Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

Chapter 7 Cryoelectron Tomography (CET) 545
and Hegerl, 2001; Jiang et al., 2003b). The main purpose of fi ltering/
“de noi si ng” is to improve the visualization of the raw data and to
facilitate interpretation primarily at the level of the ultrastructure.
However, interpretation at the molecular level requires more quantita-
tive methods.
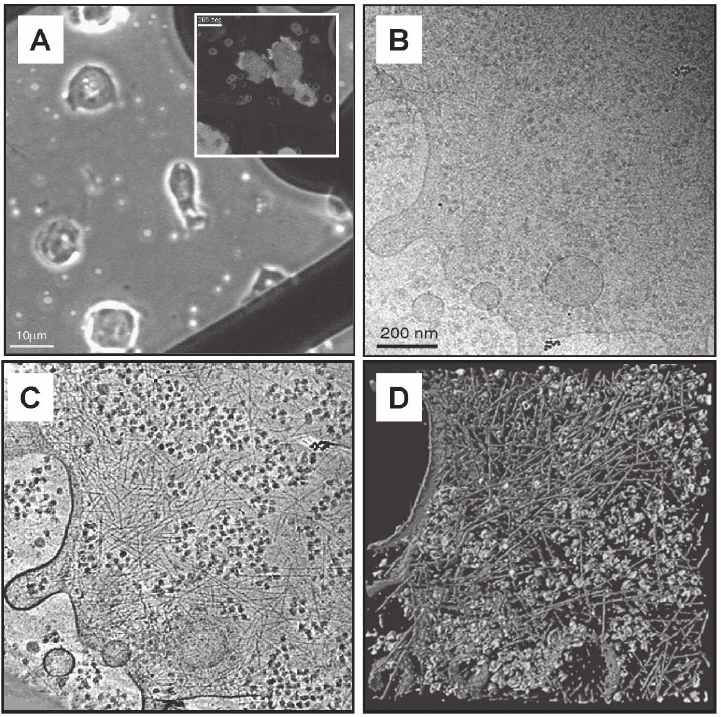
Figure 7–5. First electron tomographic investigation of a eukaryotic cell; the slime mould Dictyostel-
ium discoideum embedded in vitrifi ed ice. a) Phase contrast image and corresponding fl uorescence
image (inset) of cells on TEM grids. b) Cryo-electron micrograph at 0° tilt (conventional 2D
projection) of a ∼200 nm thin peripheral region of the cell. c) Tomographic reconstruction from a
complete tilt-series (120 images) and d) visualization by segmentation. Large macromolecular com-
plexes, e.g. Ribosomes are shown in a green color, the actin fi lament network in orange-red and the
cells’ membrane in blue. Cryo-tomograms of Dictyostelium discoideum cells grown directly on carbon
support fi lms have provided unprecedented insights into the organization of actin fi laments in an
unperturbed cellular environment. The tomograms show, on the level of individual fi laments, their
modes of interaction (isotropic networks, bundles, etc.), they allow us to determine the branching
angles precisely (in 3-D), and they reveal the structure of membrane attachment sites (Medalia
et al., 2002). (See color plate.)
546 J.M. Plitzko and W. Baumeister
A prerequisite for understanding the interaction of proteins is the
localization of macromolecules in the cell. Practical ways to achieve
this goal are labeling of macromolecules by specifi c and electron-dense
markers (Koster and Klumperman, 2003) or recognition of individual
particles by their known structural signature. The former is very hard
to realize under in vivo conditions, whereas the latter requires suffi -
ciently high resolution. Moreover, labeling techniques can be used for
only a few macromolecules simultaneously since the different labels
need to be distinguishable and specifi c. In the context of structural
proteomics this makes recognition by means of the structural signa-
ture favorable since simultaneous recognition of molecules under at
least close-to-native conditions is mandatory.
It has been shown previously that the currently achieved resolution
of cryo-ET should suffi ce to identify large protein complexes in the
range of 1 MDa (Boehm et al., 2000). Moreover, detection of protea-
somes (Lowe et al., 1995; Zwickl, 2000) and thermosomes (Klumpp
et al. 1997; Klumpp and Baumeister, 1998) in phantom cells, which
mimic real cells in size and composition with the advantage of being
biochemically well defi ned, showed that this approach is also feasible
experimentally (Frangakis et al., 2002). The approach used in these
works requires structural knowledge from other sources; matched
fi ltering of the tomograms with a structural template yields the loca-
tions and orientations of these complexes in the cell (see Section 3.5.4).
