. Hence, the planning of a technology
development program for a MGC ultra high-efficiency
turbine system is needed.
CONCLUSION
An outline of the leading research and development
for “MGC Ultra High-Efficiency Turbine System” is
given, and the current activities by the Mechanical
Engineering Laboratory have been introduced.
547

(1)
For the AI,O,/YAG binary eutectic composites,
fracture toughness for the YAG phase is the smallest,
and it decreases in the sequence of the YAG phase,
phase boundary and the Al,O, Phase. From 2-
parameter Weibull plots, a mixed Weibull distribution
is indicated and each part of the mixed Weibull
distribution corresponds
to
the YAG phase,
phase
boundary, and the AI,O, phase.
(2)
Fracture toughness for the AI2O3/YAG/ZrO2 ternary
eutectic composites show larger values than that for
the binary eutectic composites
in
the C-R plane.
Hence, higher strength and higher hcture toughness
is
accomplished with ternary eutectic composites.
(3) In the R-L orientation for the AI,O,/YAG binary
eutectic composites, which has the smallest fatigue
crack growth resistance, fatigue crack growth
resistance is the same
as
or smaller than that of
sintered Al,O, ceramics. In the L-C orientation for the
A1,OJYAG binary eutectic composites fatigue crack
growth resistance is larger than that for sintered AI,O,
ceramics.
(4)
A new materials testing system which can perform
mechanical tests in simulated gas turbine
environments is newly developed.
ACKNOWLEDGEMENT
This leading research and development is part of the
New Sunshine Program, Agency of Industrial Science
and Technology. The authors wish to thank New
Energy and lndustrial Technology Development
Organization.
The authors also wish to thank Technology Research
Association of
Gas
Turbine for Practical
Ability Progress and the cooperating contractor’s
companies (Ishikawajima-Harima Heavy Industries
Co., Ltd. Kawasaki-Heavy Industries, Ltd.,
UBE
Industries, Ltd., Japan Ultra-high Temperature Materials
Research Institute)
REFERENCES
(1) Y. Waku,
N.
Nakagawa, T. Wakamoto,
H.
Ohtsubo,
KShimizu and Y. Kohtoku, A Ductile Ceramic
Eutectic Composite Nith High Strength at 1873K3,
Nature, 389-6646,(1997) 49-52.
(2) K. Hirano, A. Kamei, and T. Suzuki, Fracture
Toughness Variation for In-sitb Single Crystal
Ceramic Eutectic Composites, Abstract for American
Ceramics Society lOlst Annllal Meeting
&
Exposition,( 1999) 191.
(3) K. Hirano, Future Prospects of R&D on Ultra-high
Temperature Structural Materials, Proc. of 7th
International Fatigue Congress-Fatigue’99,
Vo1.4,
(4) K. Hirano,
T.
Suzuki,
A.
Kamei and F. Tamai, R&D
on In-situ Single Crystal Oxides Ceramics Eutectic
Composite, Proc. of 7th Euro-Japanese Symposium
on Composite Materials and Tranwportation, (1999),
(5)
Progress Report for the Results of Leading Research
and Development for MGC Ultra High-Efficiency
Turbine System( 1999), Technology Research
Association of Gas Turbine for Practical Ability
Progress.
(1999),
2
11 9-21 26.
183-184.
548
SYNTHESIS AND PROPERTY TAILORING
OF
REACTION-BASED
COMPOSITES: THE
RBAO
AND THE 3A PROCESS
Sven Scheppokat*, Mark Roeger*, Peter Beyer*
*,
M. Leverkohne*
*,
Rolf Janssen*’*
*,
and
Nils Claussen*’*
*
(*)
Materials Engineering Hamburg GmbH
Nartenstr. 4a,
D-21079
Hamburg, Germany
(**)
Technical University Hamburg-Harburg
Advanced Ceramics Group
D-2 107
1
Hamburg, Germany
ABSTRACT
Ceramics and metal/ceramic composites are
promising materials for a variety of applications in
engines due to their unique property profile including
excellent high-temperature behavior, good tribological
properties, oxidation resistance, and low specific weight.
In order to make economic use of these materials, it is
necessary to manufacture them by a low-cost process
that can be adapted to varying demands. Two related
processes, the RBAO (Reaction Bonding of Aluminum
Oxide) and the 3A (Alumina Aluminide Alloys) process
combine low cost with broad compositional variability
and therefore with a wide range of microstructures and
properties. RBAO and 3A materials can be green
machined and provide reduced sintering shrinkage.
While the RBAO process yields purely ceramic
materials (alumina composites), the 3A process results
in metal/ceramic composites with interpenetrating
networks.
