Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

170 Charged Particle and Photon Interactions with Matter
Both provide, due to parity violation, a sensitive measure of the interactions of the muon spin with
its environment (Walker, 1983; Roduner, 1988, 1993; Kempton et al., 1991; Fleming and Senba,
1992;
Smigla and Belousov, 1994; Mozumder, 1999; Percival et al., 1999; Johnson et al., 2005).
Studies
on muon interactions with matter in the gas phase are relevant to the elds of atomic,
nuclear, and particle physics; as well as radiation chemistry, hot-atom chemistry, and studies of lin-
ear energy transfer (LET), which is the average energy released per unit path length due to ioniza-
tion
and excitation processes as shown in Mozumder, 1999.
To
maximize the lifetime of materials used in the reaction vessels for applications of radiation,
radiation chemistry in the gas phase needs to be understood. The main purpose of this section is to
review
the thermalization effects and radiation chemistry of positive muons in the gas phase.
There
are some differences in the thermalization processes of electrons and heavy charged par-
ticles in the gas phase, affecting the LET and hence affecting the nature of the radiation effects
involved. The extent of carrier gas decomposition due to ionizing radiation is a strong function of
the absorbed energy density of the incident radiation, with higher LET radiation causing decom-
position of the medium, while low LET radiation leads to little or no decomposition. Differing
LETs and stopping distances can give rise to differences in measured yields of products shown in
Mozumder
(1999).
8.1.2 Muon polarization and itS MeaSureMentS
The initial spin polarization of the μ
+
, at observation times, splits into three principal environments,
depending
on the nature of the stopping medium:
•
Paramagnetic muonium (Mu ≡ μ
+
e
−
) with polarization P
Mu
(Walker, 1983; Smigla and
Belousov,
1994).
•
Diamagnetic molecules, including molecules (like MuH, in water, shown in Percival et al.,
1999 or in hydrocarbons, shown in Kempton et al., 1991) and molecular ions, AMu
+
, where
A
is the molecule of the carrier gas (Johnson et al., 2005), with polarization P
D
.
• Muoniated
free radicals like CH
2
MuCH
2
(Walker, 1983), with polarization P
R
.
In the realm of radiation chemistry, it is important to be able to distinguish between these different
environments (P
Mu
, P
D
, and P
R
) and the timescale of their formation, provided by measurement
of their differing spin precession frequencies in a transverse magnetic eld (TF) (Walker, 1983;
Smigla and Belousov, 1994; Kempton et al., 1991; Percival et al., 1999) or by RF-μSR measurements
in
the work of Johnson et al. (2005).
In
contrast to conventional magnetic resonance studies, where bulk spin polarization is a con-
sequence of differing Boltzmann populations in high magnetic elds, in muon techniques (collec-
tively called μSR) the muon polarization is intrinsic to the probe, and is a direct consequence of
the nuclear weak interaction. Throughout this work, an external static magnetic eld (such as in TF
muon methods) is taken along the positive z-direction B
⃗
= (0, 0, B). The initial muon-spin direction
at
time t
0
= 0 is specied by the direction cosines:
ˆ
( , , ),S
0
cos cos cos= α β γ
0 0 0
(8.1)
where α
0
, β
0
, and γ
0
are the angles between the initial spin and the x-, y-, and z-axes, respectively.
Depending on which component of the muon-spin polarization is measured at observation time,
one can consider the following two congurations separately. In the rst, called the longitudinal
detector/eld conguration, the expectation value of the z component of the muon spin
σ
z
µ
/2
is
the observable quantity, where
σ σ σ σ
µ
µ µ µ
= ( , , )
x y z
represents the Pauli spin matrices for the positive
muon. The second is the transverse detector/eld conguration in which the expectation value of
Muon Interactions with Matter 171
the complex muon-spin polarization in the xy plane,
σ σ σ
µ
µ µ+
= +
x y
i ,
is relevant at observation time,
where
σ
µ
+
is the muon-spin raising operator.