Therefore, cryo-ET requires structural data from other techniques
such as X-ray crystallography or single-particle analysis to obtain
molecular information. The principal dose limitation of cryo-ET does
not permit high-resolution structure determination on the basis of the
tomogram alone. The dose fractionation theorem (Hegerl and Hoppe,
1976; McEwen et al., 1995) states that tomography images each volume
element with the same statistical signifi cance as a 2D projection
acquired with the same overall dose. This makes tomographic data
highly advantageous for location of features since the information is
distributed over three dimensions. However, the statistical signifi -
cance of each volume element is generally not suffi cient to resolve
macromolecules at the level of tertiary or quaternary structural ele-
ments initially. However, the combination of cryo-ET and single-par-
ticle averaging can offer a remedy for this; by averaging identical
copies of complexes, they can potentially be resolved to at least a few
nanometers. First realizations of the combination of cryo-ET and
single-particle averaging have been reported for isolated proteins and
yielded structures of 800-kDa protein complexes to ∼2 nm resolution
(Nitsch et al., 1998); averaging of complexes directly from tomograms
of organelles or viruses is a novel approach (Figure 7–6). First 2D
results on the envelope complexes of dried and stained retroviruses
indicated the potential of this approach (Zhu et al., 2003). Recent 3D
results on the structure of the nuclear pore complex, a membrane
complex that is diffi cult to assess by other techniques, taken from
tomograms of entire nuclei, showed that this approach can yield
structural characterization of unprecedented resolution (Beck et al.,
2004, 2007).
Chapter 7 Cryoelectron Tomography (CET) 547
3 Major Diffi culties in Cryoelectron Tomography
Despite the fact that the acquisition process for ET sounds very simple
and straightforward, the acquisition of a tomographic tilt series, espe-
cially from biological samples is, even today, a major technical chal-
lenge. The explanation will be given in the following sections addressing
the fi ve major obstacles (“core problems”) of ET: (1) sample preparation
and preservation, (2) radiation sensitivity, (3) tilting geometry, (4)
instrumentation (EM, energy fi lters, CCD detectors, etc.) and automa-
tion, and (5) alignment, reconstruction, and visualization.
3.1 Sample Preparation
3.1.1 Basics
Biological objects such as cells consist mainly of water (more than 70%).
Built up from carbon-based molecules, they form the essential frame-
work of life but for a closer structural investigation with the EM they
have to withstand a constant “bombardment” of fast electrons under
ultrahigh vacuum conditions: clearly not the best starting points
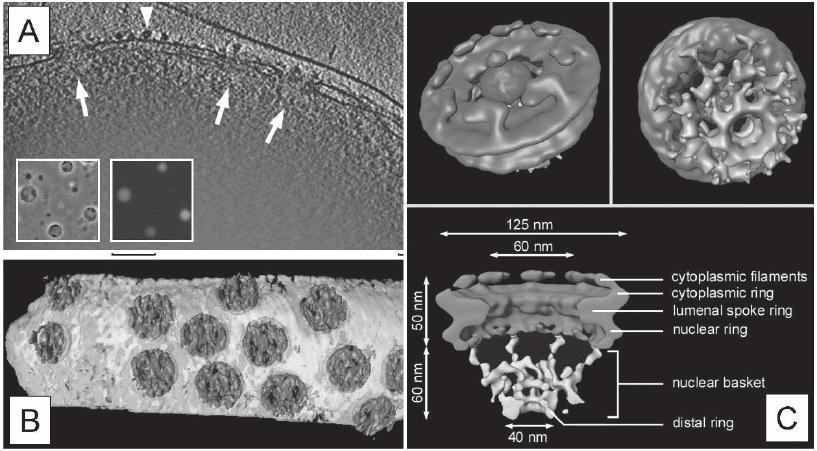
Figure 7–6. CET in combination with the single particle approach of transport-competent Dictyostel-
ium discoideum nuclei. a) Three-dimensional reconstruction of the peripheral rim of an intact nucleus.
X-y slice of 10 nm thickness along the z axis through a typical tomogram. Side views of nuclear pore
complexes (NPCs) are indicated by arrows. Ribosomes connected to the outer nuclear membrane are
visible (arrowheads). Inset displays a phase-contrast image and the corresponding fl uorescence image.
b) Surface rendered representation of a segment of nuclear envelope (NPCs in blue, membranes in
yellow). c) Structure of the Dictyostelium NPC after classifi cation and averaging of subtomograms.
