Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

180 Charged Particle and Photon Interactions with Matter
Suchionic processes involve muons, muoniated ions, and secondary electrons (formed along the
tracks, with a distribution of energies ranging from a few eV upwards) that are able to bring about
further
ionizations.
There
is a signicant gap in our knowledge about stopping power and energy loss events in liq-
uids for muons. Such knowledge is important to understand the thermalization processes of ionizing
radiation, including muon radiation. Since muon LET is higher than electron LET, this knowledge
is also of interest for medical and technological applications. There is a great need for simulations
of the inelastic collisions between bound electrons in liquids and the muons moving through it,
especially
for energies below 100
keV.
There is a limited number of published works over the last 10 years on radiation effects on
muons in molecular liquids except for Kosarev and Krasnoperov (1999) and Walker et al. (2003a).
There are previous works, however, on muonium formation in molecular liquids. The mechanism
of Mu formation in water, as opposed to other molecular liquids, has been well-studied with many
denitive and accurate experiments that are reviewed in Percival (1990).
In the case of muon irradiation of water, the key competitive reactions among transient species
in radiolysis are the following:
µ
+ −
+ →e Mu (8.16)
µ
+ +
+ →H O MuH O
2 2
(8.17)
MuH O H O MuOH H O
2 2 3
+ +
+ → + (8.18)
Mu e MuH OH
aq
+ → +
− −
(8.19)
Mu e spin depolarized Mu
aq
+ →
−
(8.20)
Mu OH MuOH
+ →
(8.21)
Mu H MuH
+ →
(8.22)
Reactions (8.16) through (8.18) determine the initial distribution of muons between Mu and the
diamagnetic fraction and mostly take place in less than a picosecond. Muon attachment to water,
Reaction (8.17), and its solvation does not prevent Mu formation. Reaction (8.16) could involve
μ
+
in a variety of solvated molecular ions. The secondary encounter of muonium with radiolysis
transients, such as hydrated electrons or H atoms, in Reactions (8.19) through (8.22) extends into
the
sub-microsecond range and leads to the “missing fraction” of muon-spin polarization in water.
As
can be seen from the reaction scheme in this model (known as the spur or radiolysis model),
the hydrated electron plays a signicant role in the processes that determine the distribution of dif-
ferent muon fractions in water.
In a recent work, a novel technique was introduced. Laser muon-spin spectroscopy can be used
to study the excited-state chemistry of muonium and muoniated free radicals and to investigate
radiation effects in muon thermalization. Experiments were performed at 298 K and 1 bar pres-
sure in water, in a 20 G transverse magnetic eld, using 532nm laser excitation with the excitation
pulse delayed relative to the muon pulse. The diamagnetic muon signal, which precesses at the
muon Larmor frequency, ν
μ
= 13.55kHz G
−1
, was detected at 20 G. Changes in μSR amplitudes,
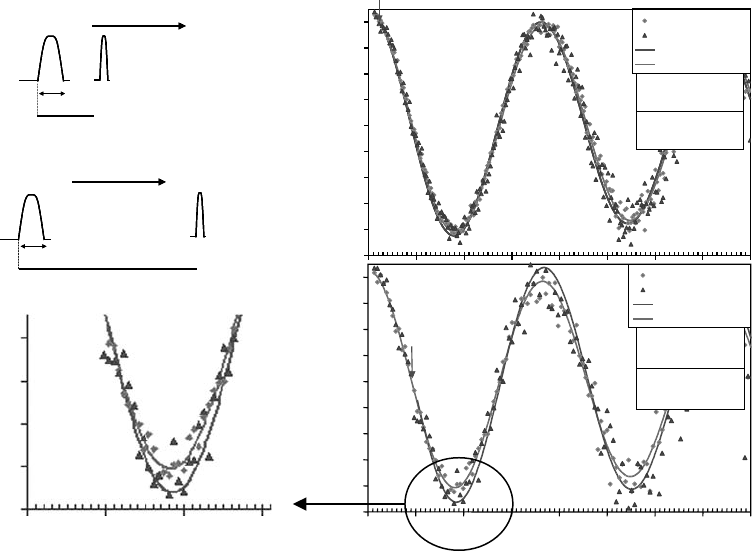
Muon Interactions with Matter 181
corresponding to the diamagnetic precession frequency in water at 20G during laser irradiation,
were detected at time delays up to 1.2 μs; selected laser delays of 0.2 and 1 μs are depicted in
Figure 8.5.
