Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

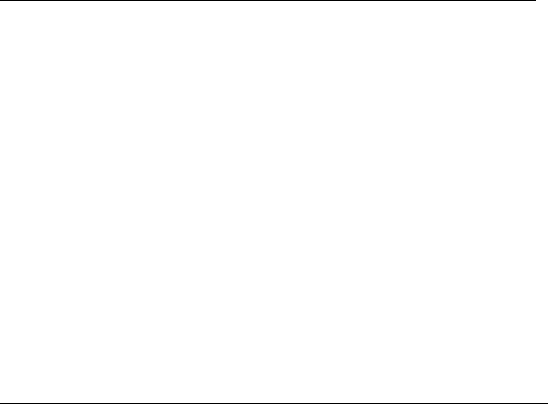
1000 Charged Particle and Photon Interactions with Matter
alkanes such as solvent extraction diluents typically react at rates of ∼10
9
M
−1
s
−1
. For these reasons,
radical redox reactions are probably less important than the nitrous acid concentration in establishing
actinide oxidation states in irradiated aqueous nitric acid.
It can be seen from the foregoing discussions that a multitude of radiolytically induced reactions
compete
to set the neptunium oxidation state in irradiated acidic solution. Vladimirova (1995) pro-
vided
the most comprehensive attempt to model the redox chemistry of neptunium in both alpha-
and gamma-irradiated acidic solution. Taking into account reactions with nitrous acid and the major
produced radicals, he calculated equilibrium concentrations of Np(IV), Np(V), and Np(VI) under
various conditions of dose rate, absorbed dose, and nitric acid concentration. High dose rates favored
the production of Np(IV), while high acidity resulted in rapid declines in Np(V) with increases in
Np(IV) and Np(VI). The reduction of neptunium to the tetravalent oxidation state was attributed
to disproportionation, and reaction with the
•
H atom. The rate constant of the reaction shown in
Equation 34.83 was calculated to increase by a factor of 100 from <5 × 10
6
M
−1
s
−1
to 2 × 10
8
M
−1
s
−1
with
an increase in nitric acid concentration from 1 to 6
M.
NpO H H Np H O
2
+ + +
+ + → +
•
2 2
4
2
(34.83)
At a dose rate of 36kGy h
−1
and a nitric acid concentration of 3M, the equilibrium percentages of
Np(IV), Np(V), and Np(VI) were 8, 72, and 20, respectively. The equilibrium yields of the three
oxidation states were the same for both types of radiation. In additional work, this same author
included renements to account for the presence of organophosphorous extractants including TBP
(Vladimirova etal., 1996). It was concluded that the presence of the organic compounds favored
higher equilibrium concentrations of Np(IV) and Np(VI) at the expense of Np(V). Sensitivity analy-
sis using a mathematical model suggested that the rate constants for the reduction of Np(VI) by
nitrous acid, and the reaction of Np(IV) with Np(VI) to produce Np(V) were decreased, possibly
due
to complexation of Np(IV) and Np(VI) by the organic complexing agents.
34.4 geologiCal disposal oF high-level radioaCtive waste
34.4.1 introduction
Management and geological disposal of high-level radioactive waste are among the most critical
issues related to nuclear technology in recent years. High-level radioactive waste, which includes
spent nuclear fuel discharged from commercial power reactors or vitried waste arising from
table 34.8
rate
Constants for r
eactions
of a
ctinide
i
ons
reaction rate Constant k (m
−1
s
−1
) references
Np
3+
+
•
OH → Np(IV)
4.00 × 10
9
Gogolev etal. (1988)
Np(IV)
+
•
OH → NpO
2
+
3.20 × 10
8
(pH 0) Shilov etal. (1982)
Np(V)
+
•
OH → ?
4.60 × 10
7
Lierse etal. (1988)
NpO
2
+
+
•
OH → NpO
2
2+
4.30 × 10
8
(pH 0) Shilov etal. (1982)
Np
3+
+
•
H → Np
4+
6.00 × 10
7
(pH 0) Gogolev etal. (1991)
Np
4+
+
•
H → ?
<1 × 10
6
(pH 0) Gogolev etal. (1991)
NpO
2
+
+
•
H → ?
<5 × 10
6
(pH 0) Shilov and Pikaev (1982)
NpO
2
2+
+
•
H → NpO
2
+
<1 × 10
7
(pH 1–3) Schmidt etal. (1983)
Np
4+
+ e
aq
−
→ Np(III)
6.00 × 10
10
(pH 0) Gogolev etal. (1991)
NpO
2
+
+ e
aq
−
→ NpO2
2.40 × 10
10
(pH 3) Schmidt etal. (1980)
NpO
2
2+
+ e
aq
−
→ NpO
2
+
1.00 × 10
11
(pH 2.5) Schmidt etal. (1983)
NpO
2
+
+
•
NO
3
→ NpO
2
2+
8.10 × 10
8
(pH < 0) Gogolev etal. (1986)
Radiation Chemistry inNuclear Engineering 1001
reprocessing of power reactor fuel, will be disposed several hundred meters (e.g., more than 300 m
in Japan’s case) below sea level in isolation from humans and their environment. This is referred to
hereafter as geological disposal. The concept of geological disposal is being considered in many
countries and is based on a system of multiple passive barriers, consisting of engineered barri-
ers and the geological environment (natural barrier), as illustrated in Figure 34.26 (JNC, 2005;
SKB, 2006a). In general, the engineered barriers consist of three different types of barriers, as
described below.
