Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

110 Charged Particle and Photon Interactions with Matter
density, plate-to-plate distance, and so forth, W-values were elaborately estimated from the mea-
sured currents at the plates. In particular, they precisely examined the backscattering effects of inci-
dent electron beams, which resulted in the determination of accurate W-values with uncertainties
smaller than 1%. The obtained characteristics of energy dependence for some atoms and molecules
(Waibel and Grosswendt, 1978, 1983, 1991) have similar features to those found by Combecher
(1980). Further, they carried out a Monte Carlo simulation for measured W-values on the basis of
data for ionization cross sections and excitation cross sections for electron collisions with mol-
ecules. The characteristic behavior of increasing W-value with decreasing energy was well claried
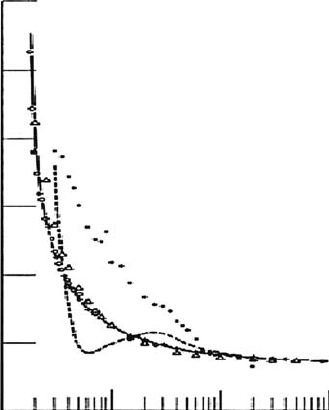
by their simulation, in particular, for the W-value of methane. Figure 6.3 exhibits a comparison of
W-values for methane between experimental and calculated data (Waibel and Grosswendt, 1983).
The experimental data obtained by Waibel and Grosswendt, and by Combecher (1980) are in good
agreement with the simulation, but earlier measured data by Smith and Booz (1978) and previous
results calculated by Dayashankar (1977) are different from this calculation. According to these
studies, this behavior in the low-energy region is clearly understood by the fact that the neutral exci-
tation probability increases with decreasing electron energy in comparison with that for ionization
in collision of electrons with molecules.
By using the ion chamber technique, Samson and Haddad (1976) measured the W-value of Xe
for electrons that were ejected from Xe by the irradiation of photons of 24–90 eV from a spark-
discharge source. They reduced the acceleration of ejected electrons by the applied eld for the sake
of the ion collection technique, which utilized the cylindrical chamber having an off-axis collector
electrode. The result obtained was well reproduced with the analytic equation by Inokuti (1975),
Equation 6.1. Further, Samson and Haddad obtained the W-value for photons using the same appa-
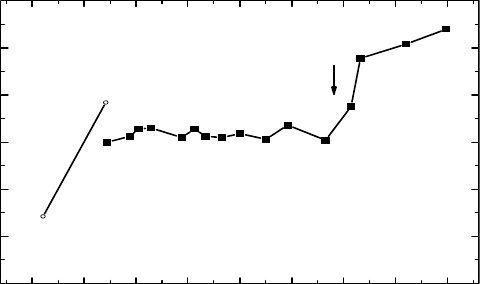
ratus. Figure 6.4 exhibits the photon W-value for Xe as a function of energy. It has been found that
the W-value remains constant between 22 and 65eV, with a distinct increase at the 4d ionization
threshold of 67.5eV. The measured result was discussed in consideration of energy levels of Xe ionic
states and of partial photoionization cross sections for different shells.
80
70
60
50
40
30
20
10
1
10
2
10
3
10
4
T (eV)
W (eV)
Figure 6.3 W-values (in eV) for methane as a function of electron energy. +: Experiment (Waibel and
Grosswendt, 1983), ○: experiment (Combecher, 1980), ●: experiment (Smith and Booz, 1978), △ and solid
curve: calculation (Waibel and Grosswendt, 1983), broken curve: calculation (Dayashankar, 1977), horizontal
arrow (27.3 eV): W-value at high energies (ICRU, 1979). (Reproduced from Waibel, E. and Grosswendt, B.,
Nucl. Instrum. Method,
211, 487, 1983.)
