Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

190 Charged Particle and Photon Interactions with Matter
The other question that was addressed by these works was as follows: How would the tempera-
ture or pressure dependence of the self-dissociation of water (Figure 8.16) affect the radiolysis of
water
and radical chemistry?
For
most of these works, The TF-μSR technique has been employed. It was expected that the
hyperne coupling constant of muonium would give information regarding interactions of Mu with
the water molecules comprising the solvent cage around it. This expectation was realized and it was
found that the cage around muonium changes signicantly under subcritical and supercritical condi-
tions,
as shown by Ghandi et al. (2000).
The
HFCs were determined from the splitting of the muonium precession frequencies in an
intermediate eld, typically 200 G. The results for the HFCs were consistent with a break in the cage
structure at higher temperatures. This could be evidence for smaller cooperative interactions among
water molecules at higher temperatures. Indeed, the hyperne coupling constant of Mu was found
to
correlate with the bimolecular collision frequency.
Very
close to the critical point, the hyperne coupling constant of Mu was found to deviate from
a smooth model. This was attributed to a local density enhancement. It is important because the
0.0E+00
4.0E–04
8.0E–04
1.2E–03
1.6E–03
2.0E–03
0 100 200 300 400
Temperature (°C)
Viscosity (Pa s)
0.E+00
5.E–05
1.E–04
η (Pa s)
0.E+00
5.E–05
1.E–04
400 bar
300 bar
224 bar
Temperature (°C)
450400350300
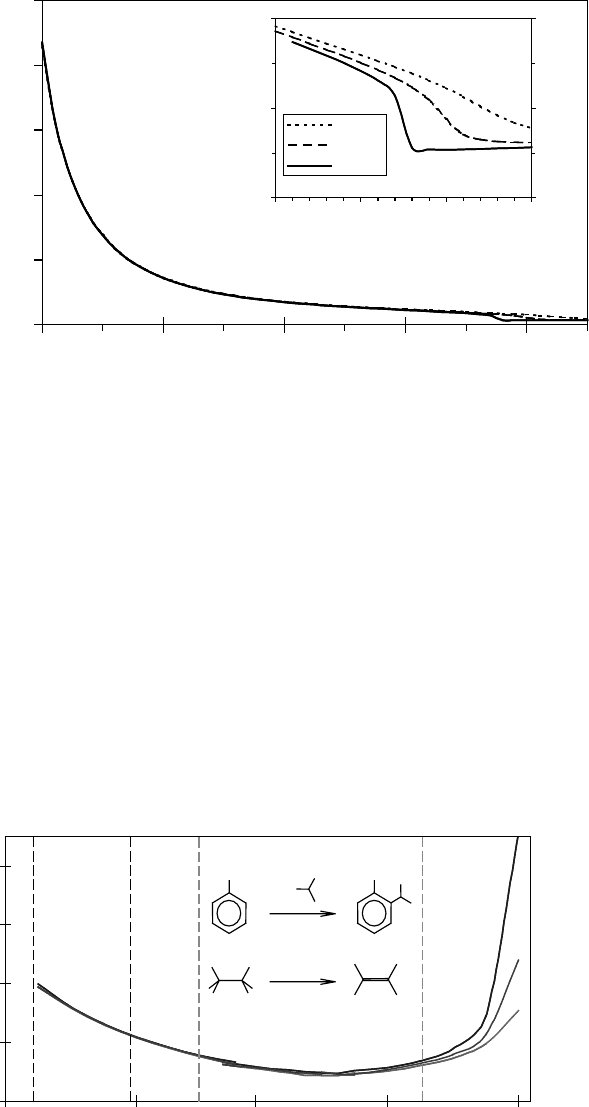
Figure 8.15 Viscosity as a function of temperature at pressures above critical point.
250 bar
300 bar
Temperature (°C)
10
12
14
16
18
200 300 4000 100
pK
w
Normal
aqueous
reactions
400 bar
Free radical
reactions
OH
OHOH
HO
H
Figure 8.16 The dissociation constant of water as a function of temperature at different pressures and the
typical
chemistry involved in each region.
Muon Interactions with Matter 191
hydrophobic nature of Mu under standard conditions makes it similar to nonpolar organic com-
pounds
and gases used in SCW oxidation reactors.
Theses
studies have led to the work on muoniated organic free radicals and muonium kinetics in
subcritical and SCW. For example, the μSR technique has been used to study radical formation in
aqueous solutions of acetone by Ghandi et al. (2003), Ghandi (2002). These investigations proved
that the μSR technique is uniquely able to provide mechanistic information for radical reactions in
SCW. The study of muon hyperne coupling constants in muoniated radicals in SCFs by Cormier
et al. (2009), Ghandi et al. (2003), and Percival et al. (2000, 2003) has also demonstrated the unique
ability of μSR to probe temperature, pressure, and solvent dependence of hyperne coupling con-
stants under extreme conditions. For the rst time, radical addition to the enol form of acetone
has been observed. The studies in supercritical CO
2
by Cormier et al. (2009) also demonstrated
the value of μSR to test theories of the temperature and density dependence of hyperne coupling
constants.