The rst barrier is the waste form itself since radioactive nuclides cannot be released until after
being dissolved. The nature of the actual waste form will be dependent on whether the spent nuclear
fuel is directly disposed (once-through) or reprocessed to recover ssile and fertile materials in
order to provide fresh fuel for existing and future nuclear power plants (nuclear fuel cycle). In
the former case, uranium dioxide pellets are encapsulated in a cladding material such as zircaloy.
Cladding tube
Fuel pellet of
uranium dioxide(a)
Copper canister
with cast iron insert
Crystalline
bedrock
Underground portion of
deep repository
Spent nuclear fuel Bentonite clay Surface portion of deep repository
500 m
Buffer
Host rock
(b)
Geological environment
HLW borosilicate glass
Engineered barrier system
Stainless steel canister
Steel overpack
Bentonite/sand buffer
Figure 34.26 Conceptual design of geological disposal systems. (a) KBS-3 type spent nuclear fuel
disposal. (Adapted from SKB, Long-term safety for KBS-3 reositories at Forsmark and Laxemar—a rst
evaluation, Main report of the SR-Can project, SKB Technical Report TR-06-09, Swedish Nuclear Fuel
and Waste Management Co., Stockholm, Sweden, 2006a.) (b) HLW disposal in Japan. (Adapted from JNC,
H17: Development and management of the technical knowledge base for the geological disposal of HLW,
Knowledge Management Report, JNC-TN1400-2005-022, Japan Nuclear Cycle Development Institute,
Tokai-mura, Japan, 2005.)

1002 Charged Particle and Photon Interactions with Matter
In the latter case, radioactive elements in the vitried high-level radioactive waste are immobilized
by matrices such as borosilicate glass, which provides a low solubility matrix in which the radionu-
clides
are homogeneously distributed.
The
second barrier is a waste package, which encapsulates the waste form to ensure its con-
nement for a certain period of time, especially so as to prevent groundwater from contact
with the waste form. In some countries which are planning to implement direct disposal, spent
nuclear fuel is packed in a copper canister with a carbon steel insert, while vitried waste in a
stainless steel canister is encapsulated in a carbon steel package. Although any metal inevitably
corrodes even in the anoxic underground environment, a metallic waste package is expected to
provide a period of complete containment during the radiogenic thermal transient, when condi-
tions are rather dynamic and may be strongly coupled (e.g., thermal and water saturation proles
in the buffer).
The third barrier is a buffer material which surrounds the waste package. The buffer material is
required to have favorable physical and chemical characteristics, such as self-sealing and mechani-
cal buffering, good thermal conductivity, low hydraulic conductivity, colloid ltration function,
and chemical buffering capacity to retard radionuclide migration. Materials like clay-based ben-
tonites are typically used for the buffer due to their low hydraulic conductivity and large sorption
capacity.
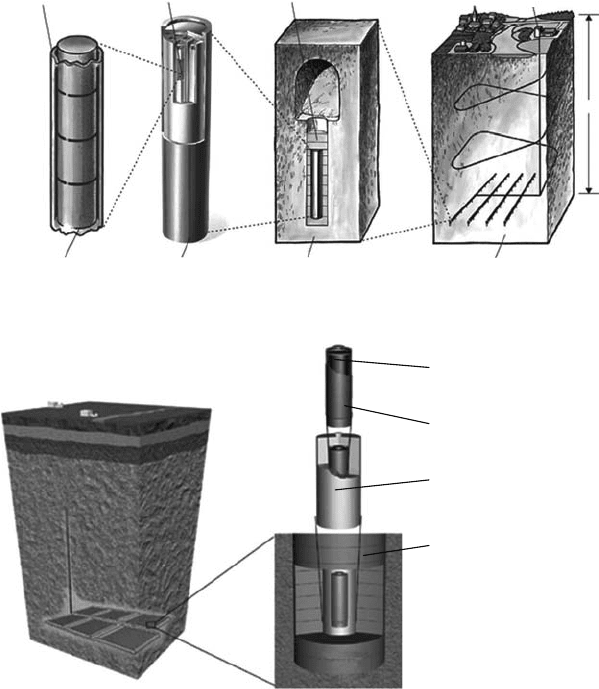
At present, bentonite is the most commonly available material for the buffer, whose mineral
composition is shown in Table 34.9. Montmorillonite is the main component of bentonite and its
properties are essential for the application of bentonite as the buffer material, including low perme-
abilities and high sorption capacities, which strongly retard migration of any radionuclides released
from
the waste package.