The percent asymmetry is shifted by 10.5% ± 3.7% after a 1μs delay and by 2.7% ± 1.5% after a
0.2μs
delay, showing the rise of asymmetry upon laser irradiation.
The
result suggests that the laser excitation can be used to manipulate the processes that deter-
mine the distribution of muon-spin polarization among the different muon fractions. This conrms
the radiolysis model as opposed to hot-atom model for muonium formation mechanism in water.
Also, the technique can shine light on the process of muonium and free-radical formation in other
molecular
liquids.
8.3 the interaCtion oF muons with superCritiCal Fluids
A supercritical uid (SCF) is any substance above its critical temperature (T
c
) and pressure (P
c
)
where only one phase exists. In SCFs, the solvent properties (density, dielectric constant, etc.) vary
with the temperature and pressure continuously. These easily tunable properties make SCFs (partic-
ularly supercritical CO
2
and H
2
O) an excellent media for chemical processes, as described in Noyori
(1999) and Rayner (2007). The change in the solvent properties can signicantly affect the radia-
tion chemistry, electronic structures of the reactive intermediates, and the corresponding transition
–10
–8
–6
–4
–2
0
2
4
6
8
Asymmetry
Laser off
Laser on
Fit to laser on
Fit to laser off
Laser on:
A
D
= 8.97(0.12)
Laser off:
A
D
= 8.71(0.06)
Laser on
–10
–8
–6
–4
–2
0
2
4
6
8
0 1 2 3 4 5 6 7 8
Time (µs)
Asymmetry
Asymmetry
Laser on
Muon pulse Data acquisition
t
80 ns
200 ns
Laser pulse
80 ns
1000 ns
Laser pulse
Muon pulse Data acquisition
t
(a)
(b)
Laser off
Laser on
Fit to laser on
Fit to laser off
Laser on:
A
D
= 9.43(0.28)
Laser off:
A
D
= 8.55(0.14)
–10
0 1 2 3
–8
–6
–4
–2
Time (µs)
Figure 8.5 (a) The asymmetry in water following excitation at 532nm with a delay of 0.2μs relative to
the muon pulse, (b) the asymmetry in water with a delay of 1μs relative to the muon pulse. The triangles are
the “laser on” data points and the dark curves are ts of Equation 8.1 to the data. The diamonds are the “laser
off” data points and the light curves are ts of Equation 8.1 to the data. A range around 2μs (minimum in
the
asymmetry) is magnied to demonstrate the increase in the diamagnetic fraction due to laser irradiation.
182 Charged Particle and Photon Interactions with Matter
states. In the last decade, muon methods have been used to investigate such tunable solvent effects
by Cormier et al. (2008), Ghandi and Percival (2003), Ghandi et al. (2000, 2002, 2003), Ghandi
(2002,
2004), McKenzie and Roduner (2009), and Percival et al. (1999, 2000, 2007).
Understanding
the radiolysis of water in subcritical and supercritical water (SCW) is essential
for the design of the next generation of pressurized water nuclear reactors. Understanding the
radiation chemistry of supercritical CO
2
is relevant to radiation-induced polymerization, waste
management applications (Clifford et al., 2001), and development of supercritical CO
2
Brayton
cycles in nuclear reactors (Moisseytsev and Sienicki, 2008). There are only a handful of studies
on electron-induced radiation chemical processes in SCFs, and there is very little report on any
higher LET radiation. The radiation chemical processes due to higher LET radiation might be very
different, such as with α-particle radiation from the radioactive decay of heavy nuclei in waste
management applications.
The studies of muon radiation chemistry can be used to explore the effects of ionizing radia-
tion in SCFs on a particle of intermediate mass. Studying radiolysis processes during muonium
formation
as a function of density (which can be easily done in SCFs) can shed light on the mecha-
nism
of muonium formation. A recent study by Ghandi et al. (2010) has compiled the results of
three previous studies on supercritical CO
2
by Ghandi et al. (2004), H
2
O by Percival et al. (1999),
and C
2
H
6
(mostly gas phase) by Kempton et al. (1991). The corrected results for C
2
H
6
using the
data in Kempton et al. (1991) and new data from Ghandi et al. (2010) are presented in Figures 8.6
through8.8.