The investigations with acetone by Ghandi et al. (2003) and Ghandi (2002) could be considered
as simple examples of multi-step synthesis. These examples show that it is possible to tune the
properties of water so as to produce the precursors of a radical under one thermodynamic condition
and then tune to another thermodynamic condition, which gives the highest concentration of the
radical. Fullcharacterization of free radicals in SCFs was carried out by a combination of TF-μSR
and ALC-μSR by Cormier et al. (2009) and Percival et al. (2003, 2005). This gave the ability to
provide both the magnitude and sign of the coupling constants in dilute solutions (Percival et al.,
2003; Cormier et al., 2009) and as well provided a tool to explore the effect of concentration of radi-
cal
precursor under extreme conditions of subcritical uids and SCFs.
The
studies on kinetics in subcritical and SCW by Ghandi and Percival (2003), Ghandi et al.
(2002) and Ghandi (2002), and Percival et al. (2007) could be considered as an extension of the work
of Troe and his coworkers (see Schwarzer et al., 1998). Troe and his group have extensively studied
kinetics over a wide range of conditions from the liquid phase to the gas phase in simple molecular
uids
such as nitrogen and simple hydrocarbons.
Although
qualitatively the ndings of the works in Ghandi and Percival (2003), Ghandi et al.
(2002) and Ghandi (2002), were similar to the ndings of Troe and his group, it was found that Troe’s
model could not describe our results in subcritical and SCW. It was also found that the analysis of the
temperature dependence by means of the model widely used by Buxton et al. (1988) and his cowork-
ers for the reactions of H and other radiolysis transient species does not give sensible parameters. The
experimental strategy was to study kinetics to separate the effect of transport (viscosity) from other
solvent effects. To do this they studied a reaction without a signicant activation barrier. This was the
spin exchange between Mu and Ni
2+
(Ghandi et al., 2002). Spin exchange reactions are expected to
be diffusion controlled, but at elevated temperatures the rate constants were found to have values far
below those predicted by Stokes–Einstein–Smoluchowski theory. For spin exchange, the nding that
the rate constants go thorough a maximum under subcritical conditions was attributed to a decrease
of the encounter time under those conditions. Such a decrease resulted in a transition from the strong
exchange to the weak exchange limit. This should be a general phenomenon for all spin exchange
reactions in subcritical and SCW. The study of temperature dependence of near-diffusion-controlled
reactions also gave similar results; the rate constants pass through a maximum under near-critical con-
ditions and then fall with an increase in temperature. In the fall-off region, the rate constant increases
with pressure. It was found that the efciency of the reactions depends on the number of collisions
over the duration of the encounter (Ghandi et al., 2002).
If this effect is general (since the temperature dependence of the encounter time depends mainly
on solvent properties), similar observations should be expected for different types of reactions.
Therefore, further reactions were studied based on three criteria: experiments to test the stability
of reactants under a wide range of temperatures and pressures, theoretical calculations, and impor-
tance of the reaction for industry. The reactions between Mu and HCOO
−
, and Mu and OH
−
were
studied because they were expected to show strong solvent dependence (Ghandi 2002). Also,the
192 Charged Particle and Photon Interactions with Matter
reactions between Mu and benzene in Ghandi (2002) and Mu and methanol in Ghandi et al. (2003)
were chosen since they were expected to have small solvent effects, and therefore the effect of
multiple collisions was expected to be more important. There are some general trends among all
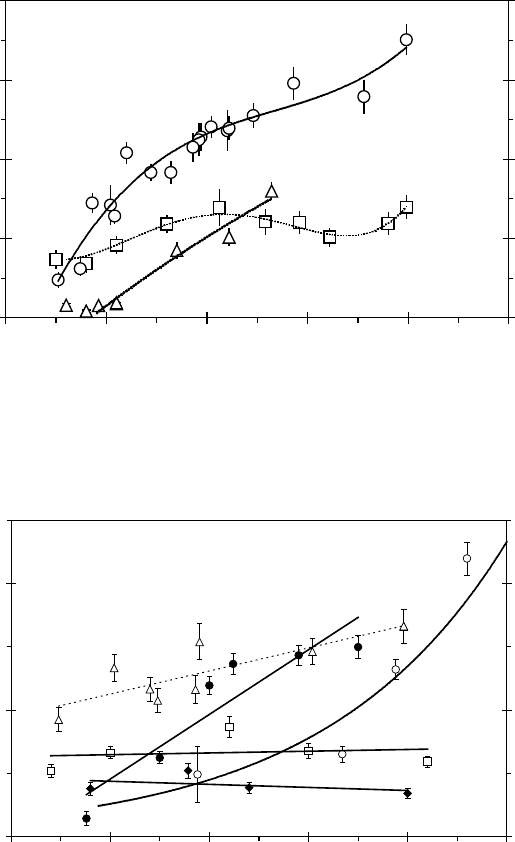
these reactions: it is clear from Figures 8.17 and 8.18 that pressure dependence is more signi-
cant at temperatures close to the critical point; there is positive pressure dependence under near-
critical conditions. For all reactions, the rate constants pass through a maximum in temperature
under near-critical conditions; both temperature dependence and pressure dependence under near-
critical conditions were consistent with the cage effect suggested for near-diffusion-controlled
0
10
20
30
40
200 250 300 350 400 450
Pressure (bar)
k
Mu
(10
9
M
–1
s
–1
)
Figure 8.17 Rate constants for the reaction of Mu with formate in water at various temperatures:
290°C(□), 378°C (○), and 385°C (△). The lines through the data only serve to guide the eyes. (From Ghandi,
K., Muonium kinetics in sub- and supercritical water, PhD thesis, Simon Fraser University, Burnaby, British
Columbia,
2002.)
