Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

150 Charged Particle and Photon Interactions with Matter
for the electron mobility measurements inradiation chemistry studies. Carbon disulde, CS
2
, has
a high electron afnity. Therefore, it was expected that the electron mobility in CS
2
should not be
high. In the initial experiments, a small mobility of electrons was obtained, as expected (Gee and
Freeman, 1989: 5399). However, very high Ps formation yields were obtained in CS
2
. The CS
2
con-
centration dependence on Ps formation was measured in many nonpolar liquids, as shown in Figure
7.12 (Jansen and Mogensen, 1977: 75). Ps formation was inhibited by the addition of a small amount
of CS
2
, as observed for many other electron scavengers. However, Ps formation enhancement was
observed at higher concentration. The only reasonable explanation for this phenomenon is the high
electron mobility at higher concentration of CS
2
. It was completely opposite of the results obtained
by the electron mobility measurement; it was then measured again, and high electron mobility was
nally
obtained (Gee and Freeman, 1989: 5399).
7.6.3 electron therMalization in water
Jonah tried to investigate the electron thermalization in water by pulse radiolysis experiments (Jonah
and Chernovitz, 1990: 935). The decay curves of the absorption of hydrated electrons in water were
measured as a function of the concentration of heavy water, as shown in Figure 7.13. The heavy
water concentration dependence of decay rates of hydrated electrons shows a linear relation with
the concentration of water with two Ds, as indicated in Figure 7.14. Therefore, it was mentioned that
H
2
O and HOD showed similar effects on the electron thermalization.
Ps formation is caused by free or quasi-free electrons, as mentioned in Section 7.6.1. Ps forma-
tion probability will increase with the decreasing distance of electron–positron pairs, because the
competitive phenomenon of Ps formation is the hydration in water. Therefore, Ps formation will be
capable of providing some information regarding the thermalization distance. Figure 7.15 shows
that Ps formation yields showed linear dependence on the concentration of D, and not on the water
0.2
15
25
23
28
29
13
8
20
1
21
6
22
18
26
24
11
16
19
5
7
12
10
9
4
3
14
17
27
2
0.5
1
2
k
Ps
(M
–1
)
5
10
20
0.5 1 2
1/C
37
(M
–1
)
5 10 20
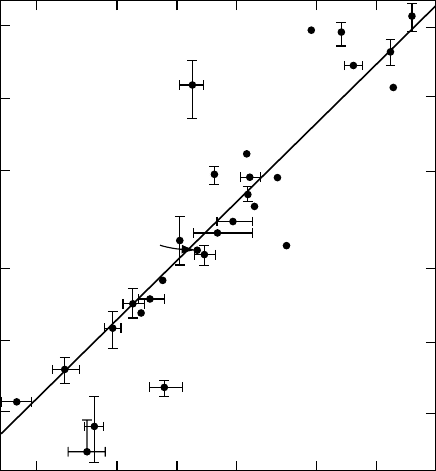
Figure 7.11 Plot of 1/C
37
(M
−1
) for the scavengers versus the Ps inhibition constant, k
Ps
(M
−1
). (Reprinted
from
Duplatre, G. and Jonah, C.D., Radiat. Phys. Chem., 24(5/6), 557, 1985. With permission.)
Positron Annihilation in Radiation Chemistry 151
0
25
30
35
40
Intensity (%)
45
50
55
0.25 0.50
Volume fraction
TMS
Neopentane
Isooctane
n-Hexane
n-Tetradecane
0.75 1.0
Figure 7.12 Intensity, I
3
, of the long-lived component versus volume fraction of carbon disulde. (Reprinted
from Jansen, P. and Mogensen, O.E., Chem. Phys., 25, 75, 1977. With permission.)
1.0
0.9
Fraction of initial OD
0.8
0 1 2
Time (ns)
a, b
c
d
e
f
3
Figure 7.13 Decay of the hydrated electron at 600nm in different mixtures of H
2
O and D
2
O: (a) 100 mol%
H
2
O, (b) 60 mol% H
2
O, (c) 50mol% H
2
O, (d) 40mol% H
2
O, (e) 20 mol% H
2
O, and (f) 0mol% H
2
O. (Reproduced
from
Jonah, C.D. and Chernovitz, A.C., Can. J. Phys., 68, 935, 1990.)
