Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

990 Charged Particle and Photon Interactions with Matter
H O HNO H NO H O
2 2 2 3 2
+ → + +
+ −
(34.52)
The loss of nitrous acid by reaction with radiolytically produced hydrogen peroxide is the main
source
of loss of nitrous acid in an irradiated system (Bhattacharyya and Veeraraghavan, 1977).
34.3.1.2
the
p
roduced
s
pecies
in the o
rganic
p
hase
Normal and branched-chain alkanes are the typical organic diluents for the ligands used in nuclear
solvent
extraction. The radiolysis of alkanes is represented by (Spinks and Woods, 1990):
CH CH CH e CH CH CH CH CH CH CH H H
sol 23 2 3 3 2 3 3 2 3
( ) ( ) ( )
n n n
i i i
i
i− +
+ + + + +
22
(34.53)
Specic yields for the products depend on the specic alkane irradiated. Branch-chain alkanes have
higher product yields, in the range of 0.2–0.6μmol J
−1
for molecular hydrogen and 0.005–0.1μmol
J
−1
for methane. Bishop and Firestone (1970) reported a yield of H
•
atom of 0.07μmol J
−1
for C
6
–C
10
hydrocarbons. The carbon-centered radical products may also be produced indirectly by hydrogen
abstraction reactions with
•
OH or
•
NO
3
radicals. Regardless of their origin, these carbon-centered
radicals react with solutes by hydrogen atom abstraction or they may undergo radical–radical addi-
tion
to create higher molecular weight products (Dewhurst, 1958):
CH CH CH CH CH CH CH CH CH
2 2 +2 33 2 2 3 2 3 2
( ) ( ) ( )
n n n
i i
+ → (34.54)
As these higher molecular weight products accumulate, they change the physical characteristics of
the solvent, including phase disengagement time, density, and viscosity. Alkane radicals may also
undergo
disproportionation to produce unsaturated products (Dewhurst, 1958):
CH CH CH CH CH CH CH CH CH CH CH CH CH
2 3 13 2 2 3 2 3 2 3 2
( ) ( ) ( ) ( )
n n n n
i i
+ → +
−
22
(34.55)
Unsaturated products are susceptible to addition reactions by
•
OH radical or N-centered radical
species.
Among the most important of carbon-centered radical reactions is oxygen addition to produce
peroxyl
radicals (Alfassi, 1997):
R O ROO
i i
+ →
2
(34.56)
Peroxyl radicals will undergo addition reactions to form tetroxides, which then decompose to pro-
duce aldehydes, ketones, and alcohols from the original compound (von Sonntag and Schuchmann,
1997). They are therefore important intermediates in the oxidative mineralization of organic com-
pounds
by radiolysis.
34.3.1.3
the
m
ixed
p
hase
Although the reactive species responsible for radiation chemical effects in solvent extraction systems
can be readily identied, additional factors must be considered to provide a quantitative understand-
ing of radiolysis in the biphasic system. Among these are that reactive species yields and reaction
rates vary with solvents, although most are known only from the aqueous phase. Further, the trans-
fer
of produced reactive species across the aqueous/organic phase boundary must be considered.
Muroya
etal. reported initial yields of solvated electrons of 0.4 and 0.2μmol J
−1
for water and
decanol, respectively (Muroya etal., 2008). Although the yield may be lower, reaction rates for elec-
tron attachment in nonpolar organic solutions are much higher than in aqueous solution (Borovkov,
2008). However, in benzene-, or dodecane-in-water microemulsions, Wu etal. (2001) found that
the yield of aqueous electrons was identical to that of pure water. Electrons, being very mobile in
Radiation Chemistry inNuclear Engineering 991
nonpolar solution, apparently crossed the interface to the aqueous phase. This resulted in a constant
yield
of aqueous electrons despite changes in the proportion of water in the emulsion.
In
contrast, it was determined in the same study (Wu etal., 2001) that water radiolysis was the only
source of
•
OH radical in irradiated emulsion, indicating that radical cations produced in the organic
phase did not cross the interface to oxidize water. The
•
OH yield in the emulsion was thus proportional
only to the water content. The rate constants for several radical reactions, including the
•
OH radical
reaction with benzene, were found to be similar in pure aqueous and microemulsion aqueous solution.
