Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.


140 Charged Particle and Photon Interactions with Matter
where
U
is the depth of the potential for Ps
r is the radius of the potential
γ
is the surface tension
p is the pressure
E
0
(U, r) is the Ps zero-point energy in the bubble. The second term is the energy of the surface, and
the third term is the volume energy. The bubble is stable at the minimum of the total energy, E.
Therefore, the bubble size is larger at smaller surface tensions, which means that it will be larger
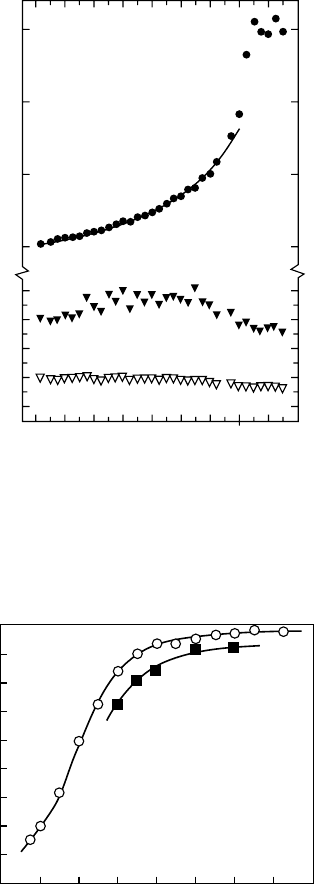
also at higher temperatures. Figure 7.3 shows the temperature dependence of o-Ps on the positron
annihilation lifetime (PAL) in neopentane (Jacobsen et al., 1982: 71). Since, at higher temperatures,
the bubble size is larger as the surface tension is lower, the lifetime of o-Ps is also longer. Above the
critical temperature, T
c
, there is no effect of the surface tension, and hence there is no temperature
dependence
on the o-Ps lifetime, that is, the bubble size.
At
lower temperatures, Ps is squeezed out from the bubble due to large surface tension. Very
interesting phenomena were observed for one liquid and some solids (Mogensen, 1994, 377;
Goworek, 2007, 318). In liquid CS
2
, Ps is squeezed out from the bubble because of the large surface
tension and large electron and positron afnities of CS
2
. The zero-point energy of Ps elevates due
to large surface tension, and then the Ps electron and positron prefer to smear out on the molecules,
even though the positron and the electron interact with each other due to Coulomb attraction. This
is called the fourth positron state. The transition from the Ps bubble state to the fourth positron state
is indicated in Figure 7.4 (Hirade, 1996: 2153). This state was conrmed by adding 1M of methanol
to CS
2
. The energy level of the fourth positron state is lower in methanol, and therefore the transition
to this state appeared at higher temperatures, as shown in Figure 7.4 (Hirade, 1996: 2153). Pressure
also
affects the bubble size and Ps formation (Kobayashi, 1992: 1869).
7.3.2 ps in SolidS
The repulsion between Ps and molecules exists even in molecular solids. If there are open volumes
like vacancies or vacancy clusters, Ps will be trapped. Moreover, positrons and electrons can be
trapped by these defects, which will affect Ps formation. Probably, Ps chemistry in solids is more
complicated
than in liquids.
100
10
1
0.1 1
Radius (nm)
Annihilation lifetime (ns)
10 100
Figure 7.2 Annihilation lifetime of the o-Ps measured in various porous materials as a function of average
pore radius. The dashed line is a calculated correlation curve. (Reprinted from Ito, K. et al., J. Phys. Chem. B,
103,
4555, 1999. With permission.)