1.0
0.8
0.6
0.4
0.2
0
0 0.2 0.4
Fraction of water molecules with two Ds
Change in amount of decay
0.6 0.8
1.0
Figure 7.14 Change in the amount of decay at 3.4ns versus fraction of D
2
O molecules in the solution.
(Reprinted
from Jonah, C.D. and Chernovitz, A.C., Can. J. Phys., 68, 935, 1990. With
permission.)
152 Charged Particle and Photon Interactions with Matter
molecules with two Ds (Hirade, 1995: 675). This shows that the thermalization distance is larger
in
heavy water. However, it does not show that H
2
O and HOD have the same effect on the thermal-
ization distance. Crowell et al. indicated that the diffusion coefcients of reactive species in D
2
O
inuenced the reactions of the hydrated electrons, and that the electron thermalization distance in
D
2
O did not need to be greater than that in H
2
O (Crowell and Bartels, 1996: 17713). Further studies
are
needed to understand the thermalization of electrons in water.
7.6.4 electric field effect (blob Model of ps forMation)
The application of external electric elds can quench Ps formation, as shown in Figure 7.16 (Stepanov
et al., 2005: 054205). Stepanov explained the electric eld dependence on Ps formation using the
modied spur model, that is, the blob model. He introduced the distribution of excess electrons and
positrons in the terminal spur of the positron track, because the number of species in the terminal
spur is large. Probably, there are more than 20–30 excess electrons. He employed the method of
Debye screening of the positron in the terminal spur. He then divided these positrans into two
positron species. One of these is thermalized in the excess electron blob, and the other is out of that
blob, as indicated in Figure 7.17 (Stepanov et al., 2005: 054205). In the blob, there are many excess
28
27
26
25
24
23
0 20 40
Concentration of D (%)
o-Ps intensity (%)
60 80
100
Figure 7.15 D concentration dependence on o-Ps intensity.
0
5
10
15
20
25
30
Chlorinated polyethylene
–(CH
2–x
Cl
x
)–
x = 0
x = 0.031
x = 0.055
x = 0.096
x = 0.209
100% EVA
60% EVA
24% EVA
0
15
(b)
(a)
20
25
30
10 20 30 40
D (kV/cm)
50 60 70
Pure
polyethylene
(0% EVA)
10 20
D (kV/cm)
I
3
, %
I
3,
%
30 40
Figure 7.16 o-Ps intensity versus electric eld in chlorinated polyethylene as indicated in (a) and polyethy-
lene (PE) and ethylene vinyl acetate copolymer (EVA) as indicated (b). Lines are ts obtained by the model
explained in the text. (Reprinted from Stepanov, S.V. et al., Phys. Rev. B, 72, 054205, 2005. With permission.)

Positron Annihilation in Radiation Chemistry 153
electrons and the external electric eld is not effective. Stepanov succeeded in explaining many
experimental results using the blob model, as shown in Figure 7.16 (Stepanov et al., 2005: 054205).
7.6.5 Slow ps forMation in SpurS (young-age broadening effectS)
A very interesting attempt has been made to clarify the Ps formation mechanism. The Stuttgart
group produced many experimental results with the use of AMOC. They showed that there exists
young-age broadening on S(t) in many materials (Stoll et al., 1995: 17). One example of S(t) without
the young-age broadening is shown as lled circles in Figure 7.18 (Komuro et al., 2007: 330). At
the positron age of zero, large S(t) appeared. It was caused by the p-Ps intrinsic annihilation. One
of the S(t) observed by the Stuttgart group is shown in Figure 7.19 (Stoll et al., 1995: 17). At very
young ages, smaller S(t) appeared. Smaller S(t) means that the energy distribution is broader. This
is the reason why it is called “young-age broadening.” They interpreted that young-age broadening
e
in
+
E
e
out
+
+
+
+
+
+
+
–
–
–
–
–
–
e
+
blob
W
S
Effective potential well for e
+
Figure 7.17 Spatial distribution of the positron density at the Ps formation stage in the presence of an exter-
nal electric eld, E. e
in
+
is the density of the positrons bounded within the blob and not perturbed by E. The
other part, e
out
+
, is biased by the eld. (Reprinted from Stepanov, S.V. et al., Phys. Rev. B, 72, 054205, 2005.)