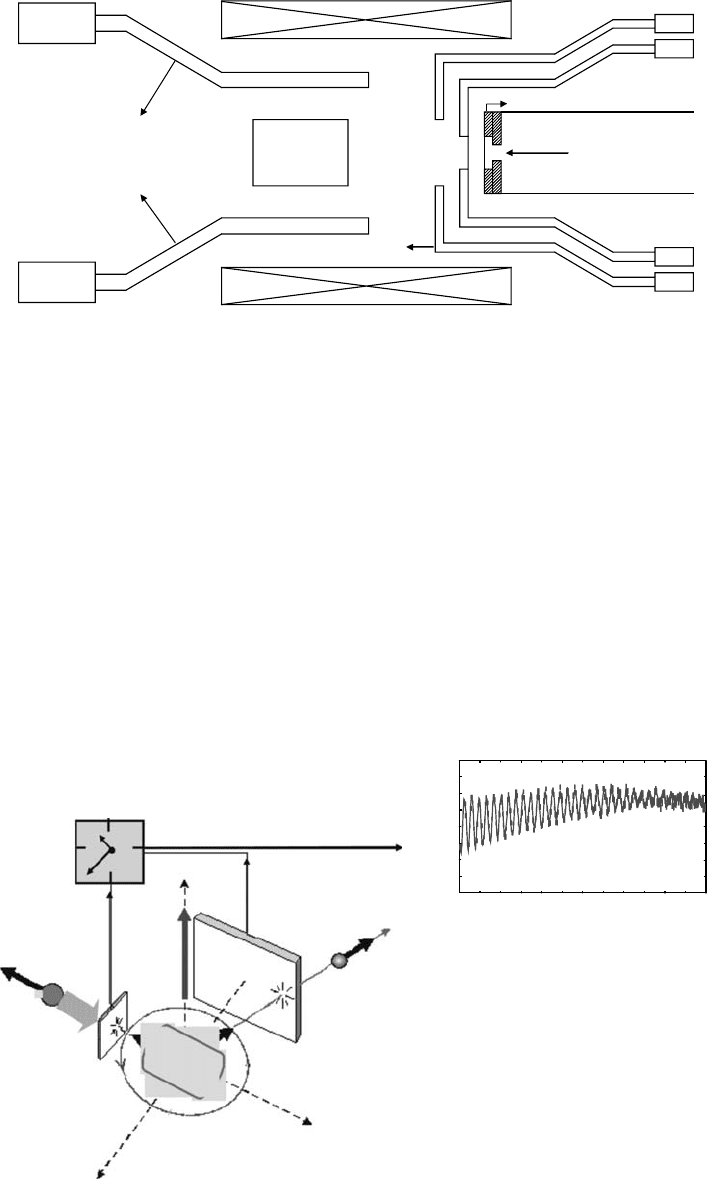
Muon beams enter and stop in the sample vessel. Detectors in coincidence detect muons entering
the sample and positrons emitted when the stopped muons decay. Positron detectors are arranged
around
the sample vessel (Figure 8.1).
In
a μSR experiment at a continuous beam facility in the time-differential mode, the elapsed
time between the stop of each muon registered through a muon coincidence (and with a time to
digital converter, TDC) and the detection of its decay positron (stopping the TDC) is measured,
and the data are collected and binned in a histogram of counts as a function of time (Figure 8.2).
In the transverse detector/static eld conguration, the probability of detecting the decay positron
in a given direction varies as the expectation value of the complex muon-spin polarization in the
xy plane oscillates in the magnetic eld. Thus, μSR histograms obtained from each positron detec-
tor contain oscillations in the muon decay spectrum, and these oscillations correspond to the time
dependence
of the muon polarization.
PMT
PMT
Target
vessel
Forward
detector
PMT
PMT
PMT
PMT
Beam pipe
Collimators
Muons
Magnet
Magnet
Backward detector
Figure 8.1 A schematic of the setup for μSR experiments.
Electronic clock
Spin-polarized
muon beam
Muon
detector
H
e
+
μ
+
Sample
Positron
detector
0.0 0.5 1.0 1.5 2.0 2.5 3.0
–0.2
–0.1
0.0
0.1
0.2
Asymmetry
Time (μs)
Figure 8.2 A schematic of the setup for TF-μSR experiments.
172 Charged Particle and Photon Interactions with Matter
Each histogram has the following form:
N t N e A t b
t
( ) ( ) ,
/
= +
+
−
0
1
τ
µ
(8.2)
where
N
0
is an overall normalization that depends on a number of factors, such as the solid angle of the
positron
detectors and the number of stopped muons
b represents random accidental (background) events
τ
μ
= 2.197μs (the muon lifetime)
A(t) represents the “asymmetry,” which represents the μSR signal of interest, and is similar to
free
induction decay (FID) in magnetic resonance
The
asymmetry parameter includes contributions from all muon environments, paramagnetic
Mu and free-radical species, as well as diamagnetic species:
A t A t w t
i i i i i
( ) exp( )cos( ),= − +Σ λ ϕ (8.3)
where
A
i
is the initial amplitude of the muon fraction i in a given environment
λ
i
is the relaxation rate of the muon spin in that environment
w
i
is the corresponding precession frequency
φ
i
is the initial phase for this fraction
The diamagnetic muon relaxation rate is invariably slow enough to be ignored in studies of Mu
reactivity.