Figure
34.27a shows the structural arrangement of montmorillonite (Bradbury and Baeyens,
2002). The unit crystal lattice of montmorillonite is built with two layers of tetrahedral silicate
units between which one layer of octahedral aluminate is sandwiched. A small part of Al
3+
in the
octahedral layer is substituted with Mg
2+
and Fe
2+
or Fe
3+
, which results in negative charges
in the layers. This charge deciency is compensated by interlayer cations. As a result, the actual
structure formula of montmorillonites from Wyoming, United States, and Tsukinuno, Japan, has
been
determined as follows:
table 34.9
mineralogical
Composition of b
entonites
kunigel v1
a
(
tsukinuno,
Japan)
mx-80
b
(wyoming,
the
united states)
Montmorillonite 46–49 75
Kaolinite <1
Mica <1
Quartz/Calcedony 29–38 15.2
Feldspar 2.7–5.5 5–8
Calcite 2.1–2.6 0.7
Dolomite 2.0–2.8
Analcime 3.0–3.5
Siderite 0.7
Pyrite 0.5–0.7 0.3
Organic
carbon 0.31–0.34 0.4
a
Ito etal. (1993).
b
Bradbury and Baeyens (2002).
Radiation Chemistry inNuclear Engineering 1003
Wyoming: Na
0.30
(Al
1.55
Fe
3+
0.20
Fe
2+
0.01
Mg
0.24
) (Si
3.96
Al
0.04
)O
10
(OH)
2
(Müller-Vonmoos and Kahr, 1983)
Tsukinuno:
(Ca
0.05
Na
0.38
K
0.01
) (Al
1.55
Fe
3+
0.09
Fe
2+
0.01
Mg
0.34
Ti
0.01
) (Si
3.90
Al
0.10
)O
10
(OH)
2
(Suzuki etal., 1992)
Figure 34.27b shows a schematic illustration of compacted bentonite after water saturation
(Bradbury and Baeyens, 2002). The typical size of a sheet of montmorillonite is about several
hundred nanometers in diameter and 1 nm in thickness. Montmorillonite sheets form stacks and
such secondary particles are randomly oriented in the compacted state together with mineral
particles. Exchangeable cations in the stack are hydrated to some extent at ambient condition
and their further hydration induces very strong volume swelling. As can be seen from the
gure, any migration of ions and particles in this compacted bentonite would experience very
tortuous pathways. Chemical reactions such as ion exchange with interlayer cations and sur-
face complexation on edge sites of montmorillonite sheets are also important for retardation of
radionuclide migration.
After the repository closure,thebuffer materialis graduallysaturated with groundwater andchem-
ical reactions in the buffer material and groundwater would occur. It is very difcult to experimen-
tally determine chemical composition of porewater in bentonite, and therefore it has been estimated
from geochemical modeling calculations. Table 34.10 shows the highest estimated concentration of
the main chemical species in bentonite porewater (JNC, 2000). These results are summarized from
various conditions of groundwater (e.g., freshwater/seawater origin, oxygen concentration, pH) and
bentonite composition and reactions (Oda etal., 1999). Carbonates are commonly found in ground-
water and the chloride concentration is high particularly in the case of groundwater with seawater
origins.
High concentrations of S(-II) are due to the dissolution of pyrite (FeS
2
).
The bedrock surrounding the engineered barriers is expected to function as a natural barrier
system since it further retards migration of radionuclides, which may be released through the buffer
material.
The long-term performance of the multiple barrier system has been assessed in several coun-
tries in an attempt to demonstrate the technical feasibility of their disposal concepts. A number of
Exchangable cations
+ Water
Interlayer water and
exchangeable cations
Calcite/Quartz/Feldspar
Double layer water
[(Si,Al)]
4
O
10
[(Si,Al)]
4
O
10
OH/O
OH/O
Al, Mg, Fe
Free water
(Bentonite porewater)
(a) (b)
Figure 34.27 Structure of (a) montmorillonite and (b) compacted bentonite. (Adapted from Bradbury, M.H.
and Baeyens, B., Pore water chemistry in compacted re-saturated MX-80 bentonite: Physicochemical charac-
terization and geochemical modelling, PSI Bericht 02–10, Villigen PSI and NTB 01– 08, Nagra, Wettingen,
Switzerland, 2002.)