New Directions in W-Value Studies 111
6.4 variation in energy dependenCe oF photonW-values
For
h
ydroCarbon
m
oleCules
6.4.1 introduction
On the basis of progress in electron beam techniques, studies on electron energy loss spectra pro-
vided excitation spectra of several molecules with a high resolution in the energy region of inner-
shell transitions, as well as those for rare gas atoms (Backx and van der Wiel, 1975; Hitchcock
and Brion, 1977; Key et al., 1977; King et al., 1977; Ungier and Thomas, 1984, 1985). According
to these spectra, the rst loss peak at the lowest energy is usually observed for a vacant molecular
orbital, which is the lowest unoccupied valence orbital, for example, π* orbital in a linear molecule.
The second peak comes from the transition into a Rydberg orbital in most cases, which is followed
by those into higher Rydberg orbitals and ionization continuum (Backx and van der Wiel, 1975;
Hitchcock and Brion, 1977; Key et al., 1977; King et al., 1977). Similar spectra were also measured
using monochromatized soft x-rays from synchrotron radiation sources (Eberhardt et al., 1976;
Brown et al., 1978; Hayaishi et al., 1984). Several years ago, highly resolved photoabsorption spec-
tra were obtained, which were compared with the electron energy loss spectra. Synchrotron radia-
tion studies have often shown energy resolving powers higher than those obtained using the electron
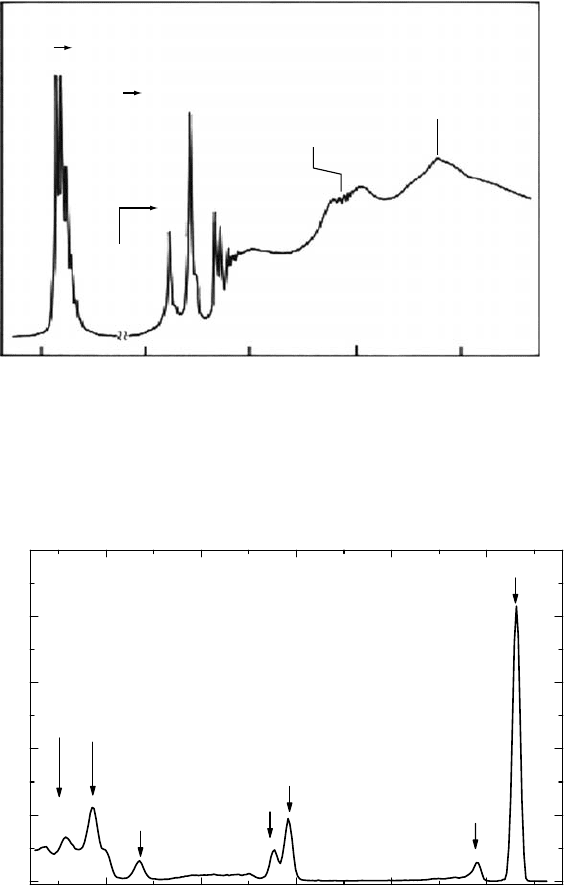
beam technique. An example of the synchrotron radiation spectra is displayed in Figure 6.5, where
a
photoabsorption spectrum of N
2
is exhibited in the N K-shell threshold region (Chen et al., 1989).
The spectrum shows a variety of peak structures corresponding to transitions of the π* orbital,
Rydberg orbitals, and ionization continua. Some peak structures have components of vibrational
excitation; in particular, π* excitation shows six or more vibrational components. Above 409.94eV
(N 1s ionization energy), some structures induced from double excitation and shape resonance are
found. Further electron emission processes were studied for many gases (Dixon et al., 1978; Rye
etal., 1978, 1980; Tamenori et al., 2004); in particular, photoelectron spectra using monochromatic
synchrotron radiation were observed for a number of atoms and molecules. Figure 6.6 shows a
photoemission spectrum of Kr with a moderate resolution at a photon energy of 410eV, where
many peaks are seen for some inner-shell photoelectrons (3s, 3p doublet, 3d, and satellites) and for
Auger electrons from the M
23
-shell hole states (Tamenori et al., 2004). These Auger electrons, that
is, electrons ejected through Coster–Kronig transitions, correspond to the decays of 3p
−1
states into
3d
−1
4p
−1
and 3d
−1
4s
−1
states.