The parameters of interest (A
i
, λ
i
, and w
i
) are extracted from ts of Equations 8.2 and 8.3 to
experimental data and give information, respectively, on Mu formation, kinetics, and hyperne
interactions
(in Mu or muoniated radicals), depending on the focus of a given experiment.
The
total (nonrelativistic) Hamiltonian describing the isotropic hyperne and Zeeman interac-
tions
of the muon and electron spin (in Mu) with an external magnetic eld B
⃗
=
(0,0,B) is given by
H
z z
= −
+
+
⋅
ω
σ
ω
σ
ω
σ σ
µ
µ
µ
2 2 4
0e
e
e
, (8.4)
where
σ⃗
e
represent the Pauli spin matrices for the electron
ω
0
/2π is the Mu hyperne frequency
the quantity ω
e
is dened by ω
e
= γ
e
B in terms of the gyromagnetic factor γ
e
of the electron
γ
e
/2π= 28.02421GHz/T
the
quantity ω
μ
is dened by ω
μ
= γ
μ
B in terms of the gyromagnetic factor γ
μ
of the muon
The energy eigenvalues ħω
1
, ħω
2
, ħω
3
, and ħω
4
are labeled in the decreasing order in the low
eld
regime:
ω
ω ω
γ γ
γ γ
ω
ω ω
µ
µ
1
0 0
2
0 0
2
4 2 4 2
1= +
−
+
, = − +
+ ,x x
( )
( )
e
e
ω
ω ω
γ γ
γ γ
ω
ω ω
µ
µ
3
0 0
4
0 0
2
4 2 4 2
1= −
−
+
, = − −
+x x
( )
( )
.
e
e
Muon Interactions with Matter 173
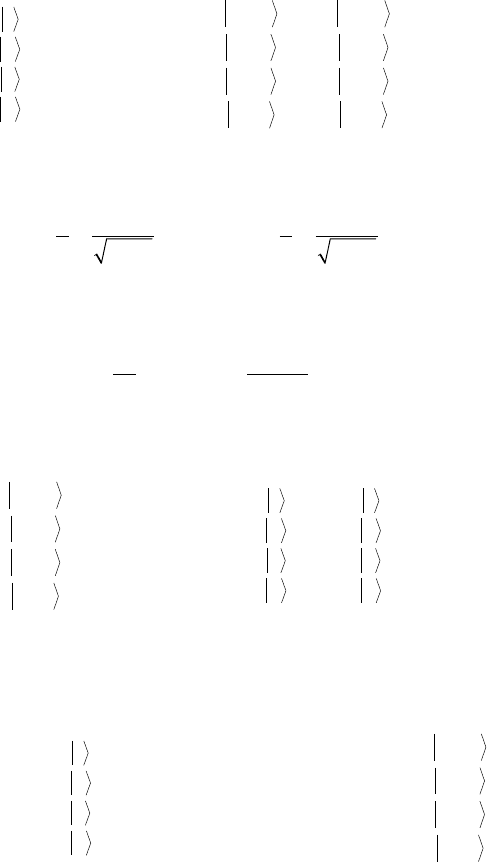
The energy eigenstates ∙1〉, ∙2〉, ∙3〉, and ∙4〉, corresponding to the energy values ħω
1
, ħω
2
, ħω
3
,
andħω
4
, labeled in the decreasing order in the low eld regime, can be expressed as superposition
of
the Mu spin basis set ∙α
μ
α
e
〉, ∙α
μ
β
e
〉, ∙β
μ
α
e
〉, and ∙β
μ
β
e
〉 as
1
2
3
4
1 0 0 0
0 0
0 0 0 1
0 0
=
−
s c
c s
α α
α β
β α
µ
µ
µ
e
e
e
ββ β
α α
α β
β α
β β
µ
µ
µ
µ
µe
e
e
e
e
≡
U , (8.5)
where
c and s are positive parameters dened by
c
x
x
s
x
x
2
2
2
2
1
2
1
1
1
2
1
1
=
+
+
=
−
+
and
in
terms of the reduced magnetic eld
x
B
B
B= =
+
.