0.6
0.55
0.5
0.45
0 0.5 1
Positron age t (ns)
S parameter
1.5 2 2.5 3
Figure 7.18 S(t) curves for a synthetic fused quartz. The solid lines were simulated by assuming that Ps is
formed at the time of positron injection. Open circles were measured under no electric elds, and lled circles
were measured under an electric eld of 48kV/cm. (Reprint from Komuro, Y. et al., Radiat. Phys. Chem., 76,
330,
2007. With permission.)
154 Charged Particle and Photon Interactions with Matter
was caused by the incompletely thermalized p-Ps (Stoll et al., 1995: 17). They also claimed that it is
possible to observe the process of Ps thermalization by young-age broadening. On the other hand,
Komuro interpreted that young-age broadening is caused by the slow Ps formation in the terminal
spur. For the electron–positron pair that acquires a larger initial distance after the thermalization,
Ps
formation time is slower. If there is slow Ps formation, the fraction of annihilation from the free
positron is large near the positron age of zero; therefore, young-age broadening appears. Komuro
applied a weak external electric eld to test the model. The young-age broadening, if caused by slow
Ps formation, will disappear because a weak external electric eld will affect the slow Ps forma-
tion. She used very pure fused quartz to observe the young-age broadening. The measured S(t) is
shown in Figure 7.18 (Komuro et al., 2007: 330). Open circles showed probably the rst young-age
broadening except for the results form the Stuttgart group. Although the effect was very small, the
external electric eld quenched the young-age broadening as the lled circles indicated (Komuro
et
al., 2007: 330).
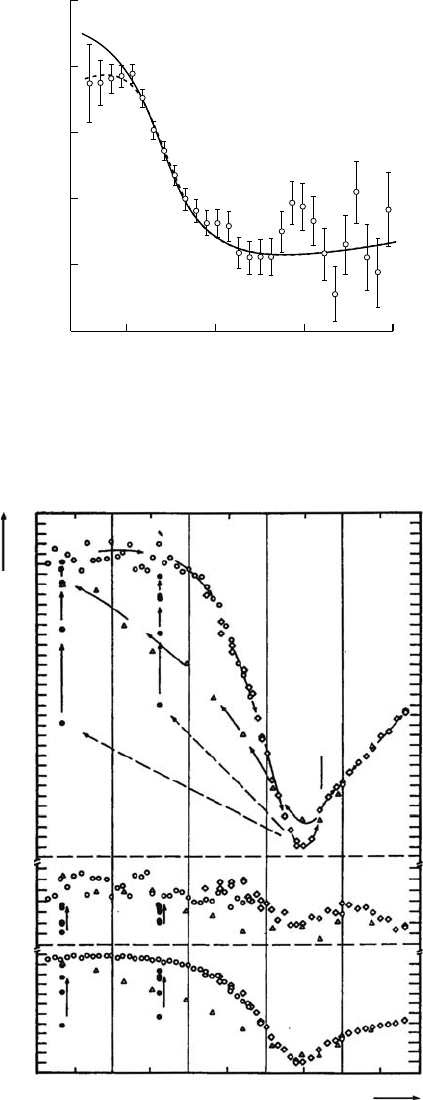
The
slow Ps formation reactions were obtained for ionic liquids. The result for N,N,N-trimethyl-
N-propylammonium bis(triuoromethanesulfonyl)imide (TMPA-TFSI) is shown in Figure 7.20
(Hirade, 2009b: 232). The solid line indicates the S(t) curve assuming Ps formation at the positron
age of zero. The dashed line indicates the S(t) curve with an electron solvation time of about 30ps. It
is a clear indication of the long lifetime of free or quasi-free electrons in ionic liquids, as indicated
by
Katoh (Katoh et al., 2007: 4770).