10 20 30 40 50 60 70 80 90
5
10
15
20
25
30
35
Photon W-value (eV)
Photon energy (eV)
Xe 4d
Figure 6.4 Photon W-value of Xe in the vacuum ultraviolet radiation region. ■: Data points. Curves con-
necting data are drawn for better presentation. The solid line in the low-energy region denotes expected values
from
physical consideration. (Modied from Samson, J.A.R. and Haddad, G.N., Radiat. Res., 66, 1, 1976.)
112 Charged Particle and Photon Interactions with Matter
As described in Section 6.3, Samson and Haddad (1976) identied a distinct variation of the
photon W-value for Xe around 70eV. This variation was ascribed to a change in the ejected electron
energy, which was caused by photoionization of the inner-shell, Xe 4d orbital. A similar variation is
expected for hydrocarbon molecules in the C K-edge region, because photoexcitation is supposed to
create several types of excited states in this energy region, yielding electrons emitted with various
energies. Recent advance in synchrotron radiation researches provides us with monochromatic soft
x-rays having continuous wavelength ranges. Therefore, we can perform studies on photon W-values
using
monochromatic soft x-rays combined with precise steps of energy scan.
Double excitations
Shape resonance
Rydberg series
×10
400 405 410
Photon energy (eV)
Absorption intensity (arb. units)
415 420
N 1s
N 1s
1π
g
*
Figure 6.5 Photoabsorption spectra of N
2
in the soft x-ray region. Excitation of 1s electrons into the
π* orbital, and 3s, 3p, and 3d Rydberg orbitals, as well as structures by double excitation and shape resonance
are seen. Note that the ordinate scale is expanded above 404eV. (Based on Chen, C.T. et al., Phys. Rev. A, 40,
6737,
1989.)
100 150 200 250 300
0.0
2.0 × 10
4
4.0 × 10
4
6.0 × 10
4
8.0 × 10
4
10.0 × 10
4
Electron counts
Kinetic energy (eV)
3d
3d satellite
3p
3/2
3p
1/2
3s
M
23
M
45
N
23
M
23
M
45
N
1
Figure 6.6 Photoelectron spectra of Kr observed in the soft x-ray region. The photon energy is 410eV.
Arrows denote 3s, 3p
1/2
, 3p
3/2
, and 3d photoelectron peaks and structures by Auger electrons. (Based on
Tamenori,
Y. et al., J. Phys. B, 37, 117, 2004.)
New Directions in W-Value Studies 113
Here, we present results of photon W-values for ethylene, methane, and propane obtained using
a proportional counter, because hydrocarbon molecules used in a gas counter indicate a clear pulse
height distribution in the detection of ionizing radiation (Suzuki and Saito, 1985a,b, 1987; Saito and
Suzuki, 1986). First, the outcome for ethylene is presented and explained in detail, and then those
for
the other molecules are given.
6.4.2 MeaSureMentS and analySiS
Synchrotron radiation from the storage ring at the Advanced National Institute of Industrial Science
and Technology (to which the Electrotechnical Laboratory was reorganized in 2001) was mono-
chromatized by a plane-grating monochromator (resolving power: about 150) (Tomimasu et al.,
1983; Saito and Suzuki, 1986). The storage ring was operated in a lower-energy mode than the
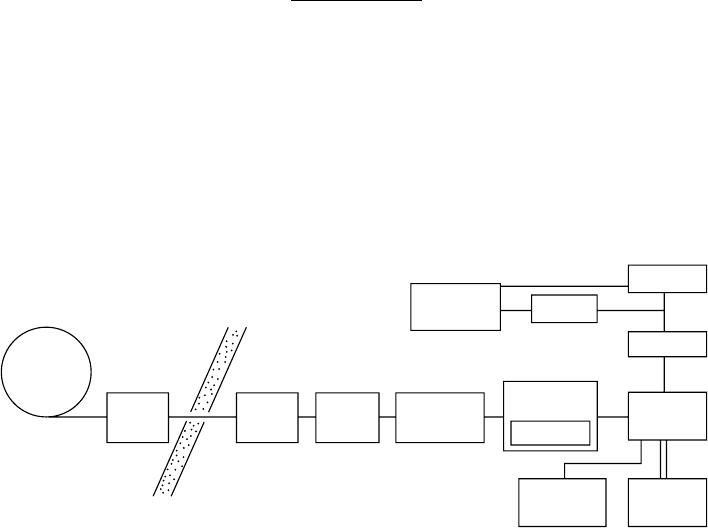
normal mode for suppressing higher-order radiation. Figure 6.7 shows the experimental setup for
measurements of photon W-values for molecules (Suzuki and Saito, 1985b, 1987; Saito and Suzuki,
1986). The monochromatized photons entered a gas-ow proportional counter (Manson-4) with a
thin window (thickness: about 1500 A). Sample gases, ethylene, methane, and propane, were sup-
plied to the counter at about 5 × 10
3
Pa. Signals from the counter were amplied and accumulated
in a multichannel analyzer. The resultant pulse height distributions of the signals were transferred
to a personal computer. Using this computer, the pulse height distributions were analyzed to obtain
the average pulse height. An example of pulse height distribution is displayed in Figure 6.8, where
the
distribution was observed under a methane pressure of 5 × 10
3
Pa at a photon energy of 388eV.