0
0
0
with
e
ω
γ γ
µ
By solving the above equation for ∙α
μ
α
e
〉, ∙α
μ
β
e
〉, ∙β
μ
α
e
〉, and ∙β
μ
β
e
〉, one obtains
α α
α β
β α
β β
µ
µ
µ
µ
e
e
e
e
=
−
1 0 0 0
0 0
0 0
0 0 1 0
s c
c s
≡
−
1
2
3
4
1
2
3
4
1
U . (8.6)
Assuming ϕ
αα
(t), ϕ
αβ
(t), ϕ
βα
(t), and ϕ
ββ
(t) are the Mu spin states that coincide, at t = 0, with ∙α
μ
α
e
〉,
∙α
μ
β
e
〉, ∙β
μ
α
e
〉, and ∙β
μ
β
e
〉, respectively. By using the time-dependent Schrödinger equation, one
canwrite
φ
φ
φ
φ
αα
αβ
βα
ββ
ω
ω
ω
( )
( )
( )
( )
t
t
t
t
U
e
e
e
i t
i t
i
=
−
−
−
−
1
1
2
1
2
33
4
3
4
0 0 0
0 0
0
11
22 23
32 33
t
i t
e
G t
G t G t
G t G
−
=
ω
( )
( ) ( )
( ) (tt
G t
)
( )
,
0
0 0 0
44
α α
α β
β α
β β
µ
µ
µ
µ
e
e
e
e
(8.7)
where
G t e
i t
11
1
( )
= ,
− ω
G t s e c e
i t i t
22
2 2
2 4
( ) ,= +
− −ω ω
G t cse cse G t cse cse
i t i t i t i t
23 32
2 4 2 4
( ) ( ) ,= − , = −
− − − −ω ω ω ω
G t c e s e G t e
i t i t i t
33
2 2
44
2 4 3
( ) ( )= + , = .
− − −ω ω ω
It should be noted that the matrix [G
jk
(t)] is a unit matrix at t = 0: G
jk
(0) = δ
jk
.
174 Charged Particle and Photon Interactions with Matter
The state in which the spin points in the positive x-direction at time t = 0 is given by
ψ α β
µ µ
( ) ( ).0
1
2
=
+ (8.8)
In magnetic elds <200G, both w
12
and w
23
(w
ij
= w
j
− w
i
) are easily observable, but w
14
and w
34
,
whose parentage is mainly the “singlet” state, are comparable to ω
0
, too fast to be seen, and hence
are averaged to zero by the experimental time resolution. The difference in frequency between
w
12
and w
23
vanishes in elds ∼10G, such that only a single frequency, w
Mu
, is observed. In this limit,
Equation 8.7 reduces to the simple result of coherently precessing “triplet” Mu (Equation 8.3) with
the “Larmor precession,” v
Mu
= 1.39MHz G
−1
. At such a low eld, the diamagnetic Larmor preces-
sion frequency, v
μ
= 13.55kHz G
−1
, cannot be seen over the short time range but shows up charac-
teristically over longer time ranges and at elds ∼20 G. At slightly larger magnetic elds, the spectra
show the fast oscillations and slower beat frequency characteristic of two-frequency Mu precession,
dened by Equations 8.9 and 8.10, and reecting allowed transitions between muon-electron energy
states
in a magnetic eld (Roduner, 1993):
v v v v v A A
12
2 2
1 2
1
2
= − − + +
+
( ) ( ) ,
/
e eµ µ µ µ
(8.9)
v v v v v A A
23
2 2
1 2
1
2
= − + + +
−
( ) ( ) .
/
e eµ µ µ µ
(8.10)
where
v
e
is the electron Larmor frequency
v
μ
the muon Larmor frequency of the coupled two-spin system, with A
μ
the corresponding
muonium
hyperne frequency
The
total Mu amplitudes are most easily found in weak elds, <10G, giving a single amplitude,
while
the diamagnetic amplitudes are usually found from larger elds (Ghandi et al., 2004).
Since
knowing the absolute muon polarization is important, studies are usually rst carried out
in the same sample vessel with a standard sample, usually N
2
for gas phase studies, since the abso-
lute polarizations for N
2
are well established over a wide range of densities as shown in Kempton
et al. (1991). Comparison of the ratio of measured muonium/diamagnetic amplitudes with these
known fractions in N
2
provides a determination both of the diamagnetic signal due to muon stops in
the cell window and walls of the sample vessel, as well as the total muon asymmetry correspond-
ing to the full polarization. The measured amplitudes in the samples of interest are then corrected
according
to established procedures (Roduner, 1993):
P
A A
A A
D
D W
S W
=
−
−
( )
( )
, (8.11)
and
P
A
A A
Mu
Mu
=
−
2
( )
,
S W
(8.12)
Muon Interactions with Matter 175
where
A
D
is the diamagnetic amplitude
A
W
is the sum of the wall and window amplitude
A
S
is the amplitude of the standard (N
2
)
A
Mu
is the muonium amplitude
The
factor 2 in Equation 8.12 accounts for non-observed singlet muonium.
The
initial stage of the energy loss process of any ion in matter is known as the Bethe–Bloch
ionization (Inokuti, 1971; Walker, 1983; Senba, 1988; Roduner, 1993; Smigla and Belousov, 1994;
Pimblott and LaVerne, 1997; Mozumder, 1999; Hatano, 2003; Senba et al., 2006). After passing
through the entrance window of the target cell (Figure 8.1), muons entering the sample still have
the MeV kinetic energies noted earlier, many orders of magnitude higher than those of chemical
interest. Most of this initial energy is dissipated in ionization and excitation processes, described
by the Bethe–Bloch stopping-power formula for −dE/dX (Walker, 1983; Senba, 1988; Smigla and
Belousov, 1994; Mozumder, 1999; Hatano, 2003; Senba et al., 2006). During this regime, there is
no
loss of muon polarization.