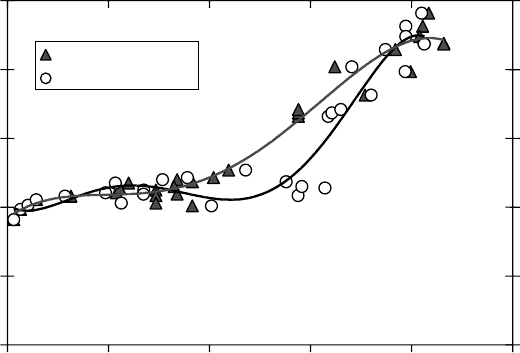
Based on a model in which hot-atom reactions of Mu and ethane determine the diamagnetic
fraction (Kempton et al. 1991), the second derivative of density of this fraction should always
be negative. Thus, when the hot-atom reactions determine the diamagnetic fraction, the diamag-
netic fraction versus density can never curve upward, in contrast to the data shown in Figure 8.6.
Founded on this model, the diamagnetic fraction in ethane can be explained on the basis of hot-
atom reactions up to ∼0.3g cm
−3
. This is in contrast to the case in SCW that above 0.2 g cm
−3
, the
radiolysis effects have the major role in the process of muon thermalization (Figure 8.8) (Percival
et al. (1999).
0.0
0.1
0.2
0.3
0.4
0.5
0 0.1 0.2 0.3 0.4 0.5
ρ (g cm
–3
)
P
D
Density
Density from EOS
Figure 8.6 Diamagnetic (P
D
) fractions in pure C
2
H
6
as a function of density (for clarity the error bars are
not shown). The data set from Kempton et al. (1991) is presented with triangles, while the dataset, presented
with
open circles, is based on corrected densities from Ghandi et al. (2010).
Muon Interactions with Matter 183
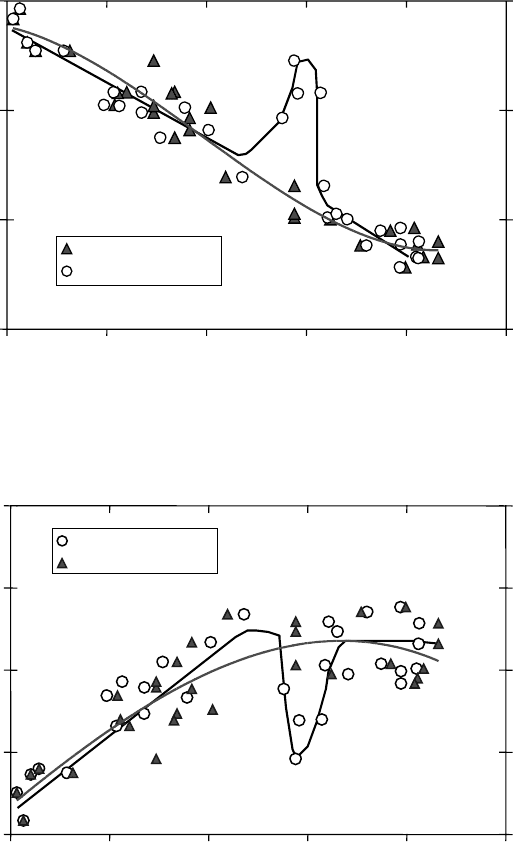
The steep increase and then decrease of the muonium fraction as a function of density in
near-critical ethane is clear in Figure 8.7. Also, there is a concomitant reverse behavior at the
same density range for the missing fraction as a function of density (Figure 8.8). This is the
only case that has revealed such drastic effects. There is a reverse density dependence of P
L
in
supercritical CO
2
, although not as pronounced, and there is no such effect in SCW (Figures 8.9
through8.11).