7.6.6 ps forMation with trapped electronS
As explained in Section 7.5, the large and slow enhancements of Ps formation at low temperatures
are caused by the accumulation of long-lived shallowly localized electrons, such as trapped elec-
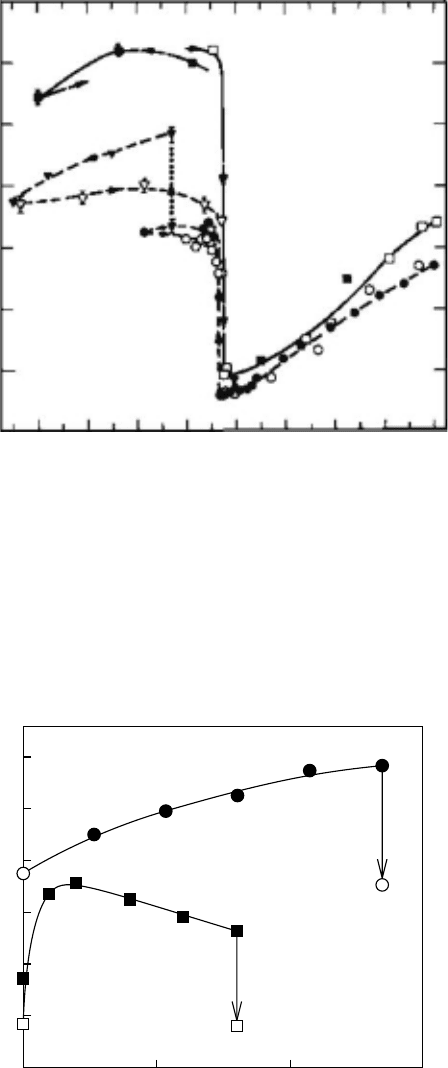
trons, by the irradiation of injected positrons. The most famous experimental result was observed
for branched polyethylene by Kindle, as shown in Figure 7.21, in 1987 (Kindle 1987: 707). As
explained in Section 7.5, some people believed that it was a special phenomenon for polymers.
However, the details of the mechanism of the slow enhancement of Ps formation were explained by
the accumulation of trapped electrons in 1998, and it was not a special phenomenon for polymers
(Hirade et al., 1998: 89; 2000: 465; Wang et al., 1998: 4654). Indeed the slow enhancement of Ps
formation in cyclohexane at low temperatures had been measured by Eldrup in 1980 (Eldrup et al.,
1980: 175). This was the rst experimental result that showed a slow Ps enhancement caused by the
accumulation of trapped electrons, as indicated in Figure 7.22 (Hirade, 2003: 375).
0.62
Fused quartz
0.60
0.58
0.56
0.54
0.52
0.50
0.48
0.0 0.5 1.0 1.5
Positron age (ns)
S
t
parameter
2.0 2.5 3.0 3.5 4.0
Figure 7.19 Annihilation γ-rays line-shape parameter, S
t
, measured at room temperature for fused quartz.
(Reprinted
from Stoll, H. et al., Appl. Surf. Sci., 85, 17, 1995. With permission.)
Positron Annihilation in Radiation Chemistry 155
0.54
0.53
0.52
0.51
0.5
0.49
0 0.5
Positron age (ns)
S(t)
1 1.5
Figure 7.20 S(t) curve for N,N,N-trimethyl-N-propylammonium TMPA-TFSI. Solid lines are obtained
by an assumption that Ps forms at the positron age = 0. Dashed lines are obtained by slow reactions in ionic
liquids.
(Reprinted from Hirade, T., Mater. Sci. Forum, 607, 232, 2009b. With permission.)