0.0
1.0
2.0
150 200 250 300 350 400
Pressure (bar)
k
Mu
(10
10
M
–1
s
–1
)
Figure 8.18 Rate constants for the reaction of Mu with hydroxide in water at various temperatures:
205°C(◆), 250°C (□), 305°C (△), 362°C (•), and 390°C (○). The lines through the data only serve to guide
the eyes. (From Ghandi, K., Muonium kinetics in sub- and supercritical water, PhD thesis, Simon Fraser
University,
Burnaby, British Columbia, 2002.)
Muon Interactions with Matter 193
reactions (Ghandi et al., 2002). At lower temperatures the solute–solvent effects were found to be
more important. In particular, in studies on reactions between Mu and OH
−
and HCOO
−
it was
found that the variation of the dielectric constant of water with temperature plays an important
role in the subcritical region (Ghandi 2002). Although both reactions between Mu and OH
−
and
HCOO
−
showed the evidence of the cage effect under near-critical conditions, the reaction of
formate showed a positive deviation from the Arrhenius extrapolation while the reaction with OH
−
showed a negative deviation.
The study of kinetics of the Mu reaction with formate can be considered as an extension of the
work of Lossack et al. (2001) to supercritical conditions. Lossack showed that in the reaction between
H and formate in water, the dipole moment of the transition state is signicantly smaller than the
dipole moment of the reactants in Lossack et al. (2001). This causes signicant destabilization of the
transition state compared to the reactants. Positive deviation from the Arrhenius temperature depen-
dence for the reaction of Mu and formate is consistent with this solvent effect under hydrothermal
conditions. At higher temperatures, the dielectric constant of water drops (Figure8.12), and there-
fore its effect on the reaction path becomes smaller. This leads to larger rate constants compared to
the predictions from Arrhenius temperature dependence with activation energy calculated from the
data
under room conditions by Ghandi (2002).
The
ab initio calculations at the density functional theory level UB3LYP with a Gaussian
6-31+G(d,p) basis set for the reaction of
Mu OH MuOH MuOH (e )
aq
+ ⇔ ⇔ +
− − −
in the presence of
three water molecules (keeping this distance between O on OH
−
and Mu xed and optimizing other
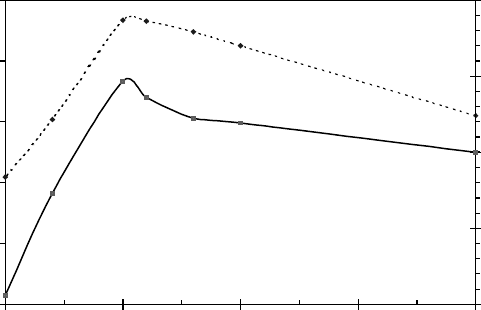
variables at each step) (Figure 8.19) suggest the dipole moment increases along the reaction path.
The larger dipole moment of the transition state compared to the reactants suggests a negative devia-
tion from predictions based on the Arrhenius model. The result of these calculations suggests that
water molecules are involved in the transition state explicitly and they are part of the reaction coor-
dinate. In addition, the effect of the dielectric constant and the cage effect are in the same direction.
The above studies led to development of a new model consistent with the temperature and pres-
sure dependence of rate coefcients over a broad range of conditions in water. The results are useful
for
modeling radiolysis of water in pressurized water nuclear reactors.
Another
new development in the eld of muonium kinetics and reaction dynamics in liquids has
been the extension of muonium kinetics to the realm of excited-state reaction dynamics by Ghandi
et
al. (2007).
0.0
0.8
1.6
2.4
3.2
4.0
1 1.5 2 2.5 3
HO–H distance (Å)
Dipole moment (Debye)
–50
–25
0
25
50
Energy/kJ mol
–1
Figure 8.19 The solid curve is the energy of the reaction complex relative to the energy of separated
reactants (OH
−
and H), and the dotted curve is the dipole moment of the reaction complex, as a function of
HO–H
distance.
