Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

535
7
Cryoelectron Tomography (CET)
Juergen M. Plitzko and Wolfgang Baumeister
Clearly, if recent improvements in electron microscopes are to be fully utilized
in biology, contrast must be enhanced without drastic molecular alteration of
the specimen or obscuration by extraneous material. . . . Thus the polytropic
montage seems to offer a means of determining the three-dimensional struc-
tures of low-contrast biological specimens at a resolution of 3 Å, or the best
resolution attainable with existing electron microscopes. (Hart, 1968)
1 Introduction
More than 70 years have now passed since the invention of the fi rst
transmission electron microscope (Knoll and Ruska, 1932; Ruska, 1987).
Many reports and publications have been written covering the techno-
logical achievements in electron microscopy (EM) and more important
the scientifi c breakthroughs related to the information disclosed by
EM studies. EM, in its various “fl avors” (see below), is now a well-
established method in life as well as in material science. Therefore,
almost every laboratory in the fi eld of structural analysis is equipped
with one or several low or intermediate voltage microscopes for routine
use and in some instances for high-end applications. However, com-
pared to light microscopy, EM is still a “young” method but with great
potential for further improvements. This has been shown, especially
in the past decade: among the highlights are the incorporation of
energy fi lters, monochromators, aberration correctors, and above all
the almost complete replacement of negative plates by charge coupled
device (CCD) cameras and imaging plates. By all means, these techni-
cal achievements have been supported by the development of the com-
puter and its availability throughout science. The various techniques
in EM, such as bright fi eld (BF) or dark fi eld (DF) imaging, weak beam
imaging, conventional transmission EM (CTEM), high-resolution TEM
or EM (HRTEM or HREM), scanning transmission electron microscopy
(STEM), energy-fi ltered TEM (EFTEM), and many others, have been
improved and, moreover, expanded with the computer power on hand.
Today’s most modern techniques are all based on automated ac quisition
536 J.M. Plitzko and W. Baumeister
and alignment procedures, reconstruction algorithms, and especially
on elaborate image processing routines.
Here, we address the advances made in biological EM and especially
in cryo-EM. Biological structures can be, by and large, characterized
as pleiomorphic. Just as every human being has a different face, cells,
proteins, and macromolecular complexes have different shapes and
forms, designed for a higher functional purpose. It is far from random
and instead of addressing them as “amorphic” or “amorphous,” as in
physics for randomly ordered solids, they are called “pleiomorphic” or
“pleiomorph.” Moreover, the inside of a cellular structure resembles
the image of a giant factory, where the single constituents act together,
building highly specifi c molecular machines and, if necessary,
change their purpose (Alberts, 1998). Therefore it is a highly variable
and dynamic environment. Every intrusion into this fragile system can
lead to changes. The suitable preparation for a fi nal characterization
with the EM is a major challenge. And it is likewise a challenge
in terms of the environment inside EMs: an ultrahigh vacuum and
electron radiation.
Despite the fact that biologists were impressed by the resolving
power of the EM, they remained very sceptical about the usefulness of
the EM in structural biology. The major drawback was and still is the
sensitivity to radiation of the biological samples. After a few minutes
exposure to the electron beam the biological substance in question is
literally “incinerated.” However, the present knowledge about the
ultrastructure of cells, viruses, and other biological substances was
accumulated by EM investigations with, at that time, suitable prepara-
tion techniques. Higher vacuum resistance was achieved by dehydra-
tion and water-substitution methods, beam resistance was increased
by staining the samples with heavy metals (Brenner and Horne, 1959),
and, in addition, enhanced contrast and transparency were obtained
by sectioning, e.g., “big” cells in slices about 200 nm thick, with ultra-
microtomes (Porter and Blum, 1953). These preparation techniques
enabled biologists to establish the basis of a common image of the cell’s
interior. Nevertheless, this “image” remained incomplete and was still
at a resolution far above that potentially available with the EM. Addi-
tionally the samples were altered by these preparation methods, thus
complicating and limiting image interpretation. In some instances the
artifacts introduced even misled scientists. Although these preparation
techniques are still used in various ways, but there was an obvious
need for far more reliable and less harmful procedures. In 1981
Dubochet and McDowall introduced a new means of sample preserva-
tion for EM investigations: the cryotechnique. Without doubt, cryo-
preparation is one of if not the greatest development in biological EM.