The pulse height distribution can be approximately expressed by the following equation
(Breyer, 1973):
f z
z z z
z
x
x
x
( )
( / )
( ).=
⋅ −
⋅
−1
exp
a
a
Γ (6.2)
where
z
indicates the number of electrons amplied by the electric eld within the counter
z
a
denotes the average gas amplication factor
x
is the average number of the electrons initially produced by the radiation effect of a photon
Γ is the gamma function
Ring
DPS
DPS
Filter
chamber
Micro
computer
SCA
MCA
Amp
Prop.
Counter
X–Z
stage
Gas-flow
control
Gas cell
Main
chamber
PGM
Shield wall
Figure 6.7 Schematic experimental setup of measurements of photon W-values using a proportional
counter. DPS, differential pumping system; PGM, plane-grating monochromator; SCA, single-channel analyzer;
Prop.Counter, proportional counter; Amp, amplier; MCA, multichannel analyzer.
114 Charged Particle and Photon Interactions with Matter
The distributions calculated using Equation 6.2 were matched with those obtained by observation.
The
average pulse height, P, is given by
P E z f z dz z
z E
W E
x( ) ( )
( )
,
p a
a p
p p
= ⋅ = ⋅ =
⋅
∫
(6.3)
where
E
p
indicates the energy of a photon
W
p
denotes the W-value of photons (Suzuki and Saito, 1985a,b, 1987; Saito and Suzuki, 1986)
Using Equation 6.3, relativeW-values were obtained for photons over the energy rangeof C K-edges.
Any change in P owing to a drift in the gas pressure was canceled by measuring the P several times at
the
same photon energy in the course of the experiment. There may be photoabsorption by hydrocar-
bon
molecules in which only neutral fragments are produced, but in which no ion pair is formed. This
process should alter the W-value, although the present technique using a proportional counter cannot
count these types of processes. At present, these processes are assumed to be negligible, in consider-
ation of the results of the ionization yield of methane and other molecules in the vacuum ultraviolet
radiation region (Backx and van der Wiel, 1975).
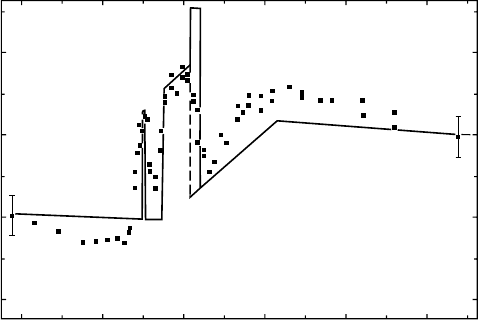
6.4.3 reSult of ethylene
The energy dependence of the W-value is shown with solid squares for photon energies from 269 to
324eV in Figure 6.9 (Suzuki and Saito, 1987). The solid curve indicates a calculated prole based
on a model described below. The measured W-value gradually decreases from 269 to 283eV as the
photon energy increases. The W-value rises to a peak at 285eV, drops to a sharp valley at 287eV,
and
again goes up a broad peak at 290
eV.