Cytoplasmic face of the NPC (upper left); the cytoplasmic fi laments are arranged around the central
channel. Nuclear face of the NPC (upper right); the distal ring of the basket is connected to the nuclear
ring by the nuclear fi laments. Cross sectional view of the NPC (bottom). The dimensions of the main
features are indicated. All views are surface-rendered (nuclear basket in brown; Beck et al. 2004). (See
color plate.)
548 J.M. Plitzko and W. Baumeister
and clearly conditions that are far from natural and extremely
inhospitable.
Indeed, the central goal in any EM study is the observation of struc-
tures down to the atomic level. Unlike material science studies, where
the samples remain steady, biological specimens tend to be more
“resistant” to an investigation with the EM. First they are exposed to
ionizing radiation, namely electrons with speeds usually above
200,000 km/s, according to the acceleration voltage used. Second, the
ultrahigh vacuum generates an ambient pressure far below the atmo-
spheric pressure, and thus literally “sucks” out every shred of liquid,
subsequently resulting in the implosion of the biological structure.
Third, they are build up from carbon-based compounds plus elements
with very low atomic numbers like hydrogen, oxygen, and nitrogen,
and minute amounts of other low Z-elements, all very weak scatterers,
in regard to their interaction with electrons, resulting in low-contrast
images. Fourth, if we regard cellular structures of higher organisms,
such as mammalian cells, they can easily reach sizes of tenth of microm-
eters in all three dimensions, and are therefore almost or effectively
impenetrable to the electron beam. Fifth, the secrets of these structures
are hidden in a highly crowded environment, the cytoplasm, where
functional units like proteins, protein complexes, or even molecular
machines are “densely packed,” literally touching each other. This
“molecular crowding” (Ellis, 2001; Ellis and Minton, 2003) makes it
diffi cult to separate them for structural characterization. Sixth and last,
every cell is different, just as every human has a different face. Thus,
single 2D projections will not provide a complete 3D characterization,
and averaging techniques as in the so-called single-particle approach
are therefore impractical. All six of these facts have to be addressed in
any life science study and especially when investigating biological
structures in a close-to-life state, quasi in vivo.
The electron beam represents a form of ionizing radiation that is
harmful to the health of any living organism. Clearly, we cannot
shield the biological substance but we can try to extend its “life-span”
during investigation before structural damage occurs. Researchers in
the past have been ingenious in inventing preparation techniques for
EM studies of biological samples. In the late 1950s staining techniques
were introduced that addressed the fi rst three issues at once (Brenner
and Horne, 1959). Staining with salts from heavy metals (usually
osmium or uranium salts) envelopes the structure of interest and after
insertion into the EM the negative imprint of the structure can be
observed. Moreover, it increases the contrast dramatically, according
to the high atomic numbers of the metals used. However, the biologi-
cal structure desiccates in the vacuum environment of the microscope
and illumination by the electron beam literally incinerates whatever
is left of the biological substance, leaving behind the imprint of the
structure outlined by the staining substance. Staining and dehumidi-
fi cation alter the structure and, because of the nanocrystalline nature
of the staining salts, limit the resolution, in the best cases, to approxi-
mately 2 nm. The major advantage of this technique is its simplicity
(no special instrumentation is required), its speed, and the fact that
Chapter 7 Cryoelectron Tomography (CET) 549
the negative “shadow” of the sample is almost unaffected by radiation
damage. The obvious disadvantage is the highly “unnatural” state in
which the investigation is performed, leading to artifacts and possible
misinterpretations.
To study larger objects such as cells or organelles staining has been
combined with embedding in epoxy resins and subsequent sectioning
with the help of ultramicrotomes (Porter and Blum, 1953). Despite the
fact that almost all our ultrastructural knowledge of the cell interior is
based on this technique, there are some obvious limitations. First the
substitution material, e.g., epoxy resin, shrinks during hardening, thus
deforming the original structure. Second the stain applied after sec-
tioning reduces the resolution (as described above) and third a second
shrinkage step of the section is observed after initial illumination in
the EM. This effect can be as large as 30–40% in the direction of the
electron beam and 5–10% in the plane perpendicular to it. Shrinkage
of the initial section dimensions is caused by outgassing of the solid
polymer in the ultrahigh vacuum and to a larger extent by the loss of
mass due to radiation damage (Luther, 1992). However, shrinkage
occurs early in the illumination process, so that preexposing the sample
until the contraction is complete can help to stabilize the section. The
total electron dose is therefore increased dramatically and can lead to
dose-induced stain aggregation. After preexposure the sample remains
steady for further investigation of the modifi ed structures and can be
reused almost indefi nitely.