This result suggests that density inhomogeneity in near-critical conditions affects the radiol-
ysis processes involved in muonium formation, the missing fraction in ethane (and probably in
other hydrocarbons), and in supercritical CO
2
in an opposite, yet less pronounced way. There is a
0.2
0.4
0.6
0.8
0 0.1 0.2 0.3 0.4 0.5
P
Mu
Density
Density from EOS
ρ (g cm
–3
)
Figure 8.7 Muonium (P
Mu
) fractions in pure C
2
H
6
as a function of density (for clarity the error bars are not
shown). The data set from Kempton et al. (1991) is presented with triangles while the dataset, presented with
open
circles, is based on corrected densities from Ghandi et al. (2010).
0.0
0.1
0.2
0.3
0.4
0.00 0.10 0.20 0.30 0.40 0.50
ρ (g cm
–3
)
P
L
Density from EOS
Density
Figure 8.8 Missing (P
L
) fractions in pure C
2
H
6
as a function of density (for clarity the error bars are not
shown). The data set from Kempton et al. (1991) is presented with triangles while the dataset, presented with
open
circles, is based on corrected densities from Ghandi et al. (2010).
184 Charged Particle and Photon Interactions with Matter
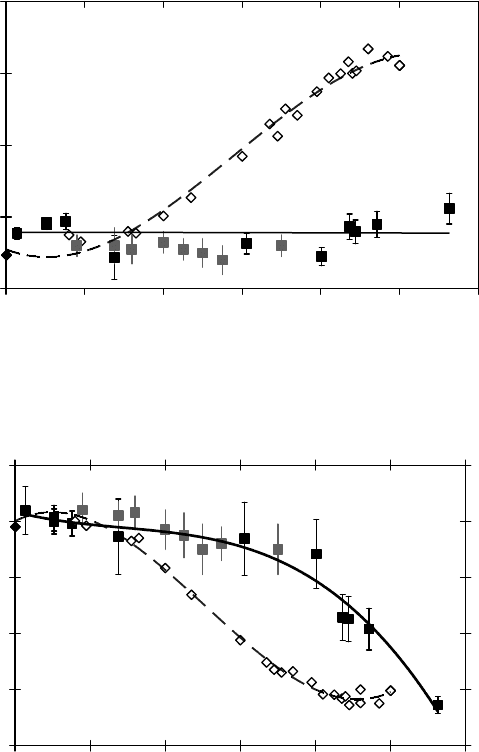
maximum in the muonium fraction at a density close to the critical density at the expense of a mini-
mum in the missing fraction in ethane. This is in contrast to the near-critical water and CO
2
. The
data in near-critical CO
2
show a small maximum in the lost fraction. This, along with the density
dependence of P
D
in ethane, suggests different mechanisms for the missing fractions in CO
2
and
ethane. The missing fraction in ethane is due to the interaction of hot Mu in low density regions with
alkyl radicals in high density regions in spurs, while in CO
2
it is due to the interaction of Mu and
solvated electrons (both in high density regions). In water there is no sign of density inhomogeneity
effects on muon polarizations. This could mean that the density inhomogeneity does not affect radi-
olysis processes in near-critical water, or it could mean that the different effects cancel each other;
for example, the effect of diffusion of Mu and a hydrated electron cancels the effect of clustering
around Mu and the hydrated electron.
Observing maxima or minima in the missing fraction as a function of thermodynamic param-
eters gives us a hint about the nature of the missing fraction (and therefore the process of muon
0.0
0.2
0.4
0.6
0.8
0 0.2 0.4 0.6 0.8 1 1.2
P
D
ρ (g cm
–3
)
Figure 8.9 Diamagnetic (P
D
) fractions in pure H
2
O and CO
2
as a function of density. Values of P
D
for H
2
O
(empty diamonds) are taken from Percival et al. (1999). The light squares are data on CO
2
from Ghandi et al.
(2010)
while the dark squares are the data taken from Ghandi et al (2010).
0.0
0.2
0.4
0.6
0.8
1.0
0
0.2 0.4 0.6 0.8
1
1.2
P
Mu
ρ (g cm
–3
)
Figure 8.10 Muonium (P
Mu
) fractions in pure H
2
O and CO
2
as a function of density. Values of P
D
for H
2
O
(empty diamonds) are taken from Percival et al. (1999). The light squares are data on CO
2
from Ghandi et al.
(2010)
while the dark squares are the data taken from Ghandi et al (2004).