Some of the most important reactive species produced in the aqueous phase, such as
•
OH or
•
NO
3
radical, must cross the phase boundary prior to reacting with ligand molecules or their dilu-
ents. This should be relatively easy for neutral species and in fact the solvent extraction system is
designed to move neutral species across the interface by providing intimate phase mixing using
pulsed columns, mixer settlers, or centrifugal contactors. For these emulsions, it may be justiable
to neglect a phase transfer diffusion gradient and to assume uniform radiolysis. However, in reality,
little information is available on the mass transfer rates of these species, and diffusion controlled
regimes may dominate when dose rates are very high or with thick phase layers due to inadequate
mixing (Macášek and Čech, 1984). In practice, the assumption is generally made that reactive spe-
cies created in either phase are available for reaction during phase mixing in solvent extraction. The
investigation
of biphasic radiolysis reactions is an area in need of more detailed investigation.
34.3.2 purex proceSS radiation cheMiStry
34.3.2.1 tbp radiolysis
The PUREX process for the extraction of the major actinides consists of 30% TBP in alkane dilu-
ent. The process can either partition uranium separately or co-extract uranium, neptunium, and/or
plutonium depending on how the valence states of the latter metals are set prior to extraction. The
metal-loaded solvent is then stripped with a mildly acidic aqueous phase and recycled (Schultz and
Navratil, 1984). However, its recycle potential is limited by the radiolytic degradation of TBP and its
diluent. It has long been recognized that the major products of TBP radiolysis are hydrogen, meth-
ane, and dibutylphosphoric acid (HDBP), with monobutylphosphoric acid (H
2
MBP) and phosphoric
acid produced in lesser amounts. The radiation chemistry of TBP was recently reviewed and the
following
discussion is abbreviated from that source (Mincher etal., 2009a,b,c).
The
accumulation of radiolytic degradation products in the PUREX solvent results in decreased
extraction performance (Lane, 1963; Neace, 1983; Stieglitz and Becker, 1985). The acidic radi-
olysis products are complexing agents that interfere with uranium and plutonium stripping and
ssion product separation factors (Davis, 1984; Tripathi et al., 1999; Tripathi and Ramanujam,
2003). Interfacial crud formation and poor phase separation have been attributed to the formation
of precipitable complexes of zirconium with H
2
MBP and phosphoric acid (Rochoñ, 1980; Stieglitz
and Becker, 1985; Miyake etal., 1990; Sugai and Munakata, 1992; Egorov etal., 2002, 2005). The
adverse affects of the buildup of these acidic phosphate products in the organic phase are mitigated
during process extractions by solvent washing with aqueous Na
2
CO
3
(Blake etal., 1963; Reif, 1988).
However, with continued recycling, washing becomes less effective and the washed solvent shows
increased retention of Pu, Zr, and Ru and increased solution viscosity (Tripathi etal., 2001a). This
has been attributed to the accumulation of higher molecular weight radiolysis products with high
organic phase solubility (Wagner and Towle, 1958; Stieglitz and Becker, 1985). The result is a per-
manently degraded and radioactively contaminated solvent, which is expensive to dispose.
Several mechanisms have been proposed to explain the production of HDBP in irradiated TBP
solutions. Zaitsev and Khaikin (1994) reported that dissociative electron capture resulted in the
production
of the butyl radical and HDBP in irradiated neat TBP:
e C H O PO C H C H O OPO
sol
− −
+ → +( ) ( )
4 9 3 4 9 4 9 2
i
(34.57)
992 Charged Particle and Photon Interactions with Matter
Jin etal. (1999) attributed the formation of HDBP under these conditions to a combination of dis-
sociative electron capture and decay of excited TBP molecules, while Haase etal. (1973) reported
that electron attachment could also result in free hydrogen atoms and a TBP carbon-centered radi-
cal.