45
40
35
I (%)
30
25
20
15
12
10
8
16
12
8
–200 –150 –100 –50 0
T (°C)
I
1
I
3
I
4
T
g
50
Figure 7.21 The relative intensities obtained by fourth-component analysis as a function of temperature.
I
4
indicates the relative intensity of o-Ps. Open triangles were measured with faster cooling rates than open
circles. Filled circles were measured with xed temperatures. (Reproduced from Kindl, P. and Reiter, G.,
Phys. Status Soldii
(a), 104, 707, 1987. With permission.)
156 Charged Particle and Photon Interactions with Matter
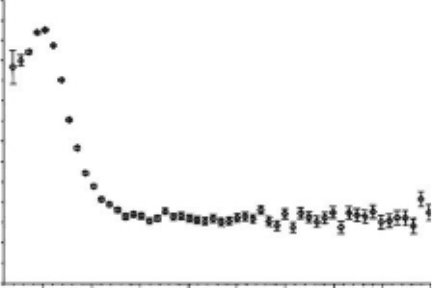
Some experiments exhibited Ps formation by trapped electrons. At rst, the visible light effect
was used. In this case, the Ps formation was inhibited immediately, because the visible light
quenched the trapped electrons. This effect was tested by Hirade at rst, and subsequently many
other investigators obtained the same effect in many materials, as shown in Figure 7.23 (Hirade
etal.,
2000: 465). It is obtained by the use of
60
Co γ-irradiation.
45
40
35
30
25
I
΄
3
20
15
10
–160 –140 –120 –100
Temperature (°C)
Intensity (%)
–80 –60 –40 –20 0
Figure 7.22 o-Ps intensity, I′
3
, as a function of temperature. The dotted line indicates the isothermal
measurement.
(Reproduced from Eldrup, M. et al., Faraday Discuss. Chem. Soc., 69, 175, 1980.)
35
30
25
20
15
10
5
0 5
Dose (kGy)
I
3
(%)
10 15
Figure 7.23 Absorbed dose dependence of the intensity of the longest-lifetime component, I
3
, in PE (■, in
darkness; □, under visible light) and PMMA6N (●, in darkness; ○, under visible light) at 77K. (Reprinted from
Hirade, T.
et al., Radiat. Phys. Chem., 58, 465, 2000. With permission.)
Positron Annihilation in Radiation Chemistry 157
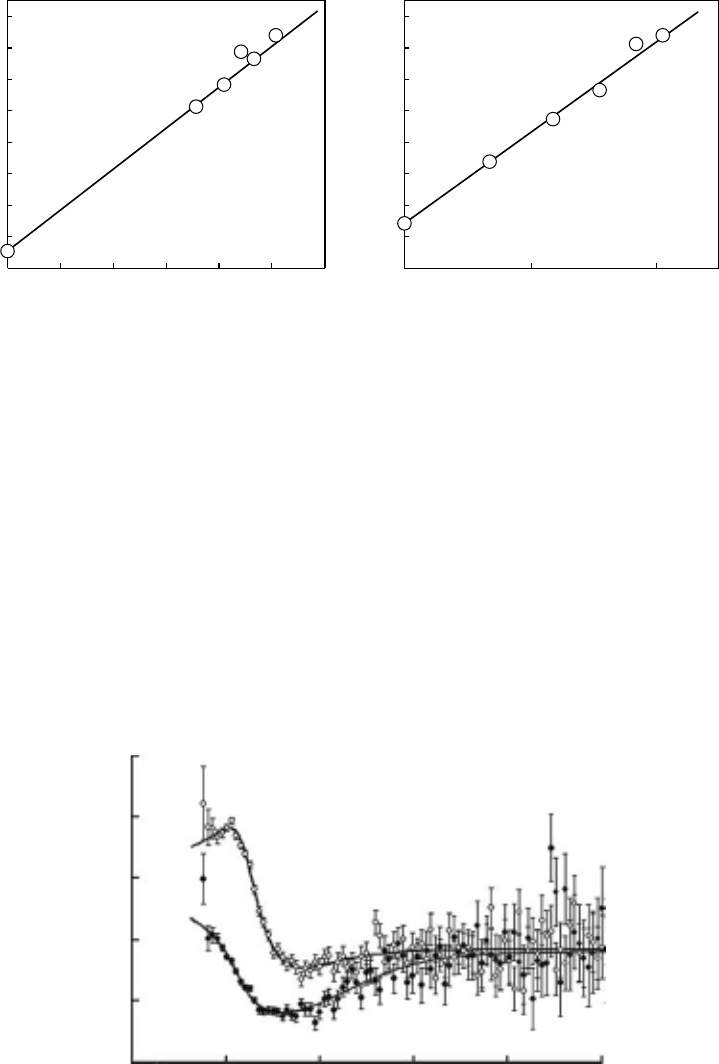
The trapped electron density dependence of Ps formation enhancement was also measured by
Hirade, as shown in Figure 7.24 (Hirade et al., 2000: 465). A combination of EPR and PAL mea-
surements gives linear relations in polyethylene (PE) and polymethyl methacrylate (PMMA). This is
the only result of measuring the Ps formation enhancements caused by the irradiation of
60
Co γ-rays.