3.1.2 Cryopreparation
In the 1970s researchers experimented on 2D protein crystals with the
possibility of preserving specimen hydration in the EM (Taylor and
Glaeser, 1973, 1974). However, In 1981 Dubochet and McDowall pub-
lished their work on the vitrifi cation of pure water for EM and in 1984
Adrian et al. reported the fi rst successful investigation of a shock-
frozen biological object, namely viruses, embedded in vitrifi ed ice, now
known as the cryopreparation technique. There is no doubt that this
invention is one of the important milestones in life science EM studies,
if not the most important. With this technique, scientists were, for the
fi rst time, able to investigate biological objects in a close-to-life state,
quasi in vivo.
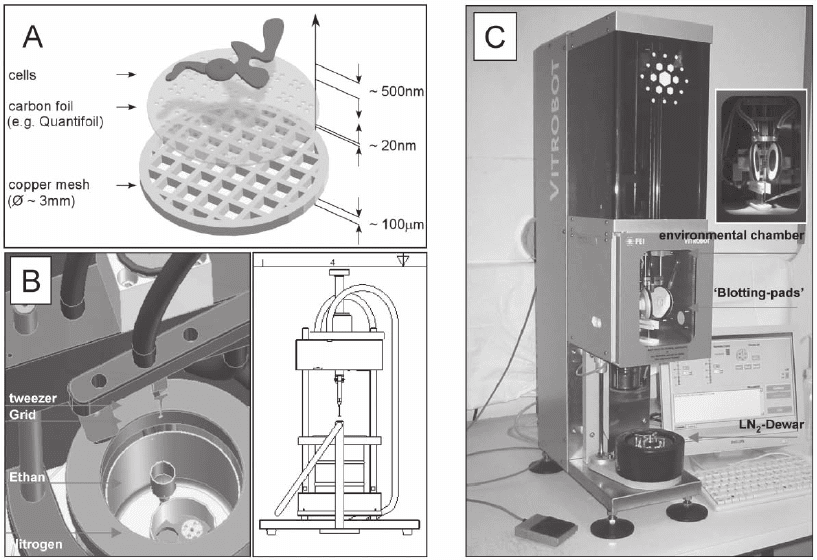
The object-holders are commercially available copper grids with a
diameter of 3 mm (Figure 7–7A). They can be purchased in different
geometries, mesh formats, and with different coatings (Plano GmbH,
Wetzlar, Germany; Ted Pella, Inc., Redding, CA; Quantifoil Micro Tools
GmbH, Jena, Germany; Ermantraut et al., 1998). Typically these grids
are coated with a very thin support foil, usually amorphous carbon
with thicknesses ranging between 4 and 20 nm. Shock-freezing has to
be done very fast and therefore a special apparatus is necessary. These
“g uillotine”-like instruments, so called plungers, permit freezing of the
sample in milliseconds to a temperature of liquid nitrogen (∼90 K). To
ensure a direct phase transformation from liquid to an amorphous
(vitrifi ed) solid, cryogens with very high cooling rates, like ethane or
less frequently propane, are used (Figure 7–7B and C).
550 J.M. Plitzko and W. Baumeister
The grid is clamped in an inverse tweezer and vertically mounted
into the plunging instrument. A droplet of a couple of microliters of
the sample solution, either isolated protein complexes or whole cells in
their buffer solution, is applied to the surface of the copper grid. Since
only thin objects in the range of a couple of hundred nanometers (t ≤
500 nm) are suitable for investigation with the EM, the sample volume,
typically in the range of 1–4 µl, has to be reduced. This is done with a
fi lter paper carefully brought either to one side or to both sides of the
grid to remove excess liquid. This process is called blotting. To prevent
any mechanically induced disruption of, e.g., cellular structures, blot-
ting can be done from the backside of the grid (Figure 7–8). Depending
on the duration of the blotting process, the viscosity of the sample
solution, and the size of the object, the thickness of the resulting ice
layer can be adjusted. It guarantees a complete vitrifi cation, without
the formation of unwanted and harmful ice crystals, which can already
be observed with fl uid layers exceeding a couple of micrometers.