Instead of replacing the water or dehydrating the whole system, the
biological substance is embedded within its original buffer solution or
simply water by rapidly freezing at very low temperatures, namely the
temperature of liquid nitrogen (∼90 K). This way the water was trans-
ferred into an amorphous state, inhibiting crystallization, and thus
disruption of the cell due to the volume increase of crystalline water.
This plunge-freezing technique revolutionized the fi eld of structural
Chapter 7 Cryoelectron Tomography (CET) 537
biology, because for the fi rst time it was possible to investigate biologi-
cal samples in their native state, without introducing any artifacts,
corroborating image interpretation.
Excluding artifacts was clearly one achievement in obtaining higher
resolved images of biological structures, but since these structures are
pleiomorphic, single two-dimensional (2D) images are clearly insuffi -
cient for a complete structural characterization, which can only be done
three-dimensionally (3D). Additionally, due to the large depth of focus
of the EM, images represent only 2D projections of the specimen, in
which almost all information about the third dimension is lost. As
mentioned, sectioning can help, and especially serial sectioning, where
the structure of interest, e.g., big cells, is serially sliced. However, serial
sectioning was previously done only on epoxy resin-embedded sam-
ples, limited in resolution to the section slice thickness (∼50 nm) and
having lost any information about the molecular organization. But it
was evident that information retrieval down to the supramolecular
level for all three dimensions is absolutely crucial for an in-depth
structural analysis of native biological samples.
In the late 1960s and early 1970s three groups independently experi-
mented with the possibility of 3D microscopy, namely electron tomo-
graphy (Hart, 1968; DeRosier and Klug, 1968; Hoppe et al., 1968; Hoppe,
1974). Perhaps inspired by the tomographic methods invented for
medical examinations (Cormack, 1963, 1980; Hounsfi eld, 1972) they
developed acquisition schemes to access the 3D information by record-
ing images from different orientations or from differently oriented
particles. DeRosier and Klug (1968) studied the helical tails of T4-
bacteriophages and with a single EM they were able to present a com-
plete 3D reconstruction. By selecting an object with the a priori
knowledge of its helical symmetry they avoided a complicated data
acquisition procedure. In the same year Hart (1968) reported very pre-
cisely on his idea: the “polytropic montage.” He investigated the rod-
shaped tobacco mosaic virus (TMV). Hart and later Hoppe have chosen
a more complicated and cumbersome acquisition process: the manual
rotation of the object perpendicular to the incoming electron beam and
the acquisition of single projections from different viewing angles.
They reported on the 3D representation from a series of images acquired
over a large angular regime. In the late 1960s, when the average elec-
tron microscopist used photographic plates, these procedures were
inevitably very cumbersome and time consuming and of course recon-
struction calculations were possible only with large supercomputers.
Nevertheless, with the introduction of CCD cameras for image record-
ing in EM in the late 1980s (Mochel and Mochel, 1986) and the intro-
duction of computer-controlled EMs attempts (Figure 7–1) were made
to improve the pioneering work with the prospect of implementing it
for routine and fast use (Typke et al., 1991; Koster et al., 1992, 1997).
With the addition of suitable preparation techniques such as cryofi xa-
tion of biological specimens, cryo-EM promised to be the method of
choice for further structural work in molecular biology. Nevertheless,
it was not until the mid-1990s that the fi rst reports on applications on
automated cryo-electron tomography appeared (Horowitz et al., 1994;
538 J.M. Plitzko and W. Baumeister
Dierksen et al., 1995; Grimm et al., 1996a; Walz et al., 1997a; Nitsch
et al., 1998), now abbreviated cryo-ET or, for the extended community,
three-dimensional electron microscopy (3DEM).
Today, cryo-EM is established in the fi eld of structural biology and
three different techniques are building the base for 3D characteriza-
tion: electron crystallography (cryo-EC), single-particle EM (cryo-EM),
and electron tomography (cryo-ET). In the following we will describe
and explain one of these tomographic approaches, namely cryo-ET
(Frank, 1992, 1996), in detail.