In
a low density gas, this is followed by a regime of cyclic charge exchange with moderator “M”
beginning
around 100
keV
for the positive muon and described by
µ
µ
σ
σ
+ +
+ −
+ → +
+ → + +
M Mu M
Mu M M e
10
01
(8.13)
with average electron capture (σ
10
) and loss (σ
01
) cross sections, together with those for elastic and
inelastic energy moderation, determining the outcome as shown in the works of Fleming and Senba
(1992), Kempton et al. (2005), and Senba et al. (2006). In this regime, lasting down to an energy
E
min
∼ 10 eV, the muon undergoes many cycles in less than 1 ns (at 1 atm in the gas phase) as
described in Fleming and Senba (1992); Senba et al. (2006), emerging as either a bare μ
+
or as the
Mu atom, prior to entering its third thermalization stage, from E
min
to k
B
T. Near the end of the cyclic
charge-exchange regime while the muon slows down, one cycle of charge exchange caninvolve
a series of complex collisions, including (1) the electron capture collision by the muonto form
muonium (Mu), (2) repeated spin exchange collisions with paramagnetic species, during the time
the projectile is in the neutral Mu state. This is when there are most likely secondary electrons pres-
ent in the radiation track, and (3) an electron loss collision by Mu to form μ
+
again.
The times taken by positive muons to slow down from initial energies in the range of ∼1 MeV, to
the energy of the last muonium formation, approximately 10eV, at the end of cyclic charge exchange,
have been measured in the pure gases H
2
, N
2
, Ar, and in the gas mixtures Ar-He, Ar-Ne, Ar-CF
4
,
H
2
-He, and H
2
-SF
6
as shown by Senba et al. (2006). At 1atm pressure, these slowing-down times,
in Ar and N
2
, vary from 14ns at the highest initial energy of 2.8MeV, to 6.5ns at 1.6MeV. Similar
variations were seen in the gas mixtures, depending also on the total charge and nature of the mix-
ture consistent with Bragg’s additivity rules. The slowing-down times were also used to determine
the
stopping powers, dE/dX, of the positive muon in N
2
, Ar, and H
2
, at kinetic energies near 2MeV.
The results demonstrated that the positive muon and proton have the same stopping power at the
same projectile velocity, as expected from the Bethe–Bloch formula (Hatano, 2003). The energy of
the rst neutralization collision that forms muonium (hydrogen), which initiates a series of charge-
exchanging collisions, was also calculated for He, Ne, and Ar. The slowing-down times through
the rst two regimes are controlled by the relevant ionization and charge-exchange cross sections,
whereas the nal thermalization regime is most sensitive to the forwardness of the elastic scatter-
ing cross sections in the low density gas phase. In this regime, the slowing-down times (to kT) at
nominal pressures are expected to be less than or similar to 100ns. In this nal stage of energy loss,
176 Charged Particle and Photon Interactions with Matter
Mumay undergo hot-atom reactions and the μ
+
as well may continue to form Mu by charge exchange
and/or form muon molecular ions, MMu
+
, both in competition with elastic and inelastic scattering.
Although most studies of diamagnetic and muonium (Mu) fractions formed in low pressure gases
have used transverse eld-μSR (TF-μSR) that provides information on sub-microsecond radiation
processes, these studies have recently been extended to the microsecond timescale using the tech-
nique
of time-delayed radio frequency muon-spin resonance (RF-μSR) in Johnson et al. (2005).
In
the RF technique, a static magnetic eld, B, is applied collinearly with the muon-spin polar-
ization, with an oscillating B
1
eld applied transversely to it. Similar to NMR, adjusting the static
eld strength and RF frequency to meet the resonance condition for the system causes the probe
spin polarization to experience a torque and precesses about the B
1
eld for its duration. μSR data
are
collected with and without the RF eld. The experiment alternates between the two conditions,
typically every 10s, to identify the effect of the RF eld on the system and reduce the effects due to
equipment
instability (Johnson et al., 2005).
Results
obtained with inert gases (nitrogen and noble gases) at pressures higher than 1atm estab-
lished the validity of the TF-μSR results, proving that formation of these species is due only to
prompt processes (sub-nanosecond timescale) resulting from charge-exchange and thermalization
processes from keV to epithermal energies. The result was also consistent with the view that the
diamagnetic environment is due to a muon molecular ion, MMu
+
, and not a bare μ
+
. The coupling
between the μ
+
and the gas suggested that the MMu
+
molecular ion is a stable species with no con-
version to a bare μ
+
. In addition, this work showed that the RF-μSR technique provides polarization
fractions in good accord with those obtained using conventional transverse-eld muon-spin reso-
nance
measurements. This is a result that will no doubt be exploited in the years to come.