Figure 7–7. Plunge freezing instrumentation. a) Typical arrangement of cells cultured on TEM grid just
a home-build plunge freezing apparatus (Images courtesy of R. Gatz, MPI of Biochemistry, Martinsried
(near Munich), Germany). The small reservoir (yellow) in the middle of the dewar (green) contains the
liquid ethane, which is chilled by a surrounding bath of liquid nitrogen. The forceps, which hold the
grid are attached to a weighted arm. When the arm is released by means of a foot-trigger, the grid is
gravity-plunged into the ethane. The cross-sectional scetch (left part of image B) illustrates the ‘guillo-
tine-like’ arrangement. c) The Vitrobot (FEI company, Eindhoven, The Netherlands), a ‘robot’ for vitri-
fi cation, allows the control of environmental and all necessary processing parameters. (See color
plate.)
prior to plunging. The schematic indicates the dimensions. b) CAD (computer aided design) image of
Chapter 7 Cryoelectron Tomography (CET) 551
Existing cryoplungers are mainly home-built and vary in design.
Quite recently commercially available instruments have been intro-
duced with the additional ability to adjust and control some of the
preparation parameters, such as humidity, temperature, and air pres-
sure, in specially designed environmental chambers. Moreover the
whole process, except for the application of the sample, which has to
be done manually, is computer controlled and automated, to facilitate
the sample preparation process and expand its reproducibility (Vitro-
bot, FEI company, Eindhoven, Netherlands).
After vitrifi cation, the sample is transferred in a cryoholder, specially
designed to ensure that the sample remains in the frozen state at
approximately liquid nitrogen temperature. If, by any chance, the tem-
perature drops below −160°C the possibility that the vitrifi ed ice will
start to crystallize increases. Therefore during transfer and during the
subsequent investigation, frozen-hydrated samples have to be kept at
all times at liquid nitrogen temperature and below −160°C, which
resulted in the name cryoelectron microscopy.
Compared to the commonly used techniques, cryopreparation allows
us to preserve the native structure of the cytoplasm and the whole
arrangement within the cell and even the physiological state at the
moment of plunging. We thus circumvent alterations or modifi cations
as seen with staining or epoxy resin embedding. The technique is
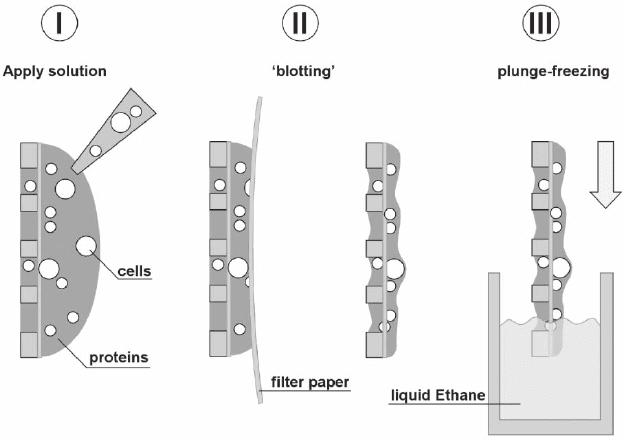
Figure 7–8. Steps to be taken in the plunge freezing process. I Apply the solu-
tion containing protein complexes or cells (TEM grid illustrated in a cross
sectional way). To remove the excess solution and thus adjust the ice layer
thickness, II blotting is done with a fi lter paper, carefully brought to the front-
or backside of the TEM grid. After blotting the remaining solution on the grid
is rapidly plunged III into liquid ethane, and afterwards kept at all times at
liquid nitrogen temperature to prevent contamination, crystallization or smelt-
ing of the amorphous (vitrifi ed) ice layer. (See color plate.)
552 J.M. Plitzko and W. Baumeister
much more sophisticated and the result of the preparation is not known
until a look is taken through the EM. The task of determining suitable
processing parameters for the material at hand, to get, in the end, the
best preparation result for an in-depth investigation of the biological
structure, can therefore be very tedious.