1004 Charged Particle and Photon Interactions with Matter
scenarios with specic features, events and coupled thermal, hydraulic, mechanical, and chemical
processes have been considered. The source model includes the radioactivity of the waste form as
it causes heat generation and ionizing radiation, which lead to material alteration and radiolysis
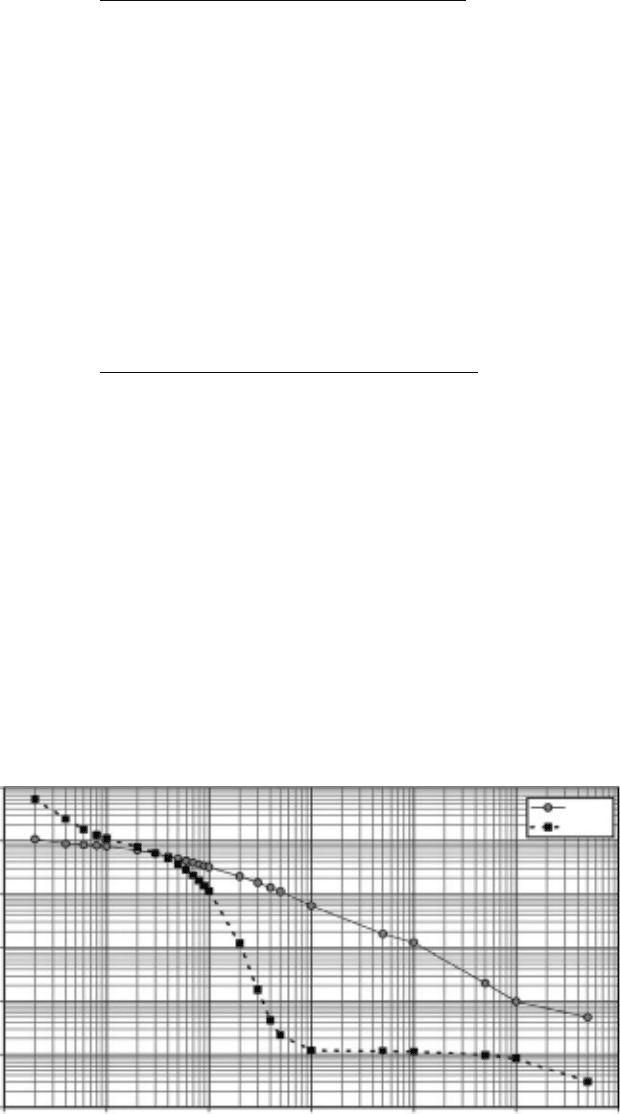
of groundwater. Figure 34.28 shows changes in radioactivity with time for a typical spent nuclear
fuel from an LWR (Poinssot et al., 2005). In the early post-closure stage, radioactivity of ssion
products is dominant. Since half-lives of most of ssion products are shorter than those of actinides,
radioactivity of actinides becomes dominant around several hundred years after repository closure.
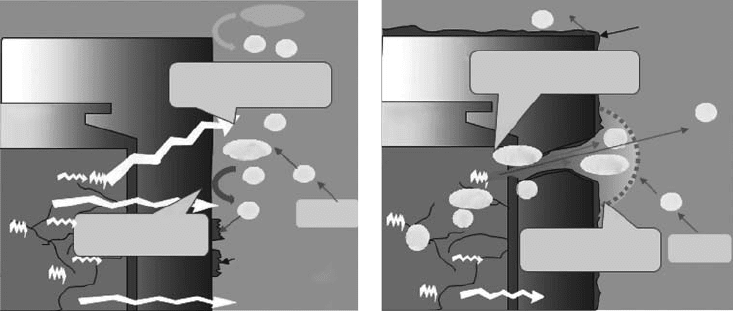
From the viewpoint of system evolution, the impact of ionizing radiation from the waste form
on the engineered barrier system can be categorized into the following two phases, as illustrated in
Figure 34.29: In the earlier post-closure stage, the radioactivity of the waste is extremely high and
the waste form itself is subjected to radiation damage including that of the damage by alpha recoil.
The buffer material would be subjected to gamma radiation, which penetrates through the waste
package. Pore uids in the buffer material would be subject to gamma radiolysis and radiolytic
products might result in oxidizing conditions in the porewater. This could lead to signicant impacts
table 34.10
range
of Chemical s
pecies
and p
h
in
p
orewater
of b
entonite
Concentration (mol dm
−3
)
HCO
3
−
/CO
3
2−
/H
2
CO
3
<7.3 × 10
−2
SO
4
2−
<6.1 × 10
−2
HS
−
/H
2
S <9.2 × 10
−2
Cl
−
<5.9 × 10
−1
P (Total) <2.9 × 10
−6
NO
3
−
0.0
NH
3
<1.6 × 10
−4
NH
4
+
<5.1 × 10
−3
B (Total) <1.7 × 10
−3
pH 5.9–8.4
1E+17
1E+16
1E+15
1E+14
1E+13
1E+12
1E+11
1 10 100 1000
Time (years)
Residual activity (Bq/tHM)
10,000 100,000 1,000,000
Beta
Alpha
Figure 34.28 Evolution of α, β and γ radiations as a function of time for a 55 GWd t
–1
UOX fuel.