8.1.3 free-radical forMation in the gaS phaSe
The identity (i.e., the molecular structure) of muoniated free radicals is usually unambiguous,
provided hyperne constants can be determined by TF-μSR or RF-μSR, and avoided level
crossing (μALCR). The spin polarization of the muon beam is perpendicular to the applied
magnetic eld for the TF-μSR experiments, perpendicular during the RF radiation and RF-μSR
experiments, and parallel to the eld for avoided level crossing-μSR (ALC-μSR) experiments
(Roduner, 1988, 1993; Smigla and Belousov, 1994). Positron counters are positioned appropri-
ately in each case.
The precession frequency of the spin in the TF-μSR varies as a function of the strength of the
magnetic eld and the electronic environment, through the hyperne interaction. At high magnetic
elds where Zeeman states are sufciently separated, only two transitions, v
12
and v
34
, are observ-
able (Roduner, 1988, 1993). Fourier transformation of the time spectrum shows three frequency
peaks, two of which correspond to the generated radical (v
12
and v
34
) and one to muons in a dia-
magnetic environment (v
d
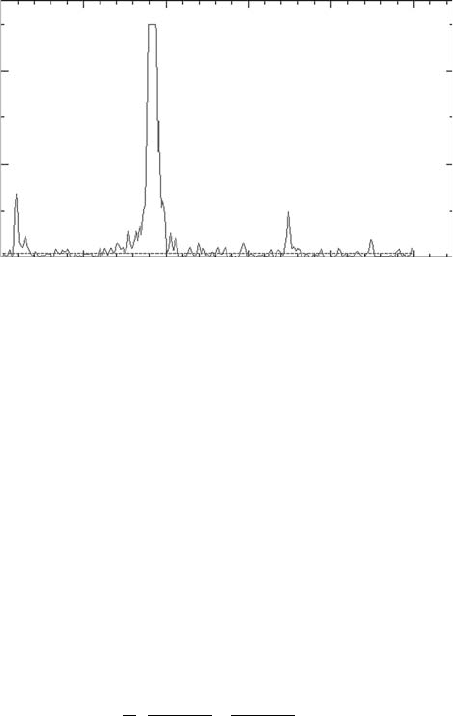
). The spectrum in Figure 8.3 is due to the muoniated ethyl radical in CO
2
(Cormier
et al., 2008).
At
high magnetic elds all energy transitions degenerate to only two, and the associated two
frequencies can be used to determine the muon hyperne coupling constant (HFC), A
μ
according
to
(Roduner, 1988, 1999):
ν ν
µ µij
A= ± ½ ,
(8.14)
where ν
μ
is the muon Larmor frequency. The HFC is proportional to the unpaired spin density at
the
nucleus.
It
is possible to observe “delayed species” that are formed up to 1 μs following muon implanta-
tion using ALC-μSR, and this makes it feasible to study samples with low concentrations of the
free-radical precursors. The spin states in large magnetic elds are the products of the unpaired
Muon Interactions with Matter 177
electron and the nuclear Zeeman states; consequently, the muon spin is time independent and the
asymmetry is independent of the applied magnetic eld. At different values of the applied mag-
netic eld, near-degenerate pairs of spin states can be mixed through the isotropic and anisotropic
components of the hyperne interaction. The muon polarization oscillates between the two mix-
ing states of the avoided crossing. This results in a resonant-like change in the asymmetry as the
magnetic eld is swept. There are three kinds of resonances, classied by the selection rule ∣ΔM∣=
0, 1, and 2, where M is the sum of the quantum numbers for the z components of the muon and
nuclear spins.
In the case of isotropic environments (∣ΔM∣ = 0), once the muon HFC is known using the TF-μSR
technique, proton HFC (A
p
) or the hyperne coupling constants of other spin-active nuclei can be
obtained
from ALC-μSR measurements through the following equation (Roduner, 1988, 1993):
B
A A A A
LCR
p
p
p
e
=
−
−
−
+
1
2
µ
µ
µ
γ γ γ
, (8.15)
where
B
LCR
is the magnetic eld on resonance
γ
p
and γ
e
are the proton and electron gyromagnetic ratio, respectively
In ALC-μSR experiments, the muons are injected into the sample with their spin parallel to the
magnetic eld. The integrated number of positrons emitted in the forward and backward directions
with regard to the incoming muon beam is measured as a function of the applied magnetic eld.