194 Charged Particle and Photon Interactions with Matter
In this work, laser-muon-spin spectroscopy in liquids has been developed, which is a technique
to study the excited-state chemical dynamics of transient species. The work has more applications
than reaction dynamics but on the reaction dynamics front, as a proof of concept reaction kinetics of
muonium and Rose Bengal in the ground and excited electronic state (triplet state) has been studied.
This work also opens the way to study chemistry of excited-state muoniated free radicals. This spec-
troscopic technique has made new directions possible both for Mu chemists and the reaction dynam-
ics community by studying kinetic isotope effects of reactions of Mu with laser pumped molecules.
8.5.3 StudieS in the gaS phaSe
The most recent and important developments in the studies of reactions of Mu and muoniated free
radicals in gas phase are as follows: (1) The time-delayed RF-μSR studies of Mu reactions with
O
2
by Johnson et al. (2005). (2) The preliminary studies of a new class of chemical bonds (ro-
vibrational bonds), where the combination of vibrational and rotational motion of nuclei would lead
to transformation of a saddle point on the electronic potential energy surface (PES) to a stable spe-
cies in the work of Ghandi et al. (2006). This was in the context of a muoniated free radical formed
from reaction of Mu with Br
2
. (3) Studies of direct abstraction reactions and kinetic isotope effects
(KIEs) in comparison with the H-atom analogue and with “heavy muonium,” Heμ (Arseneau et al.,
2009). Heμ is the muonic atom, α
++
μ
−
e
−
, where the electron is in a 1s orbital, similar to its position in
the ordinary H atom (orMu). In the “heavy muonium,” the negative muon is in the small 1s orbital,
400 times closer to α. The atom can be treated as a heavy isotope of H, with a mass 4.116amu, that
is, 37 times Mu mass! In heavy muonium, (α
++
μ
−
)
+
acts as the nucleus. (α
++
μ
−
)
+
is formed by the
negative muon capture onto He, with the ejection of two electrons by the Auger process. To form
the neutral Heμ, charge exchange with an easily ionized reactant, such as Xe or NH
3
, is required;
therefore, studies of chemical reactions of “heavy muonium” are usually performed in the presence
of one of these species. Studies of this nature demonstrate most clearly the unique sensitivity of the
muon-based-atoms to quantum mass effects in reaction dynamics, in particular to zero-point energy
(ZPE) shifts at the transition state, exemplied by late-barrier reactions with H
2
. (4) The studies of
Mu reactions with vibrational excited H
2
in the work of Bakule et al. (2009a,b) that is an extension of
the work of Ghandi et al. (2007), that is, chemical dynamics of Mu reaction with excited-state mol-
ecules in the liquid phase to the gas phase. Although the laser effect was not signicantly above the
noise level in this work, it can be an exciting development since along with the work by Ghandi et al.
(2007), they generated a new class of experimental techniques for studies of reaction dynamics.
In addition to the above-mentioned novel works in the gas phase, there have been several theo-
retical and conventional experimental studies over the last 10 years on different systems, mostly to
investigate the KIEs. These works demonstrate the unique sensitivity of the Mu atom to quantum
mass effects in reaction dynamics, to both ZPE shifts at the transition state, exemplied by late-
barrier reactions such as the Heμ + H
2
reaction described above (Arseneau etal., 2009), Mu + CH
4
(Pu and Truhlar, 2002) (KIEs k
Mu
/k
H
<< 1), and quantum tunneling exemplied by “early-barrier”
reactions like Mu + Br
2
(Ghandi et al., 2006) (KIEs k
Mu
/k
H
>> 1) at lowest temperatures and con-
comitantly
with much smaller activation energies for Mu reactions.
There
have been studies of Mu addition reactions, both in the high-pressure limit exemplied by
the Mu + C
2
H
4
(Villa et al., 1999) reaction where the high-pressure limit is easily realized at mod-
erator pressures ∼1 bar, and in the low pressure (termolecular) regime where much higher pressures
(>1000 bar) are required for stabilization, in the case of small molecules with only a few degrees of
freedom, such as Mu + NO (Pan et al., 2000), Mu + O
2
(Himmer and Roduner, 2000), and Mu + CO
(Pan et al., 2006) reactions, in which the experimental data have been compared with the predic-
tions of unimolecular (Troe) theory. In these indirect reactions, rates for addition and stabilization
are in competition with those for unimolecular dissociation, with the overall (effective) rate constant
then exhibiting different pressure limits. Such studies are important model systems for theoretical
studies (Harding et al., 2000; Marques and Varandas, 2001).
Muon Interactions with Matter 195
In general, the experimental results of Mu reactivity in these studies continue to present challenges
to reaction theory. Therefore, an interface between experimental and theoretical data on Mu is very
useful to rene reaction dynamics theories. In the realm of collision dynamics, atomic and molecular
beam studies produced under single-collision conditions at epithermal (∼1eV) energies, with their
ability to probe ro-vibrational cross sections at the state-to-state level (Althorpe et al., 2002), play an
ever increasingly important role in assessing the interface between experiment and reaction theory.