(Reprinted from Poinssot, C., et al., J. Nucl. Mater, 346, 66, 2005. With permission.)
Radiation Chemistry inNuclear Engineering 1005
on the engineered barriers’ function. For example, the corrosion of the waste package would be
accelerated under oxidizing conditions, and if the extent is signicant the waste package could be
breached earlier than expected. The reactions of radiolytic products with the buffer materials might
deteriorate
their function of retarding radionuclide migration.
It
is assumed in the scenario that the waste package would eventually be breached even under
anoxic conditions due to metal corrosion and thus would fail more than several hundred years after
repository closure. Groundwater would eventually contact the surface of the waste form and radionu-
clides would be released by dissolution of the waste form. Around this period, most of beta/gamma
radionuclides would have decayed to a level several orders of magnitude lower than the initial level,
and alpha radiation from the surface of the waste would induce radiolysis of water in a very limited
region about 30μm from that surface. In the case of spent nuclear fuel pellets, it is anticipated that
the oxidative products of alpha-radiolysis of water would react with the uranium oxide matrix and
thus increase its dissolution rate signicantly. The radiolytic products might also react with the cor-
rosion products of the waste package. Those having escaped from the reactions in the waste package
might diffuse into the buffer material and also react with reductants such as Fe(II).
Those potential processes induced by the ionizing radiation in different phases of the repository
system
life are discussed in the following sections.
34.4.2 effect of gaMMa radiation on engineered barrier SySteMS
In the near term after repository closure, gamma radiation is the dominant source of radiation impact
on high-level waste forms and their immediate environment because alpha and beta radiation can be
shielded completely by the metal waste package. The major source of gamma radiation is
137
Cs with a
half-life is about 30 years. The impact of gamma radiation on the engineered barriers can be controlled
to some extent by the engineering design. For this purpose, it has been proposed to design the waste
package so as to reduce the dose rate at its outer surface to less than 1Gy h
−1
(Werme, 1998).
34.4.2.1 effect
on Clay m
inerals
As noted above, the engineered barrier system would receive gamma radiation from the waste forms
in the early phase after repository closure. Most of iron atoms exist as Fe(III) and they are reduced
after gamma irradiation, as probed by Mössbauer and ESR spectra (Gournis et al., 2000; Plotze et
al., 2003). Observed slight decreases in cation exchange capacities after long periods (22 months)
Overpack Overpack
Canister Canister
Anoxic
corrosion
HLW waste HLW waste
α
α
α
α
α
α
β
β
β
β
γ
γ
(a) (b)
γ
γ
Accelerated
corrosion?
Redox-front
migration?
Alpha-radiolysis on
HLW surface
Corrosion
products
(Fe
3
O
4
)
Gamma-radiolysis of
porewater
H
2
O
H
2
O
2
H
2
O
2
H
2
O
2
H
2
O
2
O
2
O
2
O
2
H
2
H
2
H
2
H
2
H
2
Fe
2+
Fe
2+
Fe
2+
Fe
2+
Fe
2+
FeS
2
FeS
2
Bentonite Bentonite
Corrosion
products
(Fe
3
O
4
)
Figure 34.29 Effects of ionizing radiation on a repository. (a) Before the waste package failure and (b)After
the waste package failure. (Adapted from IRI, Radiation effects on barrier systems in high level radioactive waste
disposal, Research Project Annual Report, Institute of Research and Innovation, Tokyo, Japan, 2005. (in Japanese).)
1006 Charged Particle and Photon Interactions with Matter
may be attributed to dissipation of protons, lowering the layer charge (Plötze etal., 2003). No struc-
tural
change was observed after gamma-irradiation up to 2
MGy
(Negron etal., 2002).
Radiation-induced
defects formed in the framework of smectite clays were assigned as trapped
holes on oxygen atoms (Clozel etal., 1994, Allard and Calas, 2009). The relative amounts of the
defects are dependent on the atomic composition and arrangements and they resulted in isotope
exchange of hydroxyl ion and changes in specic surface area. However, those changes are small in
the
case of montmorillonite after irradiation up to 30
MGy
(Pushkareva etal., 2002).
Most
of the experiments summarized above were performed with powdered or dispersed smec-
tites. Pusch etal. performed experiments to simulate the actual condition of compacted benton-
ite contacting steel under high-temperature and gamma-radiation conditions (Pusch etal., 1992).
Commercial MX-80 was compacted to a dry density of 1.65g cm
−3
and saturated with simulated
groundwater. Irradiation was continued for 1 year and the total dose was 33MGy. Little change in
the framework structure and physicochemical properties were observed, although Fe(III) ions may
have
slightly been reduced.