Ps formed by the spur process and that formed by trapped electrons could be separated by observ-
ing the formation time. The Ps formation by the spur process occurred within about 1ps, and that by
trapped electrons was delayed because positron diffusion was needed. p-Ps annihilation gives a nar-
rower distribution of the annihilation γ-ray, as explained in Section 7.4.2, and a fast annihilation life-
time of 125ps. The S(t) curve observed for PE at 20K is indicated in Figure 7.25 (Hirade et al., 2007:
3714). While a simple decrement of S(t) was observed for PE with no trapped electrons, the S(t) curve
of the Ps formation by trapped electrons showed increment at rst. This means that there exists the
24
22
20
18
16
14
12
10
8
0 1 2 3
Density (spins/g)/10
15
(a)
o-Ps intensity (%)
4 5 6
36
34
32
30
28
26
24
22
0 1 2
Density (spins/g)/10
17
(b)
o-Ps intensity (%)
Figure 7.24 Relation between trapped electron density and intensity of the longest-lifetime component,
I
3
, at 77 K, (a) in PE and (b) in PMMA6N. (Reprinted from Hirade, T., et al., Radiat. Phys. Chem., 58, 465,
2000. Withpermission.)
0.54
0.52
0.5
0.48
0.46
0.44
0 1
Positron age (ns)
S(t)
2 3 4
Figure 7.25 Positron age dependence of the S parameter. The open circles were measured in darkness,
and the lled circles were measured under visible light. Solid lines are obtained by the least square ts by
considering the positron localization. (From Hirade, T., Radiat. Phys. Chem., 76, 84, 2007. With permission.)
158 Charged Particle and Photon Interactions with Matter
delayed Ps formation by trapped electrons. The solid line in Figure 7.25 shows the tted curve taking
positron trapping into consideration. It is impossible to t the S(t) curve without positron trapping.
The competitive phenomenon of Ps formation with trapped electrons, that is, positron trapping, was
successfully observed and the positron trapping rate could be obtained (Hirade et al., 2007: 3714).
There are several methods for the detection of trapped electrons or anions formed at low temper-
atures. EPR, light absorption, and glow curve measurements are often applied in radiation chemis-
try, and now the positron annihilation method can be used. The binding energy of trapped electrons
is usually 0.5–3eV, and the Ps binding energy is 6.8eV in vacuum and probably 4–5eV in materials.
Therefore, just by placing a positron in the materials, Ps can form, by picking off the trapped elec-
trons. This means that positrons have two chances of Ps formation. The rst chance is given by the
spur reactions, and the positrons that escape from Ps formation by the spur process have the next
chance
of Ps formation with trapped electrons.