Above a minimum at 293
eV,
the data show a near-linear
dependence on the photon energy between 293 and 301 eV. Above the latter energy, the W-value
seems
to have a slightly decreasing trend.
500
250
0
0 200 400
Channel number
Number of counts
600
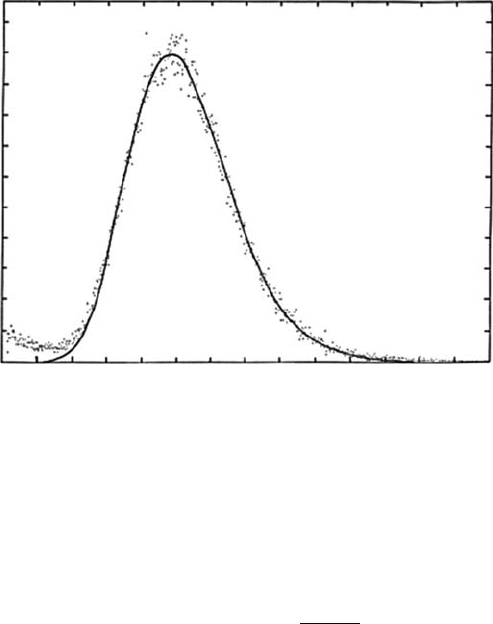
Figure 6.8 Pulse height distribution of a proportional counter for a monochromatic soft x-ray (388eV).
Dots denote measured data, and the solid curve indicates the prole calculated with a tting technique.
(Reproduced
from Saito, N. and Suzuki, I.H., Chem. Phys., 108, 327, 1986.)
New Directions in W-Value Studies 115
It is important to consider the oscillatory variation of the W-value of ethylene in connection with
the photoexcitation of the C 1s electron. Hitchcock and Brion (1977) observed electron energy loss
spectra of ethylene for the 1s electron excitation in the energy resolution of 0.2–0.5eV. The observed
spectra show strong resonant transitions of the 1s electron to the 1b
2g
orbital with vibrational excita-
tions at 284.68, 285.04, and 285.50eV. Ethylene is a pseudo-linear molecule, which has a π-type
orbital. This b
2g
is often called a π* orbital. Transitions into the 3s orbital and the 3p orbital were
found to occur at 287.4 and 287.8eV, respectively. The transition into the 3s orbital is forbidden in
a spherical eld. However, this transition is allowed in a molecule although the transition strength
is weak. Transitions to higher Rydberg orbitals and to the ionized state appeared above 288.3 and
290.6eV, respectively. They further found shake-up states at 292.6 and 295.2eV. (A shake-up state
is an ionized state having an excited valence electron.) Eberhardt and coworkers (1976) observed an
electron
yield spectrum of ethylene in the same energy region by the use of monochromatized syn-
chrotron
radiation. The same transitions were identied although the resolution was lower andthe
observed
values deviated slightly (0.3–0.7
eV).
6.4.4 SiMple Model for explanation of variation in W-value
A comparison between the inner-shell excited states and the energy dependence of the W-value
provides us with a model for interpretation as follows. For simplicity of explanation, energy regions
are divided into seven parts. In each energy region, only one photoionization process is considered,
and others are neglected owing to very small probability. Figure 6.10 illustrates a change in electron
conguration during subsequent electron emission following initial photoabsorption, together with
the nal charge state of the present molecule. Table 6.2 lists energy values necessary for the calcu-
lation of the W-value on the basis of the present model. These energies have been estimated from
available data in the literature, for example, Auger electron spectra and photoelectron spectra (Rye
et
al., 1978; Liegener, 1985; Kimura et al., 1981; Suzuki and Saito, 1987).