These
results indicate physicochemical properties of montmorillonite as the buffer materials are
little affected by gamma-ray exposure. However, signicant damage leading to amorphization has
been observed under electron-beam irradiation in electron micrographs when the accumulated dose
is increased to the order of GGy (Gu etal., 2001, Sorieul etal., 2008). Threshold values for radia-
tion-induced amorphization were strongly dependent on the temperature and structure of smectites:
Threshold values are the largest at 300°C − 450°C, which coincides with the onset of dehydroxyl-
ation. At room temperature, thresholds for amorphization were over 10 GGy, which is comparable
to cumulative doses estimated in HLW repositories after 10
6
years for radionuclides adsorbed to the
bentonite
buffer.
Several
countries are planning to build repositories in clay formations and the effect of gamma
radiation on natural clays has been studied. In particular, various tests have been performed in the
underground research facility in the Boom clay in Belgium. Mössbauer spectra of the Boom clay
indicated that while pyrite was completely oxidized after gamma-irradiation up to 30MGy, Fe(II)
remained, as in the case of the compacted bentonite (Ladriere etal., 2009). An in situ experiment in
the underground research laboratory in the Boom clay formation was performed to develop a model
to predict transient behavior of the disposal system. In the CERBERUS experiment, a
60
Co gamma-
ray source and heaters were placed in the clay formation to simulate waste forms, and changes in E
h
and
pH were monitored (Zhang etal., 2008).
34.4.2.2
effect
on Corrosion of w
aste
p
ackage
In the initial phase after the repository closure, the buffer material is unsaturated and therefore the
gamma-radiolysis of air in pores of the buffers would generate nitrogen oxides, which eventually
form nitric acid which might affect the corrosion of the canister. The amount of nitrate in the vessel
is proportional to the gas volume against the liquid volume (Linacre and Marsh, 1981). However the
estimated
amount of generated nitric acid was negligible (King etal., 2001).
As
the buffer materials are gradually saturated with groundwater, gamma-radiolysis products
like H
2
O
2
or O
2
are generated. These radiolytic products would induce oxidative corrosion of metal
packages. Increased corrosion rates of carbon steel in saline water under gamma irradiation were
observed in the 1980s in the United Kingdom. However, the effect was negligible at dose rate of
about 3Gy h
−1
(Marsh etal., 1989). The effect was also observed in compacted bentonite saturated
with the synthetic groundwater. They have performed 1D diffusion reaction model calculation to
evaluate the effect of radiation by assuming cathodic reaction of radiolytically generated oxygen.
Burns etal. have incorporated surface reactions of short-lived radiolytic products in models to
calculate gas generation in gamma-irradiated steel vessels (Burns etal., 1983). A more elaborate
mixed potential model was applied for the corrosion of HLW canisters in which homogenous and
surface reactions of radiolytic products were fully taken into account (Macdonald and Urquidi-
Macdonald, 1990). Recently, the corrosion of the carbon steel inserts of spent nuclear fuel canisters
Radiation Chemistry inNuclear Engineering 1007
under gamma radiation was studied assuming cannsiter failure (Smart etal., 2008). The effect of
gamma radiation was observed at the dose rate of 300Gy h
−1
. The predominant corrosion products
were magnetite and oxyhydroxides (or maghemite), which are thought to be formed by anoxic cor-
rosion
of iron and subsequent dissolution of Fe
2+
at pH < 7 (White etal., 1994).
3 4 4
2 4 2
Fe H O Fe O H
3
+ → + (34.84)
Fe O H -Fe O Fe H O
3 24 3
2
2
2+ → + +
+ +
γ
(34.85)
Copper is employed for the material for the canister in several countries and its corrosion under
gamma radiation has also been studied (King etal., 2001). In the early stages of disposal, the oxygen
concentration
is still high and the corrosion in saline groundwater would proceed as follows:
Anodic Cu Cl CuCl e: + → +
− − −
2
2
(34.86)
Cathodic O e H O OH
2
: + + →
− −
4 2 4
2
(34.87)
The effect of gamma radiation on the corrosion of copper embedded in a montmorillonite–sand
mixture was studied (King etal., 1992; Shoesmith and King, 1999). The copper concentration in the
mixture signicantly decreased upon irradiation, presumably due to reduction of Cu(II) to Cu(I)
which forms a weakly sorbed anionic complex. It was expected that the oxidizing species produced
by groundwater radiolysis would accelerate the cathodic reaction. Although the observed reduction
of copper by gamma-radiolysis was the opposite of expected, the effect was similar to the case of the
reduction of iron in montmorillonite (Pusch etal., 1993). King etal. conjectured that electrons pro-
duced
from montmorillonite ionization might have reduced cupric ion in the porewater. It has been
proposed that the energy of ionizing radiation deposited in nanoparticles of metal oxides dispersed
in the aqueous phase might be transferred to water to generate the hydrated electron, and eventu-
ally molecular hydrogen (Schatz etal., 1998; LaVerne and Tonnais 2003; LaVerne, 2005). The same
reaction might have occurred in the porewater of montmorillonite under ionizing radiation as the
thickness of a single layer of montmorillonite is about 1 nm and the secondary electrons produced
might have easily escaped.