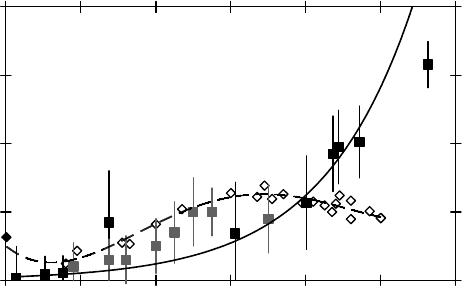
Muon Interactions with Matter 185
thermalization). As an example (Ghandi et al., 2010), the delayed muonium formation mechanism is
unlikely to be responsible for the minimum in P
L
in ethane, based on signicant clustering around
positive ions. Such a clustering would decrease the mobility of these ions and therefore have the
opposite
effect to the one observed in ethane.
8.4 muon radiation Chemistry in ioniC liQuids
This is a new eld being investigated by Ghandi’s group (Lauzon et al., 2008; Taylor et al.,
2009). The objective of these studies is to establish an understanding, at the microscopic
level, of the solvent effects in ionic liquids (ILs) on the dynamics of free-radical reactions and
free-radical formation, especially the mechanism of free-radical formation and the radioly-
sis processes in ILs in comparison with molecular solvents. ILs are two-component systems,
composed of only equimolar amounts of anions and cations. The contrasting nature of cat-
ions and anions, and the proximity of close sites of high electron deciency and high electron
richness, suggest that IL solvents could enable chemistry that may not be possible in normal
molecular solvents. ILs have a wide range of applications and their properties are tunable by
small changes in their structure (see Chapter 11). Understanding many chemical processes in
ILs requires probing reactive intermediates, such as free radicals. Kinetics and dynamics of
solvated and pre-solvated electrons and few other reactive intermediates (see Chapter 11) in a
few ILs have been studied. However, the selectivity of ILs as a media for chemical (particularly,
free-radical) reactions remains unknown. Very little is known about the structure and dynam-
ics of non-persistent free radicals in ILs and the mechanism of free-radical formation. This is
surprising since these materials offer the most disparate local environments for solvent effects
on radicals.
Most μSR studies have examined the cyclohexadienyl radical, most probably formed by the
reaction of Mu with benzene (Scheme 8.1), as shown in Taylor et al. (2009) and Lauzon et al.
(2008). Benzene was selected because the muon and proton HFCs of C
6
H
6
Mu have been measured
in a variety of reaction media (including the gas phase) and over a wide temperature range. One of
the important results is the enhanced free radical formation in trihexyl (tetradecyl) phosphonium
chloride as compared to molecular solvents. After performing careful calibration with water in
the sample cells, the diamagnetic and free-radical fractions were measured by investigating the
transverse-eld amplitudes in Lauzon et al. (2008). The diamagnetic and free-radical fractions
0.0
0.2
0.4
0.6
0.8
0
0.2 0.4 0.6 0.8
1
1.2
ρ (g cm
–3
)
P
L
Figure 8.11 Missing fractions (P
L
) in pure H
2
O and CO
2
as a function of density. Values of P
D
for H
2
O
(empty diamonds) are taken from Percival et al. (1999). The light squares are data on CO
2
from Ghandi et al.
(2010)
while the dark squares are the data taken from Ghandi et al. (2004).

186 Charged Particle and Photon Interactions with Matter
in the three solvents [propanol as a representative protic polar solvent; n-heptane, a nonpolar
solvent;and trihexyl (tetradecyl) phosphonium chloride (IL 101)] were compared, and the data
suggests free-radical formation is more enhanced in ILs compared to the other two molecular
solvents. Similar experimental conditions were used for all of the comparison experiments, spe-
cically temperature, magnetic eld–strength, and benzene concentration. In these works, the
enhanced free-radical formation in phosphonium IL is clear and dramatic. A similar result was
obtained in other aromatic compounds, which may suggest that this is a general trend of addi-
tion to aromatic rings in tetra-alkyl phosphonium ILs. In order to investigate this more directly,
we measured the rate constant by using the Mu decay rate in low concentrations of benzene in
IL 101. The rate constant at room temperature and 1 bar was ∼1.5 × 10
10
M
−1
s
−1
, almost an order
of magnitude larger than the rate constant in water. Such a large rate constant is suggestive of a
diffusion-controlled reaction.