However, at these kinds of impact energies, well above threshold, the general features of reactive col-
lision processes are often well described by classical (or quasi-classical) dynamics, so that the study
of chemical kinetics and bulk thermal rate constants, which are generally much more sensitive to
quantum mass effects in probing the details of the PES at the barrier, continue to be important. This is
truer in the case of sensitive isotopic mass probes that now extend from Mu to Heμ, a ratio of 1/37, and
hence the above-mentioned studies are unique probes of quantum mass effects in chemical reactivity.
8.6 modern slow muon beam produCtion teChniQues
The conventional muon beams are obtained from proton accelerators with proton energies higher
than 400MeV. According to the momentum of the generated muon beam, these beams are referred
to as “decay muon” or “surface muon” beams. The decay muons are obtained through the in-ight
decay of π
+
/π
−
, conned by a strong longitudinal eld of several tesla from a superconducting sole-
noid magnet. Muons obtained this way have high momentum, between 40 to several hundreds of
MeV
c
−1
. Not only positive but also negative muon beams are available (Nagamine, 1981).
The surface muons are obtained from the decay of positive pions (π
+
) stopped near the surface
of the pion production target in the proton beam line (Pifer et al., 1976). Compared to the decay
muons that have high momentum due to the in-ight decay of pions, the surface muons obtained
from the decay of π
+
at rest have a low and unique kinetic energy of 4.1MeV (corresponding to a
momentum of 29.8MeV c
−1
). Since the negative pions (π
−
) are easily captured by the nucleus, only
positive surface muon beams are available. The spin-polarized surface muons are widely used as
magnetic microprobes in materials (Schenck, 1985). The surface muon beam has a lower energy
compared to the decay muon beam, and when injected into a bulk sample, they have, therefore, an
implantation depth of just 0.1–1mm. Despite the name surface muons, it is typically used as a probe
to bulk phenomena rather than surface phenomena.
Lately, the study of surface/subsurface nanomaterials and multilayered thin lms is becoming
increasingly important. However, for that purpose, we must have muon beams that have sufciently
low energy to stop on or near the surface of the sample. To perform such studies, so called slow (low
energy) muons are required with energy that is of the order of several eV to a few tens of keV, far
lower than the energies available from the conventional muon beams. In this section we introduce
the
novel techniques to generate such slow muon beams.
8.6.1 the generation of low energy μ
+
through the cryogenic Moderation Method
The generation of low energy μ
+
through the cryogenic moderation method using van der Waals
solids,
such as solid Ar or N
2
, has been developed in the following four stages.
[Stage 1, low energy μ
+
] The rst observation of the emission of low energy positive μ
+
was
performed by Harshman et al. at TRIUMF. They could extract the so-called epithermal μ
+
with a
kinetic energy less than 10eV from solid lithium uoride (LiF), quartz, and copper with an ef-
ciency
of (1.6 ± 0.25) × 10
−7
per incident surface muon (Harshmann et al., 1986a,b).
[Stage 2, low energy μ
+
] Soon after this rst successful extraction of the epithermal μ
+
, Harshman
et al. also succeeded in observation of the epithermal muons below ∼10eV with a tail extending to
higher energies, from solid neon (Ne), argon (Ar), krypton (Kr), and xenon (Xe) moderators exposed
to the surface muons. Among the moderators, Ar was discovered to produce the highest yield of 10
−5
low
energy μ
+
per incident surface muon, as shown by Harshmann et al. (1986a,b).
196 Charged Particle and Photon Interactions with Matter
[Stage 3, low energy μ
+
] At Paul Scherrer Institute (PSI), Morenzoni et al. (1994) succeeded in
the rst measurement of the spin polarization of the low energy muon beam emitted from solid Ar
and Kr, utilizing the world’s strongest direct current (DC, not pulsed) surface muon beam. They
demonstrated that the low energy μ
+
beam generated through the cryogenic moderation method
using the van der Waals solids, such as Ar and N
2
, is a very powerful spin-polarized probe and
that there is no observable spin depolarization during the slowing-down process in the cryogenic
moderator.
The importance of this discovery should be stressed, since not only did they develop the practical
experimental technique of the low energy muon generation through the cryogenic moderation of the
surface muon beam, but also opened and established, in reality, various kinds of scientic applica-
tions,
such as surface and interface studies, using the low energy μ
+
.
[Stage 4, low energy μ
+
] Recently, Prokscha et al. (2008) constructed the new μE4 beamline
dedicated for the low energy μ
+
generation at PSI. With a solid-angle acceptance of 135msr, they
obtained the highest ux of 4.2 × 10
8
surface muons s
−1
. By constructing the most intense DC sur-
face muon beam channel, they have now prepared an intense and excellent low energy μ
+
source at
PSI
and opened up many new applications for the low energy muons.