2 Three-Dimensional Cryoelectron Microscopy
2.1 Principles of 3D Imaging
The main contrast mechanism in cryo-EM is phase contrast. In fi rst-
order approximation, the micrograph is an interference pattern of the
primary beam and the focused scattered beam. In this so-called weak
phase approximation, the electrostatic potential of the sample is the
quantity that is imaged. To create a phase delay between the primary
and scattered beams, the objective lens has to be defocused. The
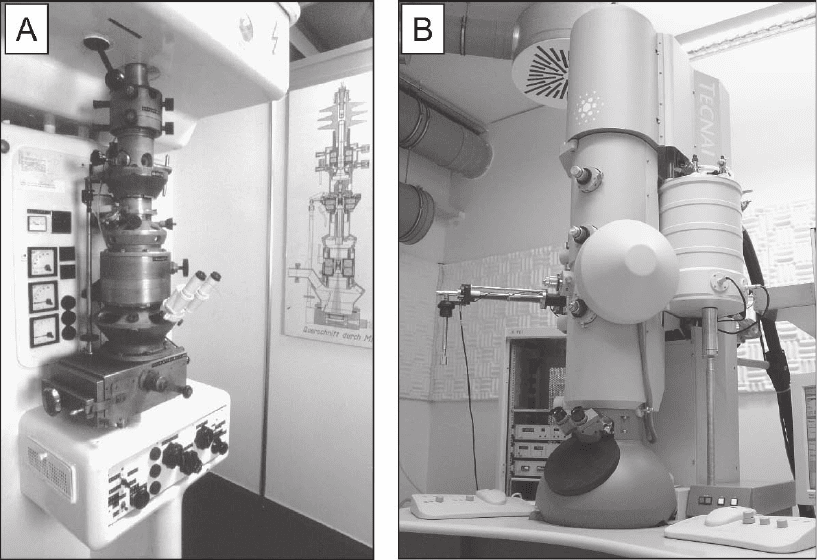
Figure 7–1. Transmission electron microscopes (TEM) in the past and today. a) 1939; First commercial
TEM (‘Siemens Super Microscope’) from Siemens & Halske Ltd., Berlin, Germany. b) 2002; Tecnai F30
Helium (‘Polara’) from FEI company, Eindhoven, The Netherlands as installed at the MPI of Biochem-
istry in Martinsried (near Munich), Germany. A computer controlled TEM dedicated for cryo-electron
microscopy, which allows cooling to liquid nitrogen (90 K) and helium temperature (∼10 K).
Chapter 7 Cryoelectron Tomography (CET) 539
imaging process of weakly scattering objects in a TEM can be sum-
marized elegantly by introducing a contrast transfer function (CTF) in
Fourier space (Reimer, 1993). In this notation, the image I
xy
of an 3D
object with an electrostatic potential V
xyz
can be written as
I CTF dzV
xy
kk
xyz
xy
=⋅
()
[]
−
∫
FF
1
,
.
Here, F denotes the Fourier transform and k
x
and k
y
denote the recip-
rocal vectors. The CTF is an oscillating function of the defocus value
∆z, which is damped by an envelope function. The envelope function
fi nally limits the resolving power of a TEM. To achieve high-resolution
data in biological EM, the use of a fi eld emission gun (FEG) instrument
with a high coherence and brightness is mandatory (Baumeister and
Typke, 1993), since the increased temporal and spatial coherence
reduces the damping of the envelope function (Frank, 1973). The oscil-
latory behavior of the CTF leads to contrast inversion of I
xy
for certain
frequency bands. Provided the signal-to-noise ratio (SNR) of I
xy
is suf-
fi cient, I
xy
can be corrected for these CTF effects, but CTF correction
will not be feasible close to the zeros of the CTF where hardly any
signal is transferred. In single particle analysis or cryoelectron crystal-
lography, images of different focus levels are recorded to avoid that
problem; the data of different focus levels can then be combined in a
so-called focus series (Schiske, 1968, 1973; Typke, 1992), which ensures
data coverage in Fourier space without gaps around CTF zeros.
Through-focus series reconstruction is particularly suitable in material
science studies where high magnifi cations combined with a high dose
can be utilized almost without restrictions to determine the atomic
positions even for very light elements in crystalline specimen (Thust
et al., 1994, 1996; Coene et al., 1996; Kisielowski et al., 2001). However,
in low-dose EM, the CTF is often not easily accessible due to the low
SNR, in particular if a CCD camera is used as a detecting device.