(i) Below 284.7 eV: Only a valence electron is ejected by photoabsorption, because the pho-
ton cannot excite an inner-shell electron. The average energy of this ejected electron is
E
p
− E
v
, where E
v
is the average binding energy of the valence electrons. Ethylene has six
orbitals for valence electrons, which are 1b
1u
, 1b
1g
, 3a
g
, 1b
2u
, 2b
3u
, and 2a
g
(Dixon et al., 1978;
270
0.96
1.0
Relative W-value
1.04
1.08
280 290
Photon energy (eV)
300 310
C
2
H
4
320
Figure 6.9 Photon W-value for C
2
H
4
as a function of soft x-ray energy. Solid squares indicate measured
data. The solid curve and a broken line denote the calculated prole based on the model explained in Section 6.4.4.
(Reproduced
from Suzuki, I.H. and Saito, N., Bull. Chem. Soc. Jpn., 60, 2989, 1987.)

116 Charged Particle and Photon Interactions with Matter
table 6.2
list
of e
nergy
v
alues
n
ecessary
for Calculation
of
the p
hoton
W-
value
of e
thylene
in the
m
odel
at s
ection
6.4.4 (in e
v)
W-value for sufciently high energy
radiation
W 25.8
Average
energy of sub-ionization
electron
for an electron with an
energy
E
U
1
for E ≥ 200
22
U
2
for E ≤ 33
11
First
ionization energy E
f
10.5
Average
of ionization energy for
valence
electron
E
v
16.1
Ionization
energy of the K-shell E
K
290.6
Average
energy of
Auger
electron E
A
245.8
Average
energy of de-excitation
electron
from the (1s)
−1
(π*)
1
state
E
Aπ
255
Average
energy of de-excitation
electron
from the (1s)
−1
(R)
1
state
E
AR
255
Average
energy gain of
Auger
electron
in a post-collision
interaction
α
E
p
− 288
Note: π* and R indicate 1b
2g
and Rydberg orbitals, respectively.
E
p
denotes photon energy.
Case
Charge of
residual ion
Final
configuration
Initial
configuration
Energy
range
E
p
< 284.7
(a)
285.1 ≤ E
p
< 287.4
V
π*
V
π*
V
V
x
x x
x
x
K
K
V
V
K
V
R
V
R
K
K
V
R
V
R
K
KK
K
1
(i) and (iii) (vi) and (vii)(ii) (iv) (v)
21 1
1
(b) (c) (d) (e)
284.7 ≤ E
p
< 285.1
287.4 ≤ E
p
< 290.6
290.6 ≤ E
p
< 291.5 291.5 ≤ E
p
K
Figure 6.10 Change in electron conguration during electron emission and nal charge state of the mol-
ecule. K, V, R, and π* indicate the K-shell, the valence orbitals, the Rydberg orbitals, and the π* orbital,
respectively.
x: occupation by an electron,
○: hole in the valence orbital or the K-shell.
New Directions in W-Value Studies 117
Kimuraet al., 1981). The value of E
v
is assumed to be 16.1eV. The ejected photoelectrons ion-
ize ambient molecules and produce a number of ion pairs. On the other hand, the W-value of
low-energy electrons (W
e
) can be approximately given by the following equation, as indicated
in Section 6.2 (Inokuti, 1975; Samson and Haddad, 1976; Combecher, 1980):
W E
E W
E U
e
( )
( )
,=
⋅
−
(6.4)
where
W denotes the W-value for radiation with a sufciently high energy for ethylene (25.8eV)
(ICRU,
1979)
U indicates the average energy of sub-ionization electrons
E
is the energy of an emitted electron
The sub-ionization electrons are the electrons that do not contribute to ionization in a sys-
tem receiving irradiation. In the present instance, the average number of ion pairs, N
e
, is
given
by
N
E
W
E U
W
e
e
= =
−( )
. (6.5)
Using
Equations 6.4 and 6.5, the photon W-value is expressed by the following equation:
W E
E
N
E W
E W E U
p p
p
e
p
p v 1
( )
( ) ( )
,=
+
=
⋅
+ − −1
(6.6)
where U
1
is assumed to be 22eV from the data for the W-value of low-energy electrons
(Combecher, 1980). Multiple photoionization of the valence electrons is assumed to occur
only
at negligibly low probability at present.