8.6.1.1
experimental
t
echnique
and Features of the l
ow
e
nergyμ
+
beam
through
the Cryogenic m
oderation
m
ethod
At PSI, the low energy μ
+
beams had been developed at πE1 and πE3 beam lines (Morenzoni etal.,
1994) and now have been extended at the μE4 by Prokscha et al. (2008). The principle of the experi-
mental setup to obtain epithermal low energy μ
+
through the cryogenic moderation method at PSI
is
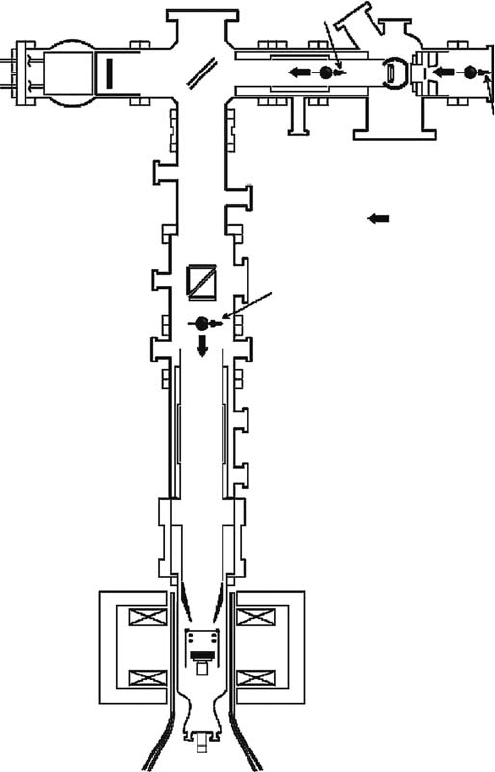
beautifully simple and is shown in Figure 8.20 (Morenzoni et al., 1994).
The
intense surface muon beam is introduced into the cryogenic moderator consisting of a high-
purity (99.999%) aluminum (Al) support foil of 250mm thickness and a thin layer of deposited solid
Ar or N
2
. In the case of Ar or N
2
(Ne), about 5 × 10
−5
(1.5 × 10
−4
) of the surface muons are converted
into the epithermal μ
+
. The epithermal μ
+
emitted from the moderator are then re-accelerated up
to 20kV and transported and focused by electrostatic Einzel lenses and a mirror into the sample.
On the way towards the sample, the accelerated μ
+
pass through a carbon foil of 10 nm thickness,
which provides a trigger signal for the TOF measurements (implantation time) at DC muon facility.
The use of such a trigger is unavoidable, but has the unwanted consequence of introducing a small
amount of energy and temporal spread. Finally, the kinetic energy of the low energy μ
+
(implanta-
tion energy) can be adjusted from 0.5 to 30 kV, by applying an accelerating or decelerating potential
up
to 12
kV
to the sample.
8.6.1.2
yield
of the l
ow
e
nergy
m
uons
at psi
According to Prokscha et al. (2008), at the newly built μE4 beam channel, the maximum 16 × 10
3
low energy μ
+
s
−1
at the Ne production target were obtained and 7.7 × 10
3
s
−1
were extracted at the
sample.
8.6.1.3 aspects
of the Charged p
article
i
nteractions
on the l
ow
e
nergy
μ
+
generation
The energy distribution of the low energy μ
+
generated through the cryogenic moderation method
has a peak at around 15eV with a tail extending to higher energies. In that sense, the low energy μ
+
through the cryogenic moderation method can be regarded as an epithermal μ
+
with the full spin
polarization. The mechanism of the emission of the epithermal μ
+
may include important features
of the “Charged Particle Interactions with Matter.” In the matter, the slowing down of μ
+
can be
proceeded by (1) Bethe–Bloch ionization, (2) cyclic charge-exchange muonium (designated as Mu,
consisting of a bound μ
+
and an e
−
, effectively a hydrogen-like atom) formation and break-up pro-
cess,
and (3) thermalization (Senba et al., 2006).
However,
in the wide-band-gap insulators such as Ar, N
2
, or Ne with band-gap energy of
between 11 and 22 eV, once μ
+
reached a kinetic energy of the order of their band-gap level, the
Muon Interactions with Matter 197
processes (2) and (3) are signicantly suppressed. At the last process of (2), epithermal μ
+
may
escape from the ionization track where excess e
−
are abundant, resulting in suppressing Mu forma-
tion and efcient emission of epithermal μ
+
into vacuum. Prokscha et al. (2008) examined Mu and
diamagnetic muon fraction in Ar, N
2
, and Ne, etc. as a function of implantation energy utilizing
the low energyμ
+
. In those cryogenic solid samples, Mu fraction was found to be suppressed near
the surface, in contrast to the diamagnetic muon fraction increasing near the surface, where the
excess e
−
are not abundant. These views occurring in the ionization track can also be reconrmed
by the dependence of the external electric eld on the Mu and μ
+
amplitudes in the solid N
2
at
20 K by Storchak et al. (1999). Both experimental results show the importance of charged particle
interactions with matter, in particular the behavior of excess e
−
in the ionization track.