3.1.3 Cryosectioning
While plunge-freezing is now almost routinely used for the prepara-
tion of purifi ed and isolated macromolecular complexes as well as for
prokaryotic and very small eukaryotic cells, larger bulky structures,
such as big mammalian cells or larger organelles, cannot be addressed
as a whole for EM investigations. However, the technique of sectioning
using ultramicrotomes, likewise known from epoxy resin-embedded
samples, has been adapted for the use in combination with high-
pressure frozen samples. To obtain a vitrifi ed sample of a specimen
tenths of micrometers in dimension, plunge-freezing as described
above is not suffi cient, because of its limited “depth of vitrifi cation,”
explained by the freezing rate, which is, for example 10
6
°C/s for pure
water. If the sample dimensions exceed approximately 10 µm, vitrifi ca-
tion is incomplete, which will result in the formation of cubic ice
crystals. Therefore, high-pressure freezing is the method of choice,
because the increase in pressure will lower the freezing rate (Moore,
1987). The lowest values are observed around 2000 bar, where the
depth of vitrifi cation increases up to 10 times as compared to freezing
at ambient pressures (Sartori et al., 1993). The sample material, e.g., a
cell suspension, is forced into a 15-mm-long copper tube with an inner
diameter of approximately 0.3 mm. Afterward a pressure of 2000 bar
builds up in a few milliseconds and, as soon as the pressure is estab-
lished, cooling of the specimen, e.g., with a jet of pressurized liquid
nitrogen, takes place. Before cutting the sections the copper jacket has
to be removed using the cryo-ultramicrotome, designed to enable
cutting at liquid nitrogen temperature. This is normally done with a
diamond trimming device to form a square face of about 150–200 µm.
Subsequent sectioning can be carried out using a diamond or a good
glass knife (Figure 7–9). Cutting conditions such as knife-angle, cutting
speed and stroke time, and temperature of the specimen, of the knife,
and of the surrounding liquid nitrogen gas can be adjusted according
to the specimen properties and the desired section thickness. However,
the optimum sectioning conditions have to be found empirically in
each experiment.
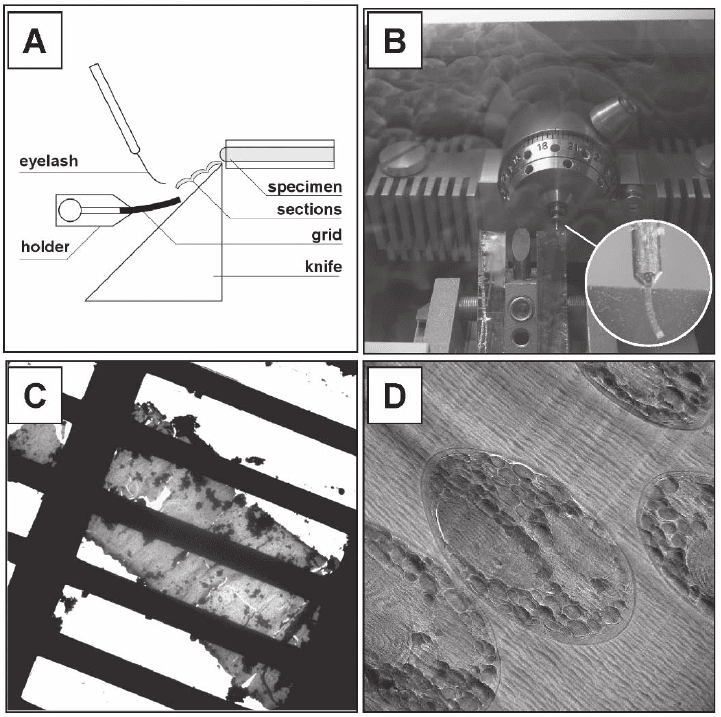
In contrast to conventional room temperature sectioning, where the
sections are fl oated on water, cryosections have to be transferred man-
ually to an EM grid with an eyelash, a tedious procedure that requires
patience and great skill. The sections tend to attract contaminating ice
crystals in the preparation chamber of the cryoultramicrotome and
during transfer to the EM. Furthermore they are generally not fl at and
exhibit cutting artifacts, e.g., deformation, crevasses, chatter, and knife
marks (for a complete description of cryosectioning and related cutting
artifacts see Dubochet et al., 1988; Frederik et al., 1991; Michel et al.,
1991; Sitte, 1996; Al-Amoudi et al., 2005).