Positron Annihilation in Radiation Chemistry 141
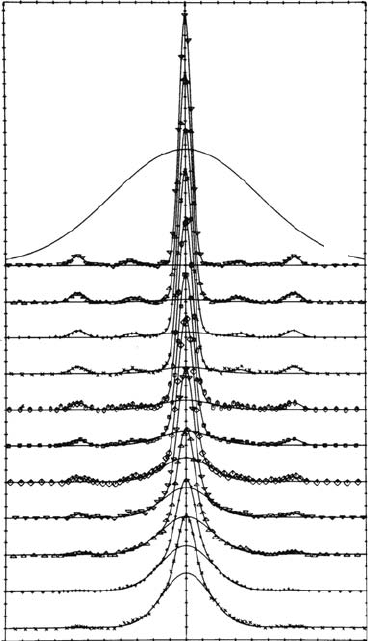
Several very interesting effects can be observed in solids. One of them is the Ps Bloch state in
crystals. It was found only in some very brittle solids. The rst experimental evidence of the Bloch
function state for Ps was found in quartz (Brandt, 1969: 522), MnF
2
(Coussot, 1970), and later for
H
2
O and D
2
O ice (Mogensen et al., 1971: 71), and in several alkali halides at LN
2
and LHe tem-
peratures (Hyodo and Takahashi, 1977: 1065; Kasai et al., 1988: 329). Ice is the only molecular
crystal in which the Ps Bloch function was found, as shown in Figure 7.5 (Mogensen and Eldrup,
1978: 85). The Bloch function state can be detected by angular correlation of annihilation radia-
tions (ACAR) (see Section 7.4.4). The curve shown in Figure 7.5 is only the component of the p-Ps
0.0
20 60 100
Temperature (°C)
Lifetime (ns)
140 180T
c
0.2
0.4
0.6
0.8
5
10
15
20
Liquid neopentane
τ
3
τ
2
τ
1
Figure 7.3 Temperature dependence of the positron lifetimes in liquid neopentane as obtained fromlife-
time spectra resolved into three exponential components. T
c
is the critical temperature. (Reprint from Jacobsen,
F.M.
et al., Chem. Phys., 69, 71, 1982. With permission.)
0
–120 –100 –80 –60 –40
Temperature (°C)
o-Ps intensity (%)
–20 0 20 40
5
10
15
20
25
30
35
40
45
Figure 7.4 Intensity of the longest-lifetime component, that is, o-Ps, versus measuring temperature.
Open circles indicate the results for pure CS
2
and lled squares are for 1 M methanol/CS
2
.
142 Charged Particle and Photon Interactions with Matter
intrinsic annihilation, that is, the broad components of free positron and o-Ps pick-off annihilation
are subtracted. Another broad component appears at higher temperatures. It is the component of the
intrinsic
annihilation of p-Ps localized on vacancies.
Irradiated
ice was also studied using positron techniques (Eldrup, 1976: 5283). Figure 7.6
shows the measured temperature dependence on PAL for irradiated ice at −196°C. In this case,
the lifetime spectra were analyzed with four lifetime components, because there were two differ-
ent vacancies that give two different lifetimes of the o-Ps pick-off annihilation. One of these had
τ
3
= 1.20 ns that was considered to be the o-Ps pick-off annihilation in mono-vacancies. The other
had τ
4
, that is, the o-Ps pick-off annihilation in vacancy clusters. The change of τ
4
can provide the
information of change of the cluster size. I
1
is the intensity of p-Ps, I
3
is the intensity of the pick-
off annihilation of o-Ps in mono-vacancies, and I
4
is the intensity of the pick-off annihilation of
o-Ps in vacancy clusters. There was inhibition of Ps formation below −180°C, which was caused
by hydroxyl radicals formed by irradiation. τ
4
was initially about 2.3ns, probably the lifetime of
the divacancies, and it increased by elevating the temperature. This indicates the increasing size
of the clusters. Then I
3
and I
4
decreased simultaneously because of the decrease in the number
density of vacancy clusters. Above 130°C, the concentration of clusters became so low that Ps
trapping could not be detected.