8.5 review oF the modern aspeCts oF muonium reaCtion
dynamiCs
and Free-
radiCal
s
tudies
by m
uons
8.5.1 introduction and techniQueS
Muonium, from a chemical point of view, is simply a light isotopic analogue of the H atom. Hydrogen
is the simplest atom in nature and consequently the study of its interactions and chemical reaction
rates
has been central to the eld of reaction dynamics.
Over
the past 10 years, studies of Mu chemical kinetics have been extended to SCW, a medium
of interest for a variety of chemical applications (including green chemistry and waste destruc-
tion) and of specic importance in some designs of future generation nuclear reactors (Gen IV
reactors) (Ghandi and Percival, 2003; Ghandi et al., 2002, 2003), Ghandi (2002). The supercriti-
cal-water-cooled reactor (SCWR) would operate at much higher temperatures than existing pres-
surized water-cooled reactors (PWRs), and a major technology gap for SCWR development has
been the lack of knowledge of water radiolysis under supercritical conditions. Accurate modeling
of aqueous chemistry in the heat transport systems of PWRs and the SCWR requires data on the
rate constants of reactions involved in the radiolysis of water and the action of water treatment
additives. Unfortunately, most experimental data do not even extend to the temperatures used in
current PWRs (typically around 320°C), which is well short of the supercritical conditions envis-
aged in generation IV designs. Many types of Mu reactions have been studied in subcritical and
SCW, and Mu kinetics have been used to deduce the nature of H-atom chemical dynamics under
extreme conditions that are very difcult to study by other techniques.
The conventional methods for detection of radicals, intermediates, and products of the
radiolysis of water are optical spectroscopy and electron spin resonance (see Chapters 12,
13, and 15). Some intermediates have been detected by optical spectroscopic methods (see
Chapter 15) under supercritical conditions, but the material composition of the optical
windows impose significant limitations on all forms of optical spectroscopy in SCW. The
Mu
Mu
H
met
H
H
H
p
H
m
H
o
+
•
sCheme 8.1 Reaction of muonium with benzene to produce the cyclohexadienyl radical.
Muon Interactions with Matter 187
limitations are imposed by the necessity to resist corrosion and to transmit a wide spectral
range under a variety of extreme conditions.
Electron paramagnetic resonance (EPR), the most conventional option for organic free-radical
characterization, is even more limited at high temperatures and pressures. Unlike the optical detec-
tion methods usually employed in pulse radiolysis studies, muon-spin spectroscopy is sensitive only
to the transient species under study (Mu in this case) and is unaffected by environmental effects (scat-
tering of light, change in extinction coefcient, etc.). Indeed, the only magnetic resonance techniques
used to study reactive free radicals in high temperature and pressure SCFs were, until recently, μSR
techniques used by Cormier et al. (2009), Ghandi and Percival (2003), Ghandi etal. (2002, 2003,
2004), Ghandi (2002), Kruse and Dinjus (2007), and Percival et al. (2003). These include the TF-μSR
technique described above and the ALC-μSR technique. It is possible to observe “delayed species”
using ALC-μSR that are formed up to one microsecond after muon implantation and this makes it
feasible to study samples with low concentrations of the free-radical precursors.
8.5.2 StudieS in Supercritical fluidS and liQuidS
Part of this section will focus on the work on muonium reaction kinetics in water from standard
conditions to SCW conditions as a typical study of reaction kinetics where muonium can be used
to probe the chemical kinetics of H atom in a complex system. To put this into context, we will
rst review the changes in the properties of water as its thermodynamic conditions change towards
supercritical conditions. At around room temperature, water behaves abnormally (compared to sim-
ple uids) due to the angular correlation existing between neighboring H
2
O molecules, as shown
in Kusalik and Svishchev (1994) and Soper et al. (1997). Some examples of abnormal behavior of
water around room temperature can be seen in Table 8.1, where some properties of water are com-
pared
with the same properties of other solvents.
Water
has a high dielectric constant that enables electrolytes to dissociate completely (Table 8.1).