Chapter 7 Cryoelectron Tomography (CET) 553
While the idea of high-pressure freezing and cryosectioning is rela-
tively old (Moore and Riehle, 1968; Bernhard, 1965) and commercially
available instruments have been available for almost 20 years, its impact
on biological and biomedical research has been comparatively small,
owing to the fact that its use in combination with cryoultramicrotomy
is still technically diffi cult, the yield of good micrographs is low, and
the cutting artifacts in most of the sections complicate subsequent
image analysis. However, in recent years cryosectioning was somehow
rediscovered, and improvements have been made to facilitate the freez-
ing and cutting procedure and to minimize the infl uence of cutting
artifacts (Studer and Gnaegi, 2000; Al-Amoudi et al., 2003). Cryosec-
Figure 7–9. Cryo-sectioning. a) Cross-sectional schematic of the cryo-sectioning process with a
magnifi cation of the trimmed copper tube and the ‘ribbon’ formed after several cutting cycles. c)
Low-magnifi cation image of a cryo-section placed on a TEM grid. d) Cryo-electron micrograph at
tesy of A. Leis and L. Andrees, MPI of Biochemistry, Martinsried, Germany). The cutting artefacts are
clearly visible.
cryo-ultramicrotome. b) View into the cryo-ultramicrotome during sectioning. The inset shows a
higher magnifi cation of the sectioned cells (unicellular red algae Cyanidioschyzon merolae; images cour-
554 J.M. Plitzko and W. Baumeister
tioning to fabricate thicker sections of 150–200 nm for cryo-ET studies
or even serial sectioning, likewise used in room temperature experi-
ments (Soto et al., 1994), in combination with cryo-ET would defi nitely
help in the 3D investigation of larger structures. The production of
undistorted vitrifi ed sections remains a specialized task and it is by
no means a routinely used technique. Nevertheless the prospects for
the future of cryosectioning in combination with cryo-ET are promis-
ing (Hsieh et al., 2002; Leis et al., 2005).
3.2 Radiation Sensitivity—Beam Damage
When biological specimens are irradiated by the electron beam in the
EM, the specimen structure is damaged as a result of ionization and
subsequent chemical reactions. Ionization occurs if energy is deposited
in the specimen as a result of inelastic scattering events. This can pri-
marily induce the heating of the sample in the irradiated area, radio-
chemical decomposition of water (radiolysis), as well as secondary
chemical reactions (breaking of chemical bonds, formation of new
molecules or even radicals). Unlike materials science, where beam
damage is almost negligible, radiation damage in life sciences is the
fundamental limitation in any cryo-EM investigation.
The damage that occurs depends on the number of electrons trans-
mitted through the sample. Therefore the current density per unit area
j (A/cm
2
) multiplied by the exposure time t (s) has been chosen as an
appropriate measure for the electron dose (C/cm
2
, or e
−
/Å
2
, e
−
= elec-
trons). This is not the same as the defi nition in radiochemistry, where
the dose is defi ned in units of gray; the energy adsorbed per unit weight
(Gy = J/kg). According to the last defi nition, a carbon sample exposed
to 50 e
−
/Å
2
generated by a 300 kV source would correspond to a dose of
1.6 × 10
8
Gy, which, e.g., can be observed in the vicinity of a nuclear
reaction!
Since the amount of damage is proportional to the applied dose, and
the amount of exposure received by the specimen escalates with the
square of the magnifi cation, it is clear that this is the fundamental
limitation in HREM of biological materials. However, several measures
can be taken to partially evade this dilemma. Although the ionization
processes are not temperature dependent, the resulting beam damage,
e.g., bubbling, mass loss, diffusion of free radicals, is. Therefore, by
lowering the temperature to, for example, liquid nitrogen temperature
(∼190°C), radiation damage effects can be drastically reduced. Cooling
the sample from room temperature to 90 K, the “lifetime” (the time the
sample can be investigated before structural damage is observed) can
be increased by a factor of approximately nine, the so called cryoprotec-
tion factor C
p
(Knapek, 1982; Conway et al., 1993; Stark et al., 1996). This
cryoprotection factor is given by the ratio of the critical dose N
e
at dif-
ferent temperatures (N
e
is defi ned as the dose at which the diffraction
intensity has fallen to 1/e (∼37%) of its original value (Hayward and
Glaeser, 1979). Further cooling to liquid helium temperature (∼4 K) has
been proven to be advantageous for very thin specimens as used for
electron crystallography or even in selected cases for single particle