–10
D
2
O
H
2
O
H
2
O
D
2
O
D
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Broad component
at –181°C
Single crystals
of ice
[0001]-direction
Counts (arb. units)
D
2
O
D
2
O
–181°C
–96°C
–80°C
–69°C
–52°C
–35°C
–26°C
–16°C
–1°C
+2°C
–5 0
Angle (mrad)
5 10
–181°C
Figure 7.5 Temperature dependence of the ACAR spectra for single crystals of H
2
O ice oriented
along the a-axis. The broad component was subtracted. (Reprinted from Mogensen, O.E. and Eldrup, M.,
Positronium Bloch function, and trapping of positronium in vacancies, in ice, Risø Report No. 366, 1977.
With permission.)
Positron Annihilation in Radiation Chemistry 143
7.4 experimental teChniQues
Positron methods can be one of the important tools to study radiation chemistry, as mentioned
above, because Ps formation is the result of the fast spur reactions and very short lifetime of posi-
trons in condensed matter. Hence, positron annihilation methods are very useful for radiation chem-
istry. Therefore, only the methods related to the positron annihilation techniques used for the study
of
radiation chemistry will be discussed here.
–200
0
4
8
12
16
20
24
28
0
4
8
12
16
20
24
0
2
4
6
0.0
4.0
Lifetime (ns)Relative intensity (%)
8.0
12.0
80 100 120 140
τ
3
=1.20 ns
τ
4
I
4
I
3
I
1
160
(K)
–180 –160
Temperature (°C)
–140 –120 –100
Figure 7.6 Lifetime results for pure H
2
O ice gamma-irradiated at −196°C and measured as a func-
tion of increasing temperature. (○) Polycrystalline samples (11 Mrad), (△) single crystals (4.1Mrad), and
(□) another single crystal (11 Mrad). (Reprinted from Eldrup, M., J. Chem. Phys., 64, 5283, 1976. With
permission.)
144 Charged Particle and Photon Interactions with Matter
7.4.1 poSitron Source
A positron is the antiparticle of an electron. We need to employ some special methods to obtain
positrons. The most convenient method is the use of radioisotopes. There are some radioisotopes
that emit positrons. A list of positron emitters is given in Table 7.1.
22
Na is the most common radio-
isotope. There are some reasons why it is often used. A γ-ray of 1.28MeV emitted almost simultane-
ously with a positron is one of the reasons. A γ-ray of 1.28MeV is very convenient because it gives
information of the time of the positron emission, and hence the time interval between the 1.28 MeV
γ-ray and 511keV annihilation γ-rays gives information of the lifetime of the positron. Another
reason why
22
Na is often used is its longer half-life of 2.6 years. It is possible to use the same source
for
several years.
The
maximum energy of positrons emitted from
22
Na is 540keV. Radioisotopes are usually
shielded by polymer or metal lms. An
22
Na source of about 400kBq shielded by Kapton or Mylar
lms is often used for PAL, Doppler broadening (DB), and age-momentum correlation (AMOC)
measurements. Kapton is a polyimide and has no Ps formation. It means that there is just one anni-
hilation-lifetime component of the free positron that is about 380ps. It is easy to subtract the PAL
component from the measured spectra. Kapton lms having a thickness of about 7.5μm areoften
used. It is necessary to know how many positrons will annihilate in the lm to subtract the annihila-
tion
component in
the
source lms from
the
spectra. It is possible to
estimate
this by calculation or
experiment. For example, the lifetime in well-annealed metals has just one short-lifetime component
and the fraction of positron annihilation in the lm can be estimated. There seems to be the effect
of back-scattered positrons, that is, the fraction of positron annihilation in the lm will be larger for
heavier samples. It is difcult to estimate it experimentally for samples with a free-positron lifetime
of about 400 ps. For these samples, it is necessary to assume the fraction of positron annihilation in
the
lms. Usually, a fraction of 10%–12% is used.
Recently,
it is possible to buy sources for these methods. A shielded
22
Na source of less than
1MBq
can be used in laboratories that are not specially installed for handling radioisotopes.
For
angular correlation measurements or mono-energetic positron beams, stronger shielded
positron sources (400–4000 MBq) are used. Pair production by high-energy radiation is also
used for creating a large amount of positrons for intense positron beams (Suzuki et al., 2000: 603;
1991: L532).