The
observation of trapped electrons by the positron methods yields some advantages. The
injected positrons induce irradiation and act as a probe. Continuous irradiation takes place during
measurement. Some interesting measurements are possible, for example, the measurement of the
apparent activation energies of local motions. The Arrhenius plots can give the apparent activation
energies, for which the isothermal decay rates should be measured at several temperatures. This
means that several samples should be prepared. However, the apparent activation energies can also
be obtained just by measuring PAL at several temperatures, because continuous irradiation exists
during measurements and the measured values are under the equilibrium condition of thermal decay
and formation that takes place through positron irradiation. The formation rate is constant; therefore,
Ps formation yields under the equilibrium condition at several temperatures can give the Arrhenius
plots, through which the apparent activation energies can be obtained (Hirade, 2003: 375).
The saturated density of trapped electrons in PE is much lower than that in PMMA, as shown in
Figure 7.24. However, Ps formation yields by the accumulation of trapped electrons are almost the
same for both of these (Hirade et al., 1998: 89). This means that the positron diffusion length in PE
is
larger than that in PMMA.
On
the other hand, the saturation of trapped electron density is controlled by the electron diffu-
sion length. A larger diffusion length gives larger probability of electron–cation recombination, and
so the saturated density becomes small. For example, a smaller diffusion length of electrons at lower
temperatures gives higher density, because the positron mobility in PE is lower at lower tempera-
tures (Brusa et al., 1995: 447). If the temperature dependence of diffusion lengths for electrons and
positrons
are similar, Ps formation yields will have very small temperature dependence.
An
interesting experiment was conducted. At a certain low temperature, PAL was measured
until the Ps formation yield saturated in a long-chain alkane. After a long time of saturation, the
temperature was elevated and measured. The density of trapped electrons was not changed imme-
diately, but the diffusion lengths of positrons and electrons had to be changed, as these are larger
at higher temperatures. The density saturated at lower temperature, and then Ps formed at higher
temperature. The Ps yields should be larger than the saturated value at that temperature. This phe-
nomenon
was successfully revealed by Zgardzinska (Zgardzinska et al., 2007: 309).
7.6.7 reactionS of o-ps with Spur SpecieS
The fourth-lifetime components in pure benzene (Consolati et al., 1991: 7739), the scintillator
NE104 (Consolati et al., 1992: 131), and a 1M solution of 2,5-diphenyloxazole (PPO) in toluene