The high dielectric constant also makes water a very good solvent for polar molecules. On the other
hand, the angular correlation between neighboring H
2
O molecules of the hydrogen bond network
demands a large amount of work to produce a cavity to accommodate a solute (note that the surface
tension of water is much larger than other conventional solvents). Therefore, if the molecule is not
polar
or ionic, its solubility at room temperature is very small.
By
increasing the temperature and/or decreasing the density of water in the liquid or super-
critical state, the angular correlation between neighboring H
2
O molecules decreases, as shown by
Svishchev et al. (1996), Liew et al. (1998), and Matubayasi et al. (1997). This causes a change in
table 8.1
Comparison
of p
hysicochemical
p
roperties
of w
ater
with o
ther
s
olvents
under r
oom
Conditions
surface tension
a
(
n m
−1
)
density
b
(g
cm
−3
)
Compressibility
c
(
gp
a
−1
)
dielectric
Constant
d
viscosity
e
(m
p
a
s)
Water 0.072 1.00 0.5 78.4 0.9
Methanol 0.022 0.79 1.2 32.7 0.5
Hexane 0.018 0.65 1.7 1.9 0.3
Pentane 0.015 0.62 2.2 1.8 0.2
a
Data taken from White et al. (1995), Riddick et al. (1986).
b
Data taken from Lemmon et al. (1998), Riddick et al. (1986).
c
Data taken from Harvey et al. (2001), Riddick et al. (1986).
d
Data taken from Mesmer et al. (1988), Riddick et al. (1986).
e
Data taken from Sengers and Kamgar-Parsi (1984), Riddick et al. (1986).
188 Charged Particle and Photon Interactions with Matter
the solvent properties of water, such that under supercritical conditions, most nonpolar molecules
are completely soluble in water. The dramatic changes in the properties of water at the microscopic
level should make its solvent properties very sensitive to temperature and pressure. The study of
solvent effects on the chemical kinetics of transient intermediates in subcritical and SCW is one of
the purposes of the work on muonium chemistry in subcritical and SCW. At the outset of these stud-
ies, there was no study on transient species in SCW. Mechanistic studies were mainly based on
end product analysis. All the kinetics information needed for modeling complex processes such
as
radiolysis of water and SCW oxidation were based on extrapolation of the low temperature data.
The
questions that these studies undertook were as follows:
Would the solvent cage change signicantly with the temperature and/or pressure?
If
it changes, then is the cage property under extreme conditions more like the cage properties of
typical
hydrocarbon solvents or is there no signicant cage effect as in high-pressure gases?
Is there any signicant cage effect at temperatures >300°C?
The
dielectric constant of water decreases with temperature and at 200°C–300°C it reaches the
values of alcohols under room conditions (Table 8.1). Under supercritical conditions it decreases to
the typical values of hydrocarbons under room conditions (Table 8.1 and Figure 8.12). Would such a
large
decrease of the dielectric constant affect radical chemistry or radiolysis of water?
A
purpose of the research of this thesis was to explore the effect of pressure on radical chemistry.
A key parameter that affects the pressure tuning of radical chemistry is the isothermal compress-
ibility.
Isothermal compressibility is dened as
κ( , ) ,T P
V
V
P
T
= −
∂
∂
1
(8.23)
where
κ is the isothermal compressibility
_
V is
the partial molar volume
P is the pressure
T
is the temperature
The
isothermal compressibility along three isobars is presented in Figure 8.13. It increases
slowly at low temperatures without any signicant pressure dependence at T < 300°C. At higher
0
20
40
60
80
100
Temperature (°C)
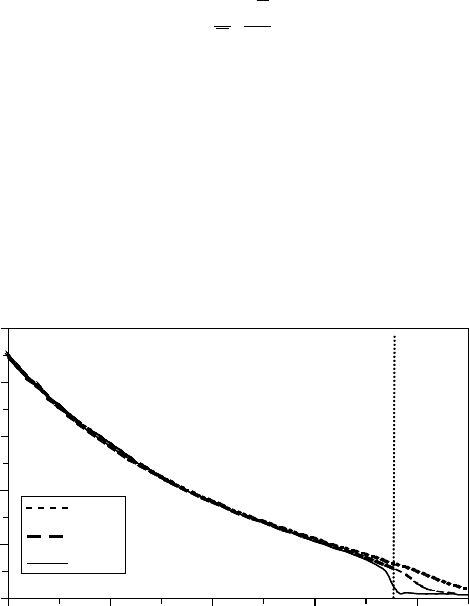
Dielectric constant
T
c
400 bar
224 bar
300 bar
0 100 200 300 400
Figure 8.12 Dielectric constant of water as a function of temperature at pressures above the critical point.