Mirror
Muon spin
Surface muon
beam
(E = 4 MeV)
Muon spin
Muon spin
Trigger
detector
Helmholtz
coils
Positron
counters
Cryostat
Cryogenic moderator
(solid, Ar, N
2
, or Ne)
Muon momentum
Figure 8.20 The principle of the experimental setup for epithermal low energy μ
+
through the cryogenic
moderation method developed at PSI (Morenzoni et al., 1994; Prokscha et al., 2008). The intense surface
muon beam is introduced into the cryogenic moderator consisting of a high-purity (99.999%) aluminum (Al)
support foil of 250mm thickness and a thin layer of deposited solid Ar or N
2
. The epithermal μ
+
emitted from
the moderator are then re-accelerated up to 20 kV and transported and focused by electrostatic Einzel lenses
and
a mirror into the sample.
198 Charged Particle and Photon Interactions with Matter
8.6.2 generation of ultraSlow μ
+
through the reSonant ionization of therMal Mu
The second method is the ultraslow muon generation through the resonant ionization of thermal
Mu atoms, developed at KEK (High Energy Accelerator Research Organization) and RIKEN-RAL
(RIKEN Muon Facility of Rutherford Appleton Laboratory) pulsed muon facilities. This method
has
been developed by the following ve stages:
[Stage 1, ultraslow μ
+
] Thermal muonium formation in vacuum.
[Stage 2, ultraslow μ
+
] Experiments for the precise measurement of Mu’s 1s-2s state for the pur-
pose
of the quantum electro dynamics (QED) test at KEK-MSL (Muon Science Laboratory).
[Stage
3, ultraslow μ
+
] The ultraslow muon generation by 1s-2p-unbound transitions, placing a
Mu
production target W in a primary proton beam at KEK-MSL.
[Stage
4, ultraslow μ
+
] The ultraslow μ
+
generation by 1s-2p-unbound transitions, utilizing an
intense
surface muon beam at RIKEN-RAL.
[Stage
5, ultraslow μ
+
] Japan Proton Accelerator Research Complex (J-PARC) ultraslow μ
+
project.
[Stage 1, ultraslow μ
+
] When surface muons are stopped at the hot tungsten (W) in the ultra high
vaccum (UHV) condition, thermal energy Mu atoms were found to be evaporated into vacuum
from a clean and hot W foil. The total Mu yield was found to increase with increasing temperature
and about 0.04Mu yield per stopped surface muons (i.e., efciency of 4%) was observed at 2300K,
according to the Mills et al. (1986) experiments. Note that it is this extremely high efciency of
converting surface muons to ∼0.2eV thermal energy muons (albeit bound to an electron) that can be
exploited
for low energy muon generation through the cryogenic moderation.
[Stage
2, ultraslow μ
+
] The second stage consisted of the experiments for the precise measure-
ment of Mu’s 1s-2s transition frequency for the purpose of the precise QED test, performed by
Chuet al. (1988). Since Mu consists of muon (lepton) and electron (lepton) pair compared with
hydrogen that is a pair of hadrons (consisting of three quarks) and electron (lepton), Mu is con-
sidered to be the ideal and simplest system to test QED theory. In this experiment, thermal Mu
atoms were produced in vacuum by utilizing a SiO
2
powder sample. The 1s-2s transition as well
as the 2s-unbound transitions were induced by light from a frequency doubled pulsed dye laser.
The 1S(F=1)–2S(F=1) transition frequency was experimentally determined to be within 300MHz
of the QED theory prediction (Chu et al., 1988; Meyer et al., 2000). In these experiments, for the
rst time, the ultraslow μ
+
was generated by the laser resonant ionization of Mu, although it was
not intended.
[Stage 3, ultraslow μ
+
] The third stage consisted of the ultraslow μ
+
generation via 1s-2p-unbound
two-photon ionization of thermal Mu at KEK. In this step, boron nitride (BN) followed by W target
was installed in the proton line. In BN, π production and πμ decay takes place. In W, a fraction of
decayed μ
+
stop and diffuse into the rear surface of W, and then pick up electrons at surface, fol-
lowing which Mu atoms evaporate into vacuum. The Mu atoms that are evaporated from W into
vacuum are then ionized by intense laser pulses to generate the ultraslow μ
+
. This concept, a new
method
to generate the ultraslow μ
+
, was successfully established by Nagamine et al. (1995).
[Stage 4, ultraslow μ
+
] The fourth stage consisted of the experiments at RIKEN-RAL at ISIS
in UK where very intense pulsed surface muon beam is available. Here, the experiment was setup
directly at the beamline delivering the intense surface muon beam, taking the advantage of the fact
that the intensity of the primary proton beam at ISIS of 200 μA is about 40 times larger than that of
KEK-MSL (Miyake et al., 2000). At the RIKEN-RAL muon facility, nally, extraction of 20 ultra-
slow μ
+
s
−1
out of 1.2 × 10
6
s
−1
surface muons was achieved with an overall efciency comparable to
the
cryogenic moderator method and many unique parameters.