The muon polarization is proportional to the experimental asymmetry. In contrast to TF-μSR, there
is no limit on the number of muons in the sample at one time; consequently, it is possible to run at
a high incident muon rate. The eld is scanned in a series of small steps. It is possible to do both
TF-μSR and ALC-μSR experiments on the same spectrometer, just by moving the counters and
changing the beam polarization. Sometimes a modulation eld (∼80G) is applied to compensate for
the background. This gives the resonance a differential lineshape; however, this is only useful for
studies
of isotropic environments where the resonances are not very wide.
Despite
the superiority of μSR to characterize free radicals (Dilger et al., 1997) and their
electronic structure in the gas phase (polyatomic radicals are generally not amenable to study by
electron spin resonance in the gas phase), gas phase studies of free radicals over the last 10 years
were not very productive. However, there has been an important study on the mechanism of the
muoniated
ethyl radical formation in the gas phase by Percival et al. (2003).
v
34
500400300200
Frequency (MHz)
0
2×10
–4
4×10
–4
Fourier power
1000
v
12
Figure 8.3 The TF-μSR spectra of ethyl radical in a low concentration ethene in supercritical CO
2
at 305K
at
85 bar.
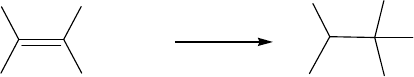
178 Charged Particle and Photon Interactions with Matter
The study tackled the following questions: Is muonium formation the rst step, followed by
addition to the alkene (Figure 8.4)? Or is there an ionic process, such as muon attachment to give
a cation, followed by electron addition? The work in reference Percival et al. (2003) explored such
questions by studying how the spin polarizations of the incident muons were transferred to the
radical product. It was possible to t the TF-μSR signal amplitudes with a model involving a single
reaction step—Mu addition to ethene. However, the rate constant deduced from the model t was
signicantly higher than the literature value for a thermal reaction (Garner et al., 1990), as deter-
mined by direct study of Mu decay in low partial pressures of ethene. The conclusion was that at
partial pressures above 1bar, the reaction (Figure 8.4) occurs before the incoming (beam) muons
are fully thermalized, that is, via a hot-Mu reaction. Comparing the experimental rate constant for
Mu + ethene with the predictions of canonical variational transition state theory (Villa et al., 1998)
suggested that Mu would have an effective temperature of about 2000K, that is, ∼0.25eV kinetic
energy. Using the reactive cross section from the thermal reaction (Garner et al., 1990) and adjusting
for epithermal energies, as shown by Senba et al. (2000), led to 4000K as an estimate of an effec-
tive reaction temperature (∼0.5eV). The rst-order muonium decay rate is 7–50 × 10
9
s
−1
, depending
on ethene pressure, and therefore the reaction timescale is of the order 10–100ps. This meant Mu
could still be epithermal; tens of picoseconds after energetic muons entered the gas at pressures
below 10 bar. This is consistent with expectations based on measurements of muon thermalization
in low pressure gases by Senba et al. (2006), where the slowing-down time to ∼10eV is of the order
of 10ns at 1 bar.
8.2 muon radiation Chemistry in moleCular liQuids
There is consensus about the mechanism of muon thermalization in the gas phase, due to the sim-
plicity of the low density gas phase interactions. However, structures are more complex in the liquid
phase and there is no consensus regarding radiation chemistry in molecular liquids. There have
been several theoretical and experimental studies of liquid muon radiation chemistry over the last
10
years, especially in muonium formation in liqueed inert gases.
The
spatial distribution of electron–muon pairs in superuid helium (He-II) was determined the-
oretically for reconstructing the muonium (Mu) formation probability by Kosarev and Krasnoperov
(1999). It was shown that because a gap is present in the excitation spectrum of He-II, the thermal-
ization time of muons and secondary electrons increases with decreasing temperature. As a result,
the average distance in the electron–muon pairs increases and, correspondingly, the muonium for-
mation rate decreases (Kosarev and Krasnoperov, 1999). Such theoretical predictions of radiolysis
effects in molecular liquids during muon thermalization were later put on rm ground using the
technique of muon-spin relaxation in frequently reversed electric elds (Eshchenko et al., 2000,
2001,
2002, 2003). Such experiments proved that the excess electrons liberated in the μ
+
ionization
track converge upon the positive muons and form Mu atoms. This process was shown to be cru-
cially dependent upon the electron’s interaction with its environment (i.e., whether it occupies the
conduction band or becomes localized) and upon its mobility in these states (Eshchenko etal., 2000,
2001). The characteristic lengths involved are quite long and in the range of 10
−6
−10
−4
cm; the char-
acteristic times range from nanoseconds to tens of microseconds. At the nanosecond timescale, the
Mu+
H
H
α
Mu
H
β
H
β
H
HH
H
•
•
Figure 8.4 Reaction of the muonium with ethene to produce the muon-substituted ethyl radical.