Therefore, the fi rst zero crossing of the CTF often limits the attainable
resolution in a cryoelectron micrograph, for example, in cryo-ET.
Since an EM image is essentially a projection, features from different
z-levels within the object overlap in the resulting micrograph and cannot
be separated. The traditional way to extract meaningful information
from an electron micrograph is to reduce the z-dimension of the object,
i.e., to image almost 2D objects. In biological imaging of cells this was
accomplished by sectioning the previously fi xed object using an ultrami-
crotome (Porter and Blum, 1953). By means of serial sectioning even 3D
data could be obtained by imaging successive sections belonging to the
same cell. In this case, the achievable resolution in z depends on the thick-
ness of the sections; the best resolution attained was in the range of
50 nm. However, the combination of serial sectioning with tomographic
data acquisition (see below) offers the possibility of improving the
resolution (Soto et al., 1994; Mueller-Reichert et al., 2003).
A different approach is to combine different views of the specimen
to derive 3D information (Figure 7–2). The cryo-EM methods for obtain-
ing 3D data can be summarized as tomographic or at least quasitomo-
graphic. They all rely on the fact that the parallel projection of a 3D
object corresponds to a slice in the 3D Fourier space of the object. This
540 J.M. Plitzko and W. Baumeister
“projection-slice theorem” and the possibility of restoring the 3D data
using their projections were fi rst discovered by Radon (1917). An
English translation of this paper can be found in Deans (1983). To
obtain the 3D information, different slices of the Fourier space, i.e.,
projections in real space, have to be gathered to sample the entire
information. Different orientations of the sample can be realized by
changing the orientation of the specimen. For this purpose the speci-
men has to be rotated, as is the case in electron crystallography or ET,
or identical copies of a specimen that occur in different orientations
can be reconstructed in a 3D model as in “single particle” analysis. In
the fi eld of EM, those tomographic methods for 3D structure determi-
nation were proposed in the late 1960s (Hart, 1968; Hoppe et al., 1968)
and realized in the pioneering work of DeRosier and Klug (1968).
2.2 The Single-Particle Approach
One powerful advantage of EM is the fact that it produces projection
images, unlike X-ray crystallography, which yields diffraction data
with no information on the position; this is known as the “phase
problem.” Thus, images may be treated in real space as well as in
Fourier space and there is no difference between those that contain
information about a single molecule without any internal symmetry
and those generated by a 2D crystalline array. This fact is exploited in
the so-called single-particle approach, which enables the electron
microscopist to determine 3D density maps of individual macromole-
cules (Frank, 1996). Depending on a variety of conditions the 3D models
show near-atomic, or, in the more characteristic case, intermediate
resolution (≥7 Å) (Jiang et al., 2003a; Spahn et al., 2001).
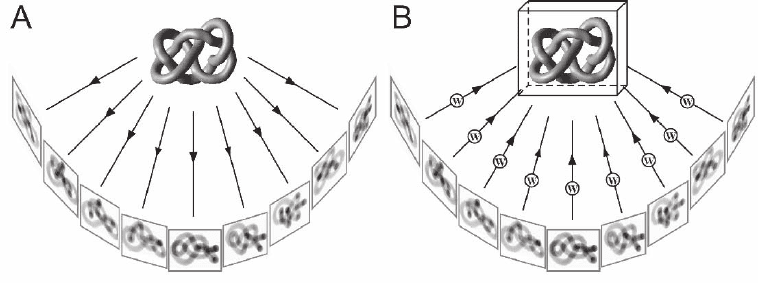
Figure 7–2. The Figure 7-shows a single axis tilt series data acquisition scheme. The object is repre-
sented by a pleiomorphic object (in this case a knot) to emphasize the fact that electron tomography
can retrieve 3D information from non-repetitive structures. a) A set of 2D projection images is recorded
while the specimen is tilted incrementally around an axis perpendicular to the electron beam, and
projection images of the same area are recorded on a CCD camera at each tilt angle. Tilt range is typi-
cally ±70° with tilt increments between 1.5–3°. b) The backprojection method explains the principle of
3D reconstruction in a fairly intuitive manner. For each weighted projection, a backprojection body is
calculated, and the sum of all projection bodies represents the density distribution of the original
object—the tomogram.