T
c
is the critical temperature of water.
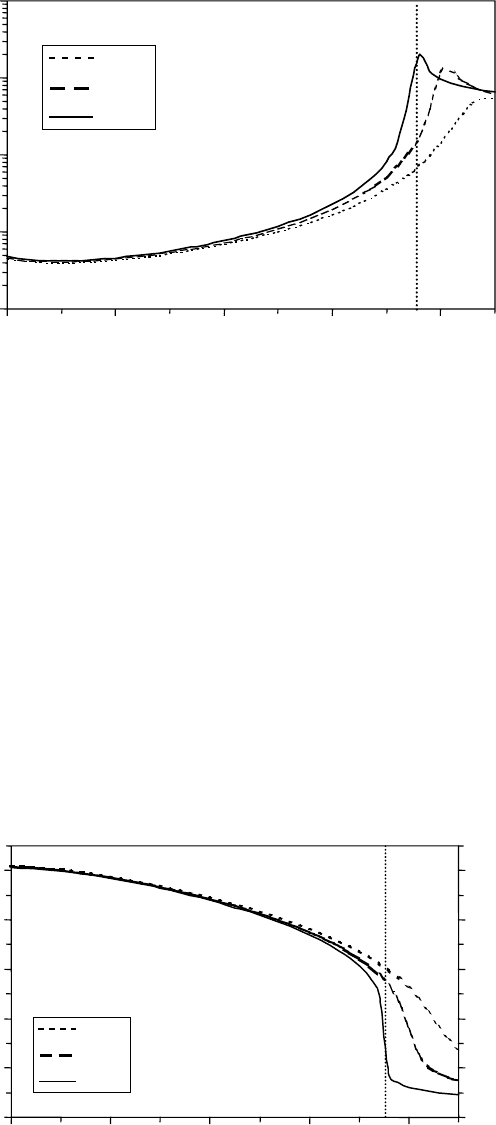
Muon Interactions with Matter 189
temperatures; however, the isothermal compressibility increases sharply until it goes through a max-
imum. The maximum becomes broader and shifts to higher temperatures with pressure. Would that
mean a maximum pressure effect at temperatures close to the critical point and at lower pressures?
Are equilibrium or dynamic properties affected and to what degree? Is pressure the signicant
parameter
in the radiation or radical chemistry?
Densities
under supercritical conditions are typical of high-pressure gas densities while at 360°C
the density of water has changed only (at 224 bar) to ∼600kg m
−3
, the density of n-pentane (Table8.1,
Figure 8.14). Would the solvent cage change signicantly with the temperature and/or pressure? If it
changes, then is the cage property under extreme conditions more like the cage properties of typical
hydrocarbon
solvents or is there no signicant cage effect as in high-pressure gases?
Due
to the fast reactions of radicals and intermediates in the radiation chemistry (radiolysis of
water), many reactions of radicals and other intermediates are believed to be diffusion controlled,
or nearly diffusion controlled. Would they remain diffusion controlled at high temperatures? There
is signicant pressure dependence of viscosity only between 360°C and 450°C (Figure 8.15). If a
reaction is diffusion controlled, then a negative pressure dependence is expected, which would be
signicant
in the temperature range of 360°C < T < 450°C.
1.E–05
1.E–04
1.E–03
1.E–02
1.E–01
0 100 200 300 400
Temperature (°C )
Compressibility (bar
–1
)
400 bar
224 bar
300 bar
T
c
Figure 8.13 Isothermal compressibility as a function of temperature at constant pressures.
0
200
400
600
800
1000
0 100 200 300 400
Temperature (°C)
400 bar
224 bar
300 bar
T
c
Density (kg m
–3
)
Figure 8.14 Temperature dependence of density at pressures above critical point.