7.4.2 poSitron annihilation lifetiMe MeaSureMent
The most important and common positron annihilation method is the PAL measurement. For PAL,
22
Na is the most common positron source, because it emits a positron and a prompt γ-ray of 1.27MeV,
almost simultaneously. The dominant decay scheme is indicated in Figure 7.7. The detection of
table 7.1
Characteristics
of s
ome
Common p
ositron-emitting
r
adioisotopes
radioisotopes Fraction of e
+
half lifetime maximum energy (mev) prompt γ energy (mev)
C-11 99% 20months 0.97 —
Na-22 90% 2.7
years 0.54 1.28
Ti-44 (as Sc-44) 88% 47 years 1.47 1.16
Ni-57 46% 36
h 0.40 1.4
Co-58 15% 71
days 0.48 0.81
Cu-64 19% 12.8
h 0.66 —
Zn-65 1.7% 245
days 0.33 —
Ga-68 (from Ge-68) 88% 275 days 0.98 —

Positron Annihilation in Radiation Chemistry 145
a1.27MeV γ-ray indicates the emission of a positron. The annihilation of a positron with an electron
can be observed by the detection of one or two annihilation γ-rays. In condensed mater, the energy
of the annihilation γ-ray is about 511keV, as almost all of the annihilations give two γ-rays. The
detection of a 511keV γ-ray indicates the annihilation of a positron. Therefore, the time interval of a
coincident event of two γ-rays, 1.27MeV and 511keV, will be the measured PAL.
This is the gamma–gamma coincidence method. There is one more method that can be applied
for measuring the PAL. It is the beta–gamma coincidence method. A positron injecting into a sam-
ple is observed by a beta-particle detector. If the energy of the positron is very high, scintillators can
be used as the detector (Castellaz et al., 1996: 457). If radioisotopes are used, thin photodiodes
can be used as the detector (Chalermkarnnon et al., 2002: 1004). There are some advantages of
thismethod. One is that a prompt γ-ray is not necessary. The second advantage is a lower random
coincidence background on the PAL spectra. However, there are some disadvantages too. One of
them is the difculty in obtaining a good time resolution because of the time-of-ight of the posi-
tron
coming into the sample (Chalermkarnnon et al., 2002: 1004).
The
most common PAL setup is shown in Figure 7.8. Fast scintillation detectors should be used to
obtain a good time resolution. Therefore, the scintillators used should be BaF
2
or fast plastic scintil-
lators. The BaF
2
scintillators have larger efciencies but a slower luminescence component. When
the counting rate is high, piling up of fast and slow luminescence components will shift theenergy
spectra; therefore, the energy windows selected by the single-channel analyzers (SCAs) of the
2+ 1.2746
β
+
90%
EC 10%
β
+
0.05%
3+
3 ps
0+
10
Ne
22
11
Na
22
2.60y
Figure 7.7 The dominant decay scheme of
22
Na.
MCACF/SCA CF/SCA
Start
Stop
Source/sample
sandwich
ns
delay
TAC
Figure 7.8 The most common PAL setup.
146 Charged Particle and Photon Interactions with Matter
constant fraction (CF) cannot detect the objective signals. The photomultiplier (PM) tubes need
quartz windows because the fast luminescence component is ultraviolet light. The PM also should
have a fast rise time of the signal. The PM tube used most often is H3378-51 (Hamamatsu) and has a
rise time of 0.7ns. The most important module for PAL is a CF discriminator, which is supplied by
many companies. The signals from PM tubes come into CF/SCA directly without inserting preamps.
These signals should be large enough to be selected by SCA. Then, two sets of detectors and CF/SCA
supply the timing signals for positron birth and death. The time interval should be converted into
amplitude of the output using a time-to-amplitude converter (TAC). Then the lifetime spectra can
be viewed using a multi-channel analyzer. This is the conventional PAL measurement technique.