34.4.3 effect on diSSolution of waSte forMS
After the assumed failure of a waste package, groundwater would penetrate to the surface of the
waste form and eventually would receive alpha-radiolysis. In this section, the effects of water radi-
olysis on dissolution of the waste form are described. The dissolution rate of the waste form is a
key parameter for the performance assessment of the disposal system as it determines the release
rates of radionuclides which eventually diffuse into the engineered and natural barrier systems.
While various factors such as pH, solutes, temperature, etc., affect the dissolution rates, the effect of
ionizing radiation is particularly important in the case of geological disposal of spent nuclear fuel.
Groundwater radiolysis would generate oxidizing species in an otherwise reductive environment
and
may increase the dissolution rate several orders of magnitude.
34.4.3.1
dissolution
of s
pent
n
uclear
Fuel
In
the case of direct disposal, most of the radioactive nuclides are retained in a uranium dioxide
matrix and their release rates are determined by the dissolution of that matrix. Uranium dioxide is
stable under the anoxic conditions of the repository and its solubility is extremely low. Figure 34.30
summarizes chemical processes on the surface of uranium dioxide (Poinssot et al., 2005; Martínez
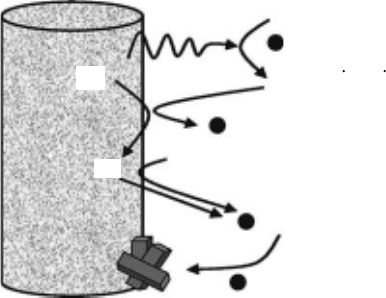
1008 Charged Particle and Photon Interactions with Matter
Esparza etal., 2005). Dissolution rates increase signicantly when UO
2
is oxidized to UO
2+x
(0 < x < 1) by dissolved oxygen or radiolytically generated oxidizing species. Dissolution of U(VI)
is facilitated by complexation with carbonate ions. Dissolved U(VI) precipitates on the UO
2
pellet
surface, although some amount would eventually migrate out of the canister to the engineered bar-
rier
systems.
Uranium
dioxide is a p-type semiconducting material and oxidative dissolution of uranium diox-
ide
can be regarded as corrosion with anodic and cathodic half reactions.
Anodic UO UO e
2
: → +
+ −
2
2
2
(34.88)
Cathodic H O e OH
2
:
2
2 2+ →
− −
(34.89)
O e H O OH
2
+ + →
− −
4 2 4
2
(34.87)
Under the condition in which fuel corrosion occurs, the redox potential of the groundwater (E
h
)
must be more positive than the equilibrium potential for fuel dissolution. When the groundwater
and the spent nuclear fuel are contacted, corrosion potential (E
corr
) is established at which the rates
of the anodic and cathodic reactions will be equal (Shoesmith, 2000, 2007)and is measured to monitor the
degree of oxidation of the sample (Johnson etal., 1983). Oxidation states of uranium on the surface
can
also be probed with x-ray photoelectron spectra (Sunder etal., 1990).
Gamma
radiation has been commonly employed to study the effect of water radiolysis on oxi-
dative dissolution of uranium oxide (Gromov, 1981; Sunder etal., 1992; Christensen and Sunder,
2000). However, alpha radiation is the main source of ionizing radiation after several hundred years
and various methods have been developed to study its effect on radiation-induced oxidative dissolu-
tion
of the fuel matrix.
The
most straightforward method is to study dissolution of spent nuclear fuels, although cur-
rently available spent nuclear fuels still have high beta/gamma radioactivity (Bruno et al., 1999,
2003; Cera et al., 2006). This activity affects water radiolysis with different G-values than alpha-
radiolysis. To eliminate the effect of beta/gamma radiation and complex chemical and morphologi-
cal changes in spent nuclear fuels, uranium dioxide doped with alpha emitters (
238
Pu,
233
U, etc.) has
been utilized to study the effect of alpha radiation (Rondinella etal., 2000; Cobos etal., 2002;
Cachoir and Lemmens, 2004; Jégou etal., 2004; Mennecart etal., 2004b; Muzeau etal., 2009).
Dissolution rates were proportional to the dose rate of the samples, which were controlled by the
H
2
O
α
1 Generation of oxidants by water radiolysis
Fuel oxidation by radiolytic oxidants
Aqueous ligands (HCO
3
–
, OH
–
...)