Chapter 7 Cryoelectron Tomography (CET) 541
Projection images from macromolecules contain information about
the 3D structure as 2D density profi les, characteristic of the particular
orientation of the particles in the electron beam (Figure 7–3). To disen-
tangle molecular substructures in the third dimension a number of
different images of known projection geometry have to be combined.
Protein complexes not too large in size are usually oriented arbitrarily
in vitrifi ed ice, so that even one EM image contains many different
projections of the isolated protein species (Figure 7–3A). Once the rela-
tive orientations of the particles are known, i.e., the three Eulerian
angles (j, Y, q) defi ning the rotation of a particle around the axes in
3D space, their projections can be placed on the surface of a sphere
centered on the origin of a common coordinate system. The Eulerian
angles exactly defi ne the positions of the projections that can now be
backprojected centrally in such a way as to superimpose the 2D densi-
ties into a common 3D volume of the particle. The most popular and
utilized reconstruction algorithm in real space is the so-called weighted
backprojection (Radermacher, 1992; see Section 3.5.2).
Very large macromolecular complexes, fi lamentous aggregates, or
proteins embedded in biological membranes usually occur in preferred
orientations in frozen samples. Here, and in cases where nonredundant
structures such as singular protein assemblies are to be investigated,
the specimen is tilted around a fi xed axis at a certain angular incre-
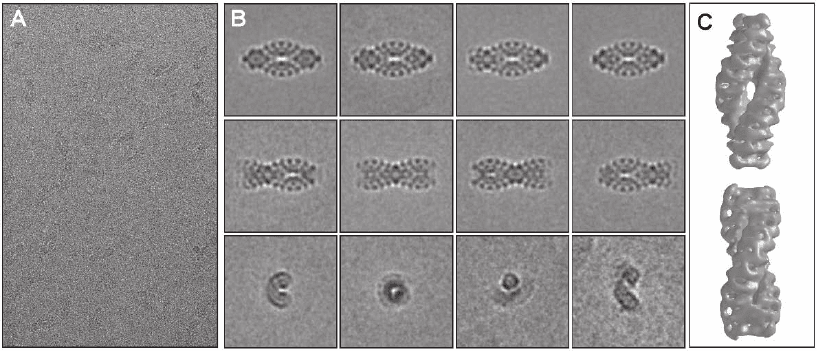
Figure 7–3. Single particle investigation of the giant protein complex TPP II from Drosophila mela-
nogaster embedded in vitrifi ed ice. In eukaryotes, tripeptidyl peptidase II (TPPII) is a crucial compo-
nent of the protein degradation pathway. The 150-kDa subunits of Drosophila TPPII assemble into a
giant proteolytic complex of 6 MDa with a remarkable architecture consisting of two segmented and
twisted strands that form a spindle-shaped structure (length 56 nm, width 24 nm). a) Cryo-electron
micrograph of isolated TPP II complexes illustrating the very weak image contrast and the high level
of noise. b) Averaging and classifi cation of a large number of equivalent projections of separate mole-
cules. Once a large set of views is available, a preliminary 3D reconstruction can be computed and
refi ned iteratively. c) The 3D model obtained by cryo-electron microscopy, reveals details of the
molecular architecture and, in conjunction with biochemical data, provides insight into the assembly
mechanism. The building blocks of this complex are apparently dimers, within which the 150 kDa
monomers are oriented head to head. Stacking of these dimers leads to the formation of twisted single
strands, two of which comprise the fully assembled spindle (Rockel et al., 2002 and 2005). (See color
plate.)
542 J.M. Plitzko and W. Baumeister
ment in the EM to obtain a series of projections. This approach of
electron tomography is applicable almost universally if the specimen
is not too thick for the electrons to penetrate.