Some important information is introduced here. It is most important to reject the piling up of the
1.27MeV γ-ray and one of the annihilation γ-rays. The geometry one can obtain for a good count-
ing rate is to place the sample and the source assembly in the middle of the detectors. One of the
detectors is used for the 1.27MeV γ-ray and the other is used for one of the annihilation γ-rays. One
should not forget that there is one more annihilation γ-ray that will go in the opposite direction of
the other annihilation γ-ray. It means that it will enter the detector for the 1.27MeV γ-ray, and then
two signals will easily pile up. This will affect the lifetime spectra, and the articial short-lifetime
component will easily appear. It is also important to have enough knowledge of the interaction
between γ-rays and the materials. When one prefers to have a large counting rate, one tends to apply
the wider energy windows. If wider energy windows are applied for the 1.27 MeV γ-ray including
the Compton area, scattered γ-rays can enter the detector for the annihilation γ-rays. It means that
articial counts appear near time zero on the lifetime spectra.
Recently, it has become possible to use digital storage oscilloscopes (DSOs) to store all of the
waves from detectors. One of the advantages of applying DSOs is that several analyses with differ-
ent parameters can be performed. For example, it is possible to select the wave data by adjusting
energy windows. Moreover, when one nds something strange on the spectra, it may be possible
to nd the cause. The biggest advantage is that the triple coincidence measurement is quite easy.
This measurement has two stop detectors for both of the annihilation γ-rays. It provides better time
resolution
(Saito et al., 2002: 612).
7.4.3 doppler broadening MeaSureMent
The peak width of the whole absorption peak of annihilation γ-rays observed by Ge detectors is
wider than those of the other γ-rays. It is caused by the Doppler shift by the momenta of an electron
and a positron just before the annihilation. It means that it is possible to obtain information of elec-
tron and/or positron momenta in the materials. p-Ps annihilation is intrinsic, and the momenta of the
electron and the positron cancel out each other. And hence, the energy of the annihilation γ-rays is
very close to 511keV, which is equivalent to the rest mass of an electron (or a positron). On the other
hand, o-Ps and free positrons annihilate by picking off one of the electrons from the surrounding
molecules. This means that the energy distribution of the annihilation γ-rays is wider because of the
Doppler shift. A so-called S parameter is often used to indicate the annihilation γ-ray energy distri-
bution. The value of S is the ratio between the counts appearing in a xed central area and those in
the entire peak area. The narrower the peak, the larger is the S value.
With spin conversion reactions, p-Ps annihilation increases and the distribution of the annihila-
tion γ-ray energy becomes narrower. These reactions are difcult to detect by just the lifetime mea-
surement. There are many researches carried out using the lifetime and Doppler shift measurements
(Komuro
et al., 2007: 330).
7.4.4 age–MoMentuM correlation MeaSureMent
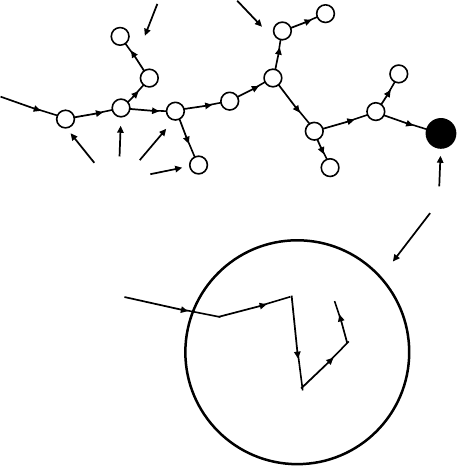
AMOC is the combination of PAL and DB, as shown in Figure 7.9. The most important use of
AMOC in radiation chemistry is in determining the time-resolved DB. Fortunately, there are two
Positron Annihilation in Radiation Chemistry 147
annihilation γ-rays in many cases. One of them is used for PAL and the other is used for DB, and
hence the DB measurement with the annihilation time stamp is possible. Afterward, it is possible
to construct a DB spectrum at the specic range of the positron annihilation time stamps. As men-
tioned above, DB is an effective tool for the investigation of the annihilation from p-Ps. This method
can provide the information of the positron annihilation process, for example, the Ps formation
process and spin conversion reactions (Castellaz et al., 1996: 457; Komuro et al., 2007: 330). The
details
are given in Section 7.6.7.