(Consolati et al., 1992: 131) have been measured by Consolati et al., who showed that the fourth
component is quite possibly caused by a Ps state due to the use of three-gamma measurements.
The lifetime spectra of many liquids showed the fourth-lifetime components, as shown in Table 7.2
(Mogensen, 1995: 73). These fourth lifetimes are shorter than the longest lifetimes, and are caused
by the reactions of o-Ps and the spur species (Mogensen and Jacobsen, 1982: 223). The most
important reactions are those of oxidation of o-Ps by cation radicals. Mogensen proposed that
Positron Annihilation in Radiation Chemistry 159
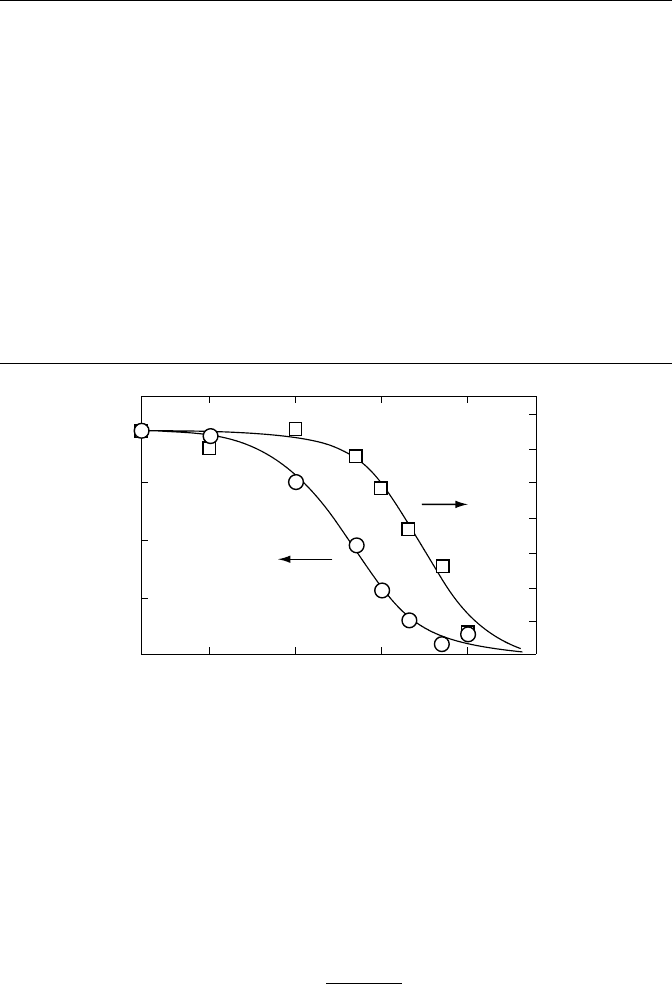
electron scavengers should inhibit the fourth-lifetime component as the o-Ps component could be
inhibited. This effect was measured directly for CCl
4
/hexane mixtures, as indicated in Figure 7.26
(Hirade and Mogensen, 1993a: 249). The interesting feature of the inhibition of the fourth compo-
nent is that the inhibition parameter, β, is larger than 1. The inhibition of Ps formation is normally
described
by the expression (Levay and Mogensen, 1980: 131; Mogensen and Jacobsen, 1982: 223)
I C
I
C
( )
( )
( )
=
+
0
1 α
β
(7.2)
where
I(C)
(I(0)) is the intensity at concentration C(0)
α and β are tting parameters
The
Equation 7.2 was proposed by Levay and Mogensen (Levay and Mogensen, 1977) by applying
empirical expression using a adjustable parameter β. The parameter β for the inhibition of the fourth
component is 1.2. The β values for 59 different combinations of inhibitors and nonpolar liquids are
found to be β ≤ 1 in all cases (Mogensen, 1995: 122). The reason of this large β is that there exist
40
30
20
10
0
0.001 0.01
Concentration (M)
o-Ps intensity (%)
Short-lived o-Ps intensity (%)
0.1 1
0
1
2
3
4
5
6
7
Figure 7.26 Intensities of the two o-Ps components in the lifetime spectra versus CCl
4
concentration in
n-hexane. Open circles indicate the long-lifetime o-Ps, and open squares indicate the short-lifetime o-Ps. The
lines are given by Equation 7.1 with α, β equal to 24 M
−1
, 1.0, and 3.5M
−1
, 1.2, respectively. (Reprinted from
Hirade,
T.
and
Mogensen, O.E., Chem. Phys., 170, 249, 1993a. With permission.)
table 7.2
results
of Four-
term
a
nalysis
of l
ifetime
s
pectra
for l
iquids
liquids I
1
(%) τ
1
(ns) I
2
(%) τ
2
(ns) I
3
(%) τ
3
(ns) I
4
(%) τ
4
(ns)
Hexane 15.2 0.127 39.8 0.410 6.0 1.7 39.0 4.18
2,2-Dimethylebutane 15.9 0.122 28.8 0.372 8.1 0.8 47.2 4.47
diethylether 15.6 0.155 49.4 0.410 8.4 2.1 26.6 4.60
3-Methylpentane 16.8 0.131 39.1 0.429 5.7 2.03 38.4 4.31
3-Methyloctane 14.9 0.132 41.7 0.407 4.5 1.70 38.9 3.87
2,2-Dimethylpentane 12.8 0.109 37.3 0.373 7.2 1.08 42.8 4.34
2,2-Dimethylhexane 13.6 0.122 46.4 0.430 4.2 1.96 35.8 4.14
Methanol 14.0 0.184 63.1 0.446 6.8 2.3 16.1 4.02
Source: Based on data from Mogensen, O.E., Positron Annihilation in Chemistry, Springer, Berlin,
Germany,
1995, 73.