Since the electron exposure must be restricted to a value low enough
not to destroy the sensitive biological material, the images contain a
high level of so-called “shot noise” (statistical variation in the number
of electrons detected at each pixel in the image). Additionally electron
scattering from elements low in atomic number (Z), like the constitu-
ents of most biological materials, e.g., carbon, oxygen, and hydrogen,
produces very weak image contrast. Based on these facts biological
structures are barely visible in single cryo-EM images. Furthermore the
exact alignment of the particles becomes a challenge, which is tackled
by averaging a large number of equivalent projections of separate mol-
ecules, thus reducing the noise level. For this purpose, the images of
some 10
3
to 10
5
particles are sorted into distinct classes of views by
multivariate statistical analysis (Van Heel and Frank, 1981). Once a large
set of views is available, a preliminary 3D reconstruction can be com-
puted and refi ned iteratively. Typically, molecular complexes must be
large in size (>200 kDa; 1 Da = 1 U = 1.66 × 10
−24
g) to provide suffi cient
signal for the alignment at high resolution. If the particles possess a
high internal symmetry, e.g., virus capsids or regular structures like 2D
crystals, the averaging and reconstruction process is simplifi ed and the
number of images necessary and the computation time are reduced.
To determine the structural basis of the working mechanism of
molecular machines we have to study the structures, positions, and
interactions of their building blocks, i.e., their distinct subunits. Since
the structure of protein–protein contacts is resolved only at near-atomic
resolution it is hardly possible to identify molecular borders in 3D
models of cryo-EM data by visual inspection. But the ability to identify
elements of secondary structure, and α-helices in particular, at a reso-
lution of about 6–8 Å, makes it possible to fi t a previously determined
(atomic) model of protein subunits or subcomplexes into 3D density
maps of larger assemblies obtained by cryo-EM (Li et al., 2002; Bottcher
et al., 1997). This approach, called “docking,” enables us to unravel the
organization of macromolecular machines that cannot be structurally
characterized otherwise. Right now researchers continue to develop
quantitative criteria that can guide this operation (Volkmann and
Hanein, 1999).
Single–particle cryo-EM is a powerful but still slow method com-
pared to X-ray crystallography, provided that for the latter well-
diffracting crystals are available. The data collection and processing
can extend over several months or longer even for specialists in the
fi eld. However, with the advent of computer-controlled electron micro-
scopes data acquisition and analysis have become subject to the devel-
opment of elaborate automation procedures that promise to reduce the
time needed to create high-resolution 3D density maps of large mac-
romolecular complexes considerably.
The real bottleneck for investigations of known or still unknown
molecular machines arose from another experimental task. Purifi ca-
tion procedures commonly used in biochemistry tend to select stable
Chapter 7 Cryoelectron Tomography (CET) 543
and abundant protein complexes only. Very large molecular machines,
rare or transient macromolecular assemblies, complexes consisting of
membrane spanning and soluble components, and all those held
together by forces too weak to withstand the purifi cation procedures,
escape isolation or even detection. The lesson is twofold: We need new
approaches for isolating or reconstituting fragile assemblies, and cryo-
EM of isolated single particles will probably not give us the whole
truth. To address the full complexity of functional macromolecular
assemblies noninvasive 3D imaging techniques have to take over to
fulfi ll the task of studying the supramolecular architecture in its native,
cellular context (Plitzko et al., 2002). The technique of cryo-ET is a
“nouvelle route” with a great potential in the fi eld of structural biology
that promises to provide noninvasive and deep insight into the func-
tional organization of the cellular proteome (Sali et al., 2003).
2.3 Cryoelectron Tomography
CET is unique in the sense that it can image nonpurifi ed macromole-
cules three-dimensionally. However, in terms of resolution, cryo-EC
and single-particle analysis are currently superior, which makes cryo-
ET a complementary method to the aforementioned. In contrast to
these methods, cryo-ET does not involve implicit or explicit averaging
of the specimen, which makes it a suitable method for imaging of
pleiomorphic objects. Therefore it is suitable to image entire cells
(Grimm et al., 1998; Medalia et al., 2002; Kuerner et al., 2005; Nicastro
et al., 2000, 2005; Scheffel et al., 2005) or nonsymmetric viruses
(Gruenewald et al., 2003) in situ (Figure 7–4). Furthermore it has the
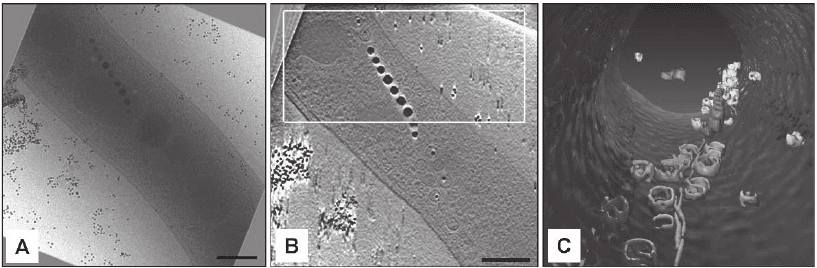
Figure 7–4. Cellular cryo-electron tomography of the magnetotactic microorganism Magnetospirillum
griphiswaldense. The entire bacterium is oriented like a compass needle inside the magnetic fi eld in its
search for optimal living conditions. The miniature cellular compass is made by a chain of single
nano-magnets, called magnetosomes (the scale bar represents 200 nm). a) The two-dimensional image
represents one projection (at 0°) from an angular tilt-series. b) x–y slices along the z axis through a
typical three-dimensional reconstruction (tomogram). c) Surface-rendered representation of the inside
of the cell showing the membrane (blue), vesicles (yellow), magnetite crystals (red) and a fi lamentous
structure (green). Until now, it was not clear how the cells organise magnetosomes into a stable chain,
against their physical tendency to collapse by magnetic attraction. However, the biochemical analysis
revealed a protein responsible for the chain formation and the 3D investigation a cytoskeletal struc-
ture, which aligns the magnetosomes like pearls on a string (Scheffel et al., 2005). (See color plate.)