7.4.5 other techniQueS
ACAR is also a very important method. It measures the angular correlation between two annihila-
tion γ-rays. The shift from 180° can indicate the shift on the sum of the momenta of the annihilated
electron and the annihilated positron. The obtained information is almost the same as DB, but the
resolution is much better. On the other hand, it is necessary to use much stronger sources so that
the positron irradiation effect is very large. Therefore, it may be difcult to apply ACAR in studies
of polymers.
The Ps Bloch state in crystals can be observed as shown in Figure 7.5. Many liquids are measured,
and the ACAR measurement could give very important information on the positron state in liquids.
Unfortunately,
now there may be no ACAR method that can be applied for liquid measurements.
Positrons
annihilate with one of the electrons from molecules or atoms, which means that a
positron can be used to create cations. This phenomenon was used in some methods. One of them is
the
Auger electron measurement. Usually,
Auger electrons
are
obtained
by the ionization produced
by electron irradiation. However, the background is usually very large, because many secondary
electrons also enter the detectors. Nevertheless, there exists no background for the positron Auger
method, because low-energy positrons are used instead of high-energy electrons. Sometimes oxy-
gen exists as an impurity on the surface, but it can be detected effectively by the positron Auger
method
due to its large positron afnity (Ohdaira et al., 1997: 177).
100,000
10,000
Counts
1,000
100
10
1
490
511
Energy (keV)
Time (ns)
532
18
16
14
12
10
8
6
4
2
0
–2
Figure 7.9 AMOC spectrum of water.
148 Charged Particle and Photon Interactions with Matter
7.5 positronium Formation
If a positron is placed in a material, Ps does not form. The binding energy of Ps is 6.8eV in vacuum,
which is smaller than the ionization potentials of molecules or atoms. This means that positrons in
materials cannot pick off electrons to form Ps. A fast Ps formation model, the so-called Ore model,
was proposed by Ore in 1949 (Ore, 1949). As mentioned above, extra energy is necessary to pick off
an electron from molecules or atoms; therefore, the idea of the Ore model is to supply the necessary
energy from the kinetic energy of the positron. This model can explain the Ps formation in rare gases.
However, this model cannot explain the Ps formation in condensed materials. In 1974, Mogensen
proposed the spur model (Mogensen, 1974: 998) that could explain the Ps formation in condensed
materials. In the case of the Ore model, extra kinetic energy from the positrons can make Ps forma-
tion possible, while in the case of the spur model, the production of free or quasi-free electrons makes
Ps formation possible. Injected positrons will make spurs on the track of positrons and will be ther-
malized at the end of the track. The structure is similar to that indicated in Figure 7.10 (Mogensen,
1974: 998). Therefore, it is quite possible to have free electrons near the thermalized positrons, which
allows Ps formation. This model could well explain qualitatively the experimental results.
However, in 1980s, a very large enhancement of Ps formation at low temperatures was reported
for many materials (Kindl and Reiter, 1987: 707). This enhancement could not be explained by the
spur model, which was the main reason why some people did not accept this model. Until almost
theend of the twentieth century, this Ps formation enhancement had not been explained, and so,
some people used other models. One of these models was called the free volume model of Ps forma-
tion, which was often applied to discuss polymer physics (Jean, 1993: 569). The basic idea of this
model was as follows. The measured o-Ps lifetime was always the longest and clearly identied by
the PAL measurement. The long o-Ps lifetime indicates that there were empty spaces for o-Ps in the
materials (see Figure 7.2). Hence, something like a hole (which does not mean the positively charged
species) was needed for Ps formation, and thus, more holes could produce more Ps. Some people
believed that the total free volume could be obtained by the free volume hole size observed by the
o-Ps lifetime (see Figure 7.2) and the number of holes observed by the Ps formation probability.