Precipitation of secondary phases
U
VI
+RN dissolution
2
3
4
U
VI
U
IV
H
2
O
2
, OH , HO
2
, H
2
, O
2
Figure 34.30 Radiation-induced processes on the surface of uranium dioxide. (Reprinted from Poinssot, C.
et al., J. Nucl. Mater., 346, 66, 2005. With permission.)
Radiation Chemistry inNuclear Engineering 1009
addition of different amounts of alpha emitters. Asimulated high-burnup spent nuclear fuel called
SIMFUEL
(Lucuta etal., 1991) was also employed for dissolution studies (Ollila, 1992).
Not
only internal alpha sources but also external sources have been applied. Alpha emitter discs
(
241
Am and
210
Po) were employed in the rst study of alpha-radiolysis affects on UO
2
dissolution by
placing them close to the surface of UO
2
pellets (Bailey etal., 1985). In the presence of alpha-radiation,
the corrosion potential of UO
2
increased to the same level as in the presence of dissolved O
2
.
In recent years, helium ion beam irradiation has also been employed to simulate alpha-radiation.
This method is advantageous in that high dose rates can be achieved and ion energy can be con-
trolled to study the LET effect. Two different types of UO
2
are employed. The rst is colloidal par-
ticles dispersed in aqueous solution (Mennecart etal., 2004a, Suzuki etal., 2006), while the other
is UO
2
discs contacted with water on one face and being irradiated from the opposite face (Corbel
etal., 2001; Sattonnay etal., 2001). The dissolved U(VI) concentration due to ion beam irradiation
was higher than the value following the addition of the same amount of H
2
O
2
generated by the ion
beam,
indicating the effect of radical species is not negligible (Corbel etal., 2006).
Radiation-induced
oxidative dissolution of UO
2
was rst modeled by taking into account the
reactions of radical species with UO
2
(Christensen and Bjergbakke, 1987). Reaction rates at the
UO
2
surface were estimated from the calculated number density of surface U(IV) atoms and homo-
geneous reaction rates for U(IV) in solution. The model was further rened (Christensen etal.,
1994) and one-dimensional diffusion–reaction calculations of UO
2
dissolution were performed by
setting different regions of water subjected to radiation (Christensen, 1998). The model was then
extended to the case of UO
2
dissolution in salt brine (Kelm and Bohnert, 2000a,b). Dissolution rates
of alpha-emitter-doped UO
2
were recently calculated (Christensen, 2006). The reaction rates of UO
2
and radical species were estimated to be about two orders of magnitude larger than for molecular
species. Difculties in setting appropriate values of surface-to-volume ratio were pointed out in the
calculation of the dissolution rates of UO
2
. One-dimensional diffusion–reaction model calculations
of the UO
2
surface were performed to simulate helium ion beam irradiation experiments assum-
ing alpha-dose rate proles from the surface (Poulesquen and Jégou, 2007). The calculated values
of U(VI) and H
2
O
2
were lower than the measured values in both aerated and deaerated cases.
Measured values remained relatively high even in the presence of hydrogen, in contrast to the case
of dissolution experiments under dissolved hydrogen. The authors attributed this to a high dose rate
used in the calculation, which may have overestimated radical–radical recombination to form H
2
O
2
.
Another model of radiolytic oxidative dissolution of UO
2
has been developed in which the reac-
tions of UO
2
were limited to those with H
2
O
2
and O
2
(Eriksen, 1996). The reaction of H
2
O
2
is the
most important and consumption rates of oxidants rapidly decreased possibly due to accumulated
H
2
, which scavenged OH
•
and suppressed H
2
O
2
formation. They also measured dissolution rates of
PWR spent nuclear fuel pellets (Eriksen etal., 1995). In the early stage of the experiments, mea-
sured concentrations of radiolytic products were lower than estimated by the model, and they were
therefore
assumed to have decomposed on the surface.
The
model has been further developed by Jonsson in recent years (Jonsson etal., 2007). Oxidative
dissolution was facilitated by U(VI) carbonate complex formation, and dissolution rates became
proportional to surface oxidation rates when [HCO
3
−
] > 10
−3
mol dm
−3
, typical of groundwater. One-
electron oxidation of UO
2
is considered to be the rate-determining step as the reaction rates of a
series of oxidants with UO
2
were found to be logarithmically proportional to one-electron reduction
potentials (Ekeroth and Jonsson, 2003). Based on UO
2
dissolution rates under gamma-irradiation
and reaction model calculations, the relative contributions of various oxidants to UO
2
oxidation by
water radiolysis were evaluated. It was concluded that contribution of H
2
O
2
was more than 99.9%
and other oxidant species were negligible, which supports the reaction model proposed by Eriksen
(1996)
and (Ekeroth etal., 2006).
One-dimensional
reaction–diffusion model calculations for the surface were also performed.
Dose rate distributions were provided from calculated inventories and geometrical considerations
on the dissipation probability of alpha particles (Nielsen and Jonsson, 2006). Steady-state H
2
O
2