Muon Interactions with Matter 179
thermalization timescale is similar to the gas phase thermalization time but the longer timescales
are
much longer than in the gas phase.
In
general, there have been two types of theoretical (computational) modeling of muon thermal-
ization in molecular liquids. One type is based on electron number density calculations (Kosarev
and Krasnoperov, 1999; Gorelkin et al., 2000) and is similar to the kinetic theory of plasma, while
the other one is based on a stylized initial track structure comprised of many ion pairs, where
the trajectory of each charged particle is followed under the competing inuences of Coulomb
forces and Brownian diffusion (Siebbeles et al., 1999, 2000). Both types of studies are limited to
the low permittivity liquids, where Coulomb forces are long range. The later works of Siebbeles
et al. (1999, 2000) were on liquid hydrocarbons that had accessible electron mobility and electron
thermalization distances. The former works were on liquid rareed gases by Gorelkin et al. (2000)
and Kosarev and Krasnoperov (1999). The studies on liquid hydrocarbons suggested that delayed
muonium formation could account entirely for the missing polarization or lost fraction (Siebbeles
etal., 1999, 2000), P
L
= 1−P
Mu
−P
D
in the absence of free-radical formation, as opposed to the inter-
action of Mu with solvated electrons in the spur from the muon radiation track. For liquids with no
unsaturated bonds, P
R
= 0 because free radicals are not formed. For most saturated hydrogenated
materials, P
L
has been found to be similar in magnitude to P
Mu
according to Walker et al. (2003a,b).
Such theoretical studies set the stage for both experimental and more advanced theoretical inves-
tigation of muon radiolysis effects. The theoretical extension is certainly necessary, in particular a
computational study where the track structure and radiation chemical kinetics simulation would be
extended to include charge cycling, muon trapping as RMu where R is the alkyl radical, and the
effect of the R group on trapping, hot muonium formation, and hot muonium reactions.
There have been preliminary experimental tests carried out by Walker et al. (2003a,b) of the theo-
retical predictions of the radiolysis processes in hydrocarbons performed by Siebbeles et al. (1999,
2000). In the experiments that were carried out by Walker et al. (2003a,b), instead of using Equations
8.6 and 8.7, which is the most accurate way to study the diamagnetic and Mu fraction, the computer-
tted A
D
values were converted to fractional muon yields, P
D
, using P
D(x)
= (A
D(x)
/A
D(water)
) × 0.62, based
on the diamagnetic yield in water of 0.62. Although such a method does not give a denitive test of the
delayed muonium formation predicted by Siebbeles et al. (1999, 2000), the experimental results suggest
that muonium forms on a much shorter timescale than the proposed delayed mechanism (microseconds)
for a fraction of formed Mu. Certainly a more accurate measurement of both diamagnetic and muonium
fractions, a study of magnetic and electric eld dependence and the effect of scavengers on both Mu and
diamagnetic fractions, and RF-μSR investigation of liquid hydrocarbons are needed as denitive tests
of the theoretical studies by Siebbeles et al. (1999, 2000). Such studies are also useful to distinguish
between the spur and hot-atom models in liquids. Indeed, if electron–muon (or muoniated molecular
ion) recombination has a signicant role in muonium formation, the muonium amplitude in a transverse
magnetic eld could be magnetic eld-dependent. Such investigations along with the measurements of
muon polarizations as a function of electric eld, RF, and laser frequencies and delay times will be use-
ful to shed light on mechanism of muon thermalization in molecular liquids.
Two things that are agreed upon regarding the muon thermalization process in liquids between sci-
entists (radiolysis model proponents such as Roduner, 1988; Kosarev and Krasnoperov, 1999; Percival
et al., 1999; Siebbeles et al., 1999; vs. hot-atom model proponents such as Walker, 1983; Walker et al.,
2003a,b) are that (1) the initial stage of the thermalization is ionization and excitation, and (2) the charge-
exchange process (Equation 8.8) follows the established understanding of radiolysis in molecular liq-
uids, where, when a charged particle (except for electrons of energy >100MeV) is being thermalized, it
loses energy by ionizing and exciting the molecules of the medium (Salmon, 2003).
The question now is, what is the thermalization process after this initial stage? There are two
schools of thought. One suggests the nal thermalization steps are only due to hot-atom reactions
of Mu* that determine the nal diamagnetic and muonium fractions proposed by Walker (1983)
and Walker et al. (2003a,b). The other school of thought is based on ionic processes that may arise
at the end of the radiation track (Eshchenko et al., 2000, 2001, 2002, 2003; Ghandi et al., 2007).