(ii) Between 284.7 and 285.1
eV: The 1s electrons can be excited to the π* orbital (1b
2g
), and
a hole is formed in the C K-shell. After this excitation, an Auger transition is supposed to
occur, because uorescence yield is extremely low in a light element. A variety of Auger
transitions have been observed in ethylene (Rye et al., 1978; Liegener, 1985). The average
energy of Auger electrons, E
A
, is estimated here to be 245.8eV, according to the spectrum
by Rye et al. (1978). However, the average Auger electron energy in the present instance
is supposed to be somewhat higher than 245.8eV owing to the existence of that electron
in the π* orbital. This average energy, E
Aπ
, is assumed to be 255eV, by analogy from a
resonance-type Auger electron spectrum of CO (Ungier and Thomas, 1984, 1985). The
W-value
is expressed by
W E
E
N
E W
E W U
p p
p
e
p
A 1
( )
( ) ( )
.=
+
=
⋅
+ −1
π
(6.7)
When Equation 6.7 is compared with Equation 6.6, E
Aπ
(255eV) is lower than E
p
− E
v
(e.g.,270.9eV
for the photon with an energy of 287
eV).
This fact implies that the W-value
at 284.7eV becomes higher than that at 287eV. In the present model, it is assumed that the
photoabsorption probability involving valence electrons can be negligibly low in this pho-
ton energy region on account of the spectra of the inner-shell excitation (Eberhardt et al.,
1976; Hitchcock and Brion, 1977; Brown et al., 1978; Henke et al., 1993). This assumption
is
also applied to the energy region above 287.4
eV
(case iv to case vii).

118 Charged Particle and Photon Interactions with Matter
(iii) Between 285.1 and 287.4 eV: Photons can excite only valence electrons, because there is
no inner-shell excited state in this region. Ejected photoelectrons ionize ambient molecules
and
produce a number of ion pairs. W
p
is expressed by Equation 6.6.
(iv) Between 287.4 and 290.6
eV: Photons can excite inner-shell electrons to the Rydberg orbit-
als, and then the formed inner-hole is lled with a valence electron through the subsequent
Auger transition. The average energy of the Auger electrons, E
AR
, is assumed to be 255eV.
(v) Between 290.6 and 291.5
eV: Inner-shell electrons can be ejected from the molecule by
photons,
but these ejected photoelectrons leave slowly. In the case of CO, the K-shell pho-
toelectron
with low energy is retrapped by the molecular ion when a second electron with
higher energy is ejected by an Auger transition (post-collision interaction [PCI]) (Key
etal., 1977; Hayaishi et al., 1984). Since there has been no detailed study on these mol-
ecules, the slow photoelectron in the present case is assumed to be retrapped at the instant
of the Auger transition. The Auger electron obtains a slightly higher energy owing to the
existence of the slow photoelectron than the normal Auger electron. This energy gain of
the Auger electron is equal to the energy loss of the trapped electron. The average energy
of the Auger electron is assumed here to be 245.8 + α eV (α = E
p
− 288eV), because of no
available data in molecules. This PCI is tentatively assumed to occur between 290.6 and
291.5eV, in consideration of the energy giving a minimum W-value (293eV) and of the
photon
energy resolution.
(vi) Between 291.5 and 301.1
eV: The inner-shell electrons can be ionized and can promptly
escape from the molecular eld. This photoelectron ejection is followed by a normal Auger
transition. The photoelectron has an energy of E
p
− 290.6eV. However, this electron cannot
ionize other ambient molecules because the electron energy is lower than the rst ioniza-
tion energy of the valence electron (E
f
= 10.5eV). The Auger electron (E
A
) has an average
energy
of 245.8
eV.
(vii) Above 301.1
eV: The photoelectron released from the inner shell has an energy higher
than the rst ionization energy of ethylene. This electron and the Auger electron produced
subsequently can ionize ambient molecules. The W-value is expressed by the following
equation:
W E
E
N N
E W
E E W E U U
p p
p
e1 e2
p
p A K 1 2
( )
( ) ( )
,=
+ +
=
⋅
+ + − − −2 2
(6.8)
where U
2
denotes the average energy of the sub-ionization electrons for the electron with
kinetic energy between 11 and 33 eV. U
2
is assumed to be 11eV on the basis of the study by
Combecher
(1980).