Secondary tracks
Spurs
Terminal spur
e
–
e
–
e
–
e
–
e
+
M
+
M
+
M
+
M
+
Figure 7.10 Structure of terminal spur of the positron track.
Positron Annihilation in Radiation Chemistry 149
The measured slow enhancements of Ps formation at low temperatures were used for the study of
polymer physics. It was believed that the slow enhancement observed at low temperatures showed
the
physical change of polymers that could not be observed by other methods.
Ps
can be in the delocalized state in crystals. It means that the empty spaces are not needed for
Ps formation. There is no Ps formation in some polymers, such as Kapton, which is a polyimide,
even though there must exist some free volume. Indeed, any reasonable explanation has not been
obtained
by the free volume model until now.
In
1998, a new explanation of the appearance of Ps formation enhancement at low tempera-
tures was given by Hirade (Hirade et al., 1998: 89; 2000: 465; Wang, 1998: 4654). The Ps for-
mation enhancement, observed by the PAL measurement at low temperatures, appeared slowly.
He explained it by the accumulation of shallowly localized electrons, such as trapped electrons.
However, shallowly localized electrons are stable and live for a long time at low temperatures
because the molecular motions are frozen. Positrons in materials cannot pick off electrons from
molecules or atoms to form Ps, as mentioned above, because of the larger ionization potentials of
molecules or atoms than the Ps binding energy. The binding energy of shallowly localized electrons
is usually 0.5–3eV, which is smaller than the Ps binding energy. It means that positrons can pick off
shallowly localized electrons without the need of extra energy to form Ps. Thus, Ps can form just by
placing
positrons in the materials where many shallowly localized electrons exist.
This
Ps formation process proposed by Hirade can explain many phenomena, such as Ps formation
quenching by visible light (Hirade et al., 1998: 89; 2000: 465), relationship between the density of the
shallowly localized electrons and Ps formation enhancement (Hirade et al., 2000: 465), and delayed
Ps formation caused by the diffusion of positrons (Suzuki et al., 2003: 647; Hirade et al., 2007: 3714).
The free volume model often used for Ps formation at low temperatures cannot explain the Ps
formation quenching by visible light, while the Ps formation process proposed by Hirade and the
spur model proposed by Mogensen could explain Ps formation at any temperatures. It was accepted
that radiation chemistry processes are very important for Ps formation (Hirade, 2007: 84).
7.6 radiation Chemistry studies by positron annihilation
7.6.1 coMpariSon of the yieldS of hydrated electronS obServed by pulSe radiolySiS
Positron annihilation methods can provide some information regarding fast reactions in spurs, as
pulse radiolysis experiments can. Duplatre and Jonah tried to compare the positron and pulse radiol-
ysis experiments. The electron scavenger effects observed in aqueous solutions are studied for both
these methods. The inhibition of hydrated electron formation and Ps formation showed very similar
tendencies, as shown in Figure 7.11, and hence it was claried that the precursor of Ps was mainly
free electrons or quasi-free electrons (Duplatre, 1985: 557). As the Ps formation time is about 1ps in
common materials, the yields of Ps formation will give information of free or quasi-free electrons
similarly as the yields of hydrated electrons observed by pulse radiolysis experiments. The most
important detection method applied for pulse radiolysis is light absorption, which can make very
fast experiments possible. This implies that only the species that absorb light can be detected by the
fast pulse radiolysis experiments. On the other hand, Ps formation yields can beobtained as the
longest-lifetime component by the PAL measurement, which means that the precursor of Ps, that is,
free
or quasi-free electrons, can be detected by positrons.
7.6.2 coMpariSon of electron Mobility experiMentS
There is one more advantage of applying positron methods, which is the short lifetime of positrons.
In condensed materials, the longest lifetime is given by o-Ps, which is usually less than 4–5ns. This
means that small amounts of impurities do not affect the yields of Ps very much. Therefore, it is not
necessary to have very pure samples. In some cases, very pure samples are needed, for example