THE RBAO PROCESS
In the RBAO (Reaction Bonding of Aluminum
Oxide) process, mixtures of A1 and A120; are intensively
milled, compacted to high green density, and fired in
oxidizing atmosphere.[ 1-61 During this heat-treatment,
A1 is oxidized completely to A120;. The newly formed
A1203 particles sinter and bond the originally added
A120;. ZrOs is normally added to the precursor powder
in
order to improve the microstructure and the
mechanical properties of the final product. The milling
is
performed in organic liquids such as ethanol or
acetone in order to avoid excessive hydrolyzation of the
Al. The milled material is dried and then compacted.
Due to the presence of ductile Al which forms metallic
ligaments in the green bodies,
RBAO
green compacts
achieve exceptionally high green strengths that allow
extensive green machining. This provides an economical
advantage by saving costs (e.g. elimination of need to
manufacture dies for pressing samples of specific
geometry) and is also suitable for fast manufacturing of
small numbers of components or spare parts.
Fig.1: Green machined
RBAO
compacts
Typical green bodies compacted unidirectionally at
50
MPa and subsequently isostatically pressed at
300MPa reach strength values of about 20 MPa.The
green bodies are then heated in air slowly to 1000°C to
allow the A1 oxidation to take place, after that they can
be fired fast with a typical sintering temperature of
1550°C. Sintered
RBAO
components achieve typical
strength values
>
650 MPa and a fracture toughness of
3.5
MPadm. Increasing the zirconia content results in
fracture toughness values
>
5
MPadm. The RBAO
process can be modified by adding other phases, e.g.
additions of Sic to the precursor lead to mullite
materials and make zero shrinkage possible.
3A MATERIALS
The Alumina Aluminide Alloys (3A) process is a
novel route for low-cost manufacturing of metaVceramic
composites with interpenetrating networks.[7-
101
While
the densification of metal/ceramic composites usually
requires the application of pressure, [11,12] the 3A
process allows pressureless densification. The process
549

consists of a redox reaction between Al and a metal
oxide
M,Ob
followed by the formation of an intermetallic phase
between the metal and excess A1 in the material:
A]+
MaOb
+
M
i-
AI2o3 (1)
AI+M
+
M,Al,
(2)
Due to its very high affinity to oxygen, A1 reduces
the oxides of many other metals, these will be called the
reactive oxides here. Examples
of
reactive metal oxides
that have been used in this process include Ti02, Fe203,
Cr103, NiO, Zr02, and Nb205. The composition of the
aluminide formed is determined by the ratio of residual
A1 to the metal
M
in the material after completion of the
redox reaction. For example, in the system Ti02/Al,
materials containing TiAl, Ti3A1, and Ti have been
made. Alumina is always present in the final product as
a product of the redox reaction. Depending on their
composition, aluminides can be very refractory (T,
NbA13
=
1657"C, T, Nb3AI
=
1960"C), as well
as
low
specific weight
(p
TiAI3
=
3.4 g/cm3). Depending on the
type and volume fraction of the metal in the final
product, 3A materials reach strength values
>
600 MPa
and fracture toughness values >10 MPadm [8]. There
are two basic variants of the 3A process: One of them is
a powder metallurgical, or
sintered,
route (s-~A), the
other one involves metal
infiltration
of a porous ceramic
preform (i-3A). The basic reactions in both cases are the
same. The s-3A process starts from intensively milled
precursor mixtures of Al, A1203, and a reactive metal
oxide
MaOb.
Due to the presence of a ductile metal
phase (Al), the powder compacts made from these
mixtures exhibit high strength and can be green
machined. Densification is achieved by pressureless
sintering
in
inert atmosphere or vacuum. At
temperatures
>
5OO0C, the redox reaction between A1
and the reactive metal oxide takes place. This reaction
proceeds mainly as a solid state reaction below the
melting point of A1 (660°C). Because the redox reaction
is generally highly exothermal, carefkl process control
(slow heating ramps around the reaction temperature) is
needed in order to avoid thermal runaway. The
densification of the materials typically takes place at
temperatures between 1400 and 1550°C. A variant
of
the s-3A process is the
so
called 3AMC (3A Metal
Composites) process. In this case, the precursor
mixtures contain a metal, such as Fe or Cr instead of the
reactive oxide, and only a small amount
of Al. The
primary objective of the A1 addition here is to improve
the sintering behavior of the material by reducing the
native oxide layer on the metal. Because only a small
amount of
Al
is present, and the redox reaction takes
place only on a very small scale, much less energy is
released, and thermal runaway is not likely to occur.
Therefore, this version of the process requires less
careful control of process parameters, and faster heating
rates can be employed. In the case of Fe/AI2O3
composites, the usehlness of
A1
as a sintering aid has
been established [S], in the case of Cr/A1203 and
Nb/AI2O3 composites, dense materials have also been
attained without the addition of A1 [13,14]. However,
recent experiments indicate that A1 also promotes
densification in the case of Cr.
Fig.
2:SEM
micrograph
of
a
30
Vo1.-YO
Cr(A1)
/
70
Vo1.-YO
A1203
composite
Fig.