544 J.M. Plitzko and W. Baumeister
potential to unravel not only the structure but also the molecular inter-
actions of the various macromolecules inside a cell (Beck et al., 2004).
Whereas tomography of plastic embedded specimens (Marsh et al.,
2001; Murk et al., 2003) reveals primarily data on the ultrastructure and
morphology of a cell cryo-ET aims to resolve the cell at a molecular
level. Although the SNR of a tomogram of a frozen hydrated specimen
is generally worse than that of a plastic embedded specimen, more
biologically signifi cant information is contained in the high spatial
frequencies since the preparation technique does not limit the inter-
pretable information.
A cryo-electron tomogram essentially depicts the entire proteome of
a cell. Therefore, cryo-ET can make signifi cant contributions to the
larger enterprise of structural proteomics (Sali et al., 2003). It has the
potential to unravel the interactions of the various proteins that reside
not only in the cytoplasm but also in the cell membrane. Moreover, it
can provide medium resolution structural characterization of com-
plexes that are largely undescribed to date. A prerequisite for those
contributions is suffi cient resolution to recognize macromolecules in a
cellular tomogram.
The fi rst published cryo-electron tomogram of a eukaryotic cell
(Figure 7–5), the slime mould Dictyostelium discoideum, demonstrated
that cryo-ET is able to resolve individual macromolecular complexes
such as ribosomes or the 26S proteasome in the context of a cell (Medalia
et al., 2002). Currently, cellular cryo-ET is effectively confi ned to rela-
tively thin samples. Cells of a thickness beyond 1 µm are not transpar-
ent to the electron beam at a voltage of 300 kV. These cells are typically
prokaryotic cells or unusually thin eukaryotic cells. Electron tomogra-
phy of frozen hydrated sections of cells (Hsieh et al., 2002; Leis et al.,
2005) can possibly extend the specimen class to thicker cells and even
tissue cells. Unfortunately, the sectioning process still introduces severe
artifacts; in particular, the forces that arise during the sectioning
process tend to compress the cells strongly. The use of vibrating
cryoknifes can possibly offer some remedy from these shortcomings
(Al-Amoudi et al., 2003). The technique is promising, but it is far from
being a routine preparation technique, as yet.
To date, cryo-ET is effectively the only 3D imaging technique that
can image cells or organelles in a close-to-native state at molecular
resolution. It is of vital importance to have an imaging method that can
image biological macromolecules since many of the complexes present
in a cell tend to be fragile or transient. The detection of statistically
signifi cant distribution patterns can provide unprecedented informa-
tion about the interaction of macromolecules, in transient complexes,
for example. The basic problem that cryo-ET has to face is to derive
statistically signifi cant information from the initially very noisy tomo-
grams. Interpretation of the raw data can be performed only by low-
pass fi ltering the data to a resolution that is regarded as signifi cant.
Application of so-called “denoi si ng” algorithms can extend this limit
gradually and greatly improve the visualization of tomograms. These
techniques mainly perform nonlinear fi ltering of the data (Frangakis