[Stage
5, ultraslow μ
+
] The fth step is the ultraslow muon source at J-PARC. The muon science
facility (MUSE), along with the neutron, hadron, and neutrino facilities, is one of the experimental
areas of J-PARC, which was approved for construction at the Tokai JAEA site. The MUSE facil-
ity is located in the Materials and Life Science Facility (MLF), which is a building integrated to
Muon Interactions with Matter 199
include both neutron and muon science programs. Construction of the MLF building was started
in the beginning of 2004, and the rst muon beam was delivered in the autumn of 2008. A super
omega muon channel with a large acceptance to extract the world’s strongest pulsed surface muon
beam is planned to be installed. Its goal is to extract 4 × 10
8
surface muons s
−1
for the generation
of the intense ultraslow μ
+
, utilizing laser resonant ionization of Mu by applying an intense pulsed
VUV laser system. As a maximum, 1 × 10
6
ultraslow muons s
−1
will be expected, which will allow
for the extension of μSR into the eld of thin lm and surface science (Miyake et al., 2009). All the
experimental apparatus that have been installed at RIKEN-RAL muon facility are planned to be
transferred
to J-PARC when the super omega muon beam channel is ready at J-PARC.
8.6.2.1
the
u
ltraslow
μ
+
generation by resonant ionization of mu
The ultraslow pulsed muons are generated by the resonant ionization of thermal muonium atoms
generated from the surface of a hot W foil, placed at the intense surface muon beam line. In order to
efciently ionize the Mu near the W surface, a resonant ionization scheme via the {1S-2P-unbound}
transition was adopted. This requires a laser system to generate Lyman-α photons (in the vacuum
ultraviolet (VUV) region around 120nm) and 355nm photons (for the ionization step from the 2P
state). Since there are only very few solids that are partly transparent in VUV region, the genera-
tion of the 120nm photons is the most challenging part of such experiment. For the experiments
both at KEK-MSL and at RIKEN-RAL, a very efcient technique of nonlinear up-conversion in
Kr gas, the so-called resonant four-wave frequency mixing (ω
VUV
= 2 ω
r
− ω
t
), has been adopted.
This wave mixing technique generates the Lyman-α photon through a parametric process of add-
ing energies of two 212.55nm photons (ω
r
) and subtracting energy of one tunable infrared photon
(ω
t
). This allows the generation of a VUV pulse that is easily tunable and has the bandwidth of
∼200 GHz required to cover the Doppler broadening of the Mu atoms moving at thermal veloci-
ties corresponding to 2000 K. The 212.55nm UV beam is generated as a fourth harmonic of the
fundamental laser operating at 850nm. This single-mode 850nm light with a bandwidth of 0.5–
1.0 GHz is generated from an OPO system (Continuum Mirage800) and amplied by series of
Ti:sapphire ampliers. The output energy of the single-mode beam at 850nm is as high as 300mJ
p
−1
at 25 Hz repetition rate and pulse duration of 5ns. The amplied 850nm light is quadrupled by
using two β-Ba
2
BO
4
crystals, generating two beams at 212.55nm wavelength with pulse energy of
10 mJ each. The difference tunable infrared beam was generated by a broadband OPO laser system
(Continuum Mirage3000). Finally, in order to ionize the Mu atom from the excited 2P state, a third
harmonic of a Nd:YAG laser (Continuum Powerlite 9025) with a wavelength of 355nm was used
(Miyake et al., 1995).
Figure 8.21 (top) shows a resonant ionization scheme via the 1s-2p-unbound transition for the
hydrogen atom isotopes and the scheme for the Lyman-α generation via the four-wave frequency
mixing method using two 212.55nm (ω
r
) photons for the two-photon resonant excitation of the
4P
5
5P[1/2,0] state in krypton, subtracted by a photon with a tunable difference wavelength.
Experiments have been performed using “slow-ion optics,” which consists of an immersion lens
(SOA), a magnetic bend for mass separation, an electrostatic mirror, and ve sets of electrostatic
quadrupoles. Charged particles ionized by the laser pulses are extracted by the SOA lens with an
acceleration voltage of 9.0kV (20 kV to be prepared), and transported to a micro-channel plate
(MCP) detector located about 3 m from the target. Any ions produced at the W target region can
be identied through the mass/charge (Q) ratio by setting the bending magnet in the ion optics,
and by observing the time-of-ight (TOF) spectrum, consequently minimizing the background
signicantly. Additionally, the electrostatic deector selects the energy of the particles that are
transported to the sample, thus signicantly suppressing the background. The laser pulses are
delayed by typically 400ns relative to the surface muon pulse to allow time for Mu to move away
from the target, since the mean thermal velocity in only 20 mm μs
−1
. A high-purity (99.9999%)
W foil with 50 μm thickness (obtained from Metallwerk Plansee GmbH) is placed inside the SOA
target chamber. The W foil is heated up to 2300K by a pulsed DC current that is turned off for