2
shows an SEM micrograph of a 3AMC
composite containing 30 Vo1.-% Cr(AI) and
70
VoL-%
A1203. The material is dense with few pores. The metal
phase shows a high degree of interconnectivity, and the
material is electrically conductive. Grain sizes are in the
range of up to
5
pm. The metal content has a strong
influence on the mechanical properties of the final
product. In general, strength decreases, but toughness
increases with increasing metal content.
In the i-3A process, a porous preform containing
A1203 and the reactive oxide is infiltrated with liquid A1
and subsequently annealed at higher temperature in
order to allow the reaction to take place. The i-3A
process is a net shape process that allows low
temperature synthesis of materials that are suitable for
high temperature applications due to the formation of
the refractory intermetallic phase. It is also particularly
suitable for local reinforcement of components and can
be used in existing industrial processes such as die
casting or squeeze casting.
With its different versions, the 3A process and its
derivates are very versatile. They allow the cost-efficient
manufacturing
of materials covering a wide range of
compositions with good properties.
ACKNOWLEDGEMENT
The authors wish to thank NED0 and the
Innovation Foundation Hamburg (Innovationsstihng
der Stadt Hamburg) for their support.
REFERENCES:
(1) N. Claussen, T. Le und
S.
Wu: ,,Low-Shrinkage
Reaction-Bonded Alumina",
J.
Eur. Ceram. SOC.
5
29-35 (1989)
550

(2)
N. Claussen, N.A. Travitzky und
S.
Wu: ,,Tailoring
of Reaction-Bonded A120; (RBAO) Ceramics",
Ceram. Eng. Sci. Proc.
1
1
806-820 (1990)
(3)
S.
Wu, D. Holz und N. Claussen: ,,Mechanisms and
Kinetics
of
Reaction-Bonded Aluminum Oxide
Ceramics";
J.
Am. Ceram. SOC.
76 [4] 970-980
(1
993)
(4)
D. Holz,
S.
Wu,
S.
Scheppokat und
N.
Claussen:
,,Effect of Processing Parameters on Phase and
Microstructure Evolution in RBAO Ceramics";
J. Am. Ceram. SOC.
77 [lo] 2509-25 17 (1 994)
(5)
D. Holz: "Herstellung und Charakterisierung von
reaktionsgebundenen A1203-Keramiken (RBAO-
Verfahren) am Beispiel des Systems A1203/Zr02",
PhD Thesis (in German), VDI Verlag Fortschritt-
Berichte Reihe
5
Nr.367
(1994)
(6)
M. Roger: "Aufbereitung und Verarbeitung von
RBAO-Precursormischungen
zur Herstellung
hochfester Bauteile", PhD Thesis (in German), VDI-
Verlag Fortschritt-Berichte Reihe
5
Nr.524,
(1
998)
(7)
N. Claussen, D.E. Garcia, and R. Janssen, "Reaction
sintering of alumina-aluminide alloys (3A)", J. Mat.
Res.
11
[
1
11
2884-2888 (1996)
(8)
S.
Schicker,
T.
Emy,
D.E: Garcia, R. Janssen, and
N.
Claussen, "Microstructure and Mechanical
Properties of Al-assisted Sintered Fe/A120;
Cennets",
J.
Eur. Ceram. SOC.
19
[13/14], 2455-
2463 (1999)
(9)
S.
Schicker, D.E. Garcia, J. Bruhn, R. Janssen, and
N. Claussen, "Reaction Processing of A1202
Composites Containing Iron and Iron Aluminides"
J.
Am Ceram. SOC.
80
[9], 2294-2300 (1 997)
(1
0)
D.E. Garcia,
S.
Schicker, J. Bruhn,
R.
Janssen, and
N.
Claussen, "Synthesis of Novel Niobium
Aluminide-Based Composites", J. Am Ceram. SOC.
80
[9], 2294-2300 (1997)
(1
1)
X.
Sun, J.A. Yeomans, "Microstructure and
Fracture Toughness
of
Nickel Particle Toughened
Alumina Matrix Composites", J. Mat. Sci.
31,
875-
880 (1996)
(12)
P.D. Djali and K.R. Linger, "The Fabrication and
Properties
of
Nickel-Alumina Cermets", Proc.
Br.
Ceram. SOC.
26
113-127 (1978)
(13)
D.E. Garcia,
S.
Schicker, J. Bruhn, R. Janssen, and
N. Claussen, "Processing and Mechanical Properties
of
Pressureless-Sintered Niobium-Alumina-Matrix
Composites",
J.
Am Ceram.
SOC.
80
[9], 2248-2252
(1 997)
Claussen,
"Nb-
and Cr-A120; Composites with
Interpenetrating Networks", J. Eur. Ceram.
SOC.
18
(14)
D.E. Garcia,
S.
Schicker, R. Janssen, and
N.
601-605 (1998)
55
1