By
using the present model, the relative W-value has been calculated and shown with the solid
curve in Figure 6.9. The resolving power of the monochromator was not included in this calcula-
tion. Energy dependence of the calculated W-value is in agreement with the experimental results.
Therefore,
the present model is essentially correct.
There
is a slight discrepancy between the experimental data and the calculated result at a few
energies. The discrepancy at 286eV is ascribed to the low resolving power of the present monochro-
mator, while that between 291 and 293eV is presumed to originate from two reasons, other than low
resolving power. First, although the energy of the electron may be lowered through a post-collision
interaction below 291.5eV, there is a possibility that some fraction of slow photoelectrons are not
retrapped by the molecular ion. If this is the case, the nal conguration is that having two holes in
the valence orbital. The calculated W-value becomes lower than that shown in Figure 6.9 between
290.6 and 291.5eV. Second, slightly distorted pulse height distributions in the proportional counter
New Directions in W-Value Studies 119
around 291eV have been observed. The width of the distribution was broader than those at other
energies. This phenomenon is supposed to be brought about by a contamination of the used grating
in the soft x-ray monochromator, which resulted in a decrease of output intensity near the C K-edge.
This should cause the effective resolving power to be lower. From the minimum at 293eV (not at
290.6eV), it can be said that the post-collision interaction takes place just above the C K-edge.
However, it is impossible at present to estimate the size of the energy region where it occurs. The
broken line between 290.6 and 291.5eV in Figure 6.9 shows the calculated result in case of no
occurrence
of the post-collision interaction.
The
reason why the experimental data are slightly lower than the calculated curve near 280eV
is not clear at present. The photoabsorption cross section near 280eV is very low owing to no exci-
tation of inner-shell electrons. The electron cloud induced by photoabsorption is distributed more
homogeneously in the proportional counter near 280eV than other energies. This fact may have a
small effect on the average pulse height distribution through a possible change in the gas amplica-
tion
factor.
The
shake-up states were reported to exist at 292.6 and 295.2eV in the literature (Hitchcock and
Brion, 1977). In contrast to the transition to the π* orbital, the transition probability to these states
was considerably lower than to the normal continuum state. Thus, this transition was not expected
to have an appreciable effect on the W-value. The experimental W-value does not show a change at
the
energies in Figure 6.9 within experimental uncertainty.
6.4.5 Methane and propane
Methane is usually used as a counter gas for the measurement of ionizing radiation. Since the
photoabsorption spectrum of methane shows a ne structure near the C K-edge due to transitions
of C 1s electrons (Eberhardt et al., 1976; Brown et al., 1978), the W-value of methane may also
have a ne structure related to these transitions.
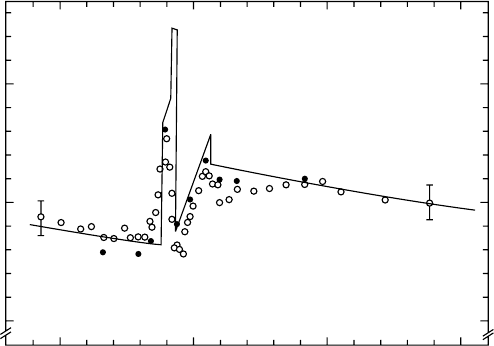
Figure 6.11 shows the relative W-value of methane for photon energies between 240 and 400 eV
(Saito and Suzuki, 1986). Open and solid circles denote the data obtained by using Al and the
copolymer of vinyl chloride and vinyl acetate (VYNS) windows, respectively. The W-value gradually
250
0.95
1
Relative W-value
1.05
300
Photon energy (eV)
350 400
Figure 6.11 Photon W-value for CH
4
as a function of soft x-ray energy. Solid and open circles indicate
measured data using the VYNS window and the Al window, respectively. The solid curve denotes the cal-
culated prole based on the model explained in Section 6.4.5. (Reproduced from Saito, N. and Suzuki, I.H.,
Chem. Phys.,
108, 327, 1986.)