However, the electron-initiated reactions are unlikely to be of consequence in the acidic mixed
phase due to the fast reactions shown in Equations 34.36 through 34.38, and the direct excitation of
TBP becomes less important when TBP is dissolved in a diluent. These may not be important reac-
tions
in the solvent extraction process.
Burr (1958) proposed that HDBP was formed by the decay of the TBP carbon-centered radical:
( ) ( )C H O ( C H O)PO C H C H O OPO
8 8
+
4 9 2 4 4 4 9 2
i
i
→ +
−
(34.58)
This TBP radical could be produced by hydrogen atom abstraction (Burr, 1958) by reaction with
either
the radiolytically produced
•
H atom or
•
OH radical:
( ( ) ( )
i
i
i
HO) H C H O ( C H O)PO H O H
8
+ → →TBP
4 9 2 4 2 2
k
OH
M s M i n c h er et al.,= ×
− −
5 0 10 2008
9 1 1
. ( )
(34.59)
k
H
M s Mincher et al.,= ×
− −
1 8 10 2008
8 1 1
. ( )
Besides direct decay to HDBP, the TBP radical could also undergo hydrolysis to again produce
HDBP
(von Sonntag etal., 1972):
( ) ( )C H O ( C H O )PO H O C H OH C H O POO H
8 84 9 2 4 2 4 4 9 2
i i
+ → + +
− +
(34.60)
Khaikin (1998) suggested that HDBP was also the stable product of dissolved oxygen addition to the
TBP
radical, which gives the TBP peroxyl radical:
( ) ( )C H O ( C H O)PO O C H O) P(O) O (C H OO
8 9 2 44 9 2 4 2 4 8
i i
+ → – – (34.61)
Superoxide elimination followed by hydrolysis produces HDBP and butyraldehyde, also a measured
product
(Clay and Witort, 1974).
( ) ( )C H O P(O) O (C H )OO O C H O) P(O) O (C H
8
2
9 2 44 9 2 4 4 8
– – – –
i i
→ +
− +
(34.62)
( ) (C H O P(O) O (C H ) H O C H O) P(O) O C H CHO H
8
+
9 24 9 2 4 2 4 3 7
2– – –+ → + +
− +
(34.63)
Wilkinson and Williams (1961) proposed yet another mechanism for HDBP formation based upon
direct
TBP radiolysis:
( ) (C H O PO e C H O) PO
sol 34 9 3 4 9
− +
+
i
(34.64)
( ) (C H O PO C H O) P(OH)OH CH CHCH CH
24 9 3 4 9 2 3
i i+ +
→ +
(34.65)
( ) (C H O P(OH)OH C H O) POOH H
24 9 2 4 9
+ +
→ +
(34.66)
Radiation Chemistry inNuclear Engineering 993
Intramolecular hydrogen bonding between the phosporyl oxygen and a butoxy hydrogen atom
of the radical cation formed in Equation 34.64 forms a ring structure, which decays as shown
in Equations 34.65 and 34.66. This mechanism, although dependent on direct TBP radiolysis
cannot be entirely discounted since TBP is used at a rather high concentration of 30% in the
PUREX process. Thereactions described above all lead to the production of HDBP, and occur
competitively. Continued irradiation produces H
2
MBP and phosphoric acid from HDBP via
analogous reactions.
Among the less abundant but still important radiolysis products are the higher molecular weight
acid phosphates. These species with varying alkane chain lengths suggest that radical addition
reactions occur between TBP, HDBP, and alkane solvent radicals, including the production of
TBP dimers (Rochon´, 1980; Adamov etal., 1990). These higher molecular weight compounds are
among those species that are not adequately removed from irradiated solvent by aqueous carbonate
washing.
Additional products of TBP radiolysis in the presence of HNO
3
are nitrated phosphates, which
also impede stripping efciency. He etal. (2004) proposed that
•
NO
3
reacts with TBP by hydrogen
atom
abstraction, producing the TBP radical
i i
NO TBP C H O C H O PO HNO
M s M i n c h er et a
3 4 9 2 4 8 3
6 1 1
4 3 10
+ → +
= ×
− −
( ) ( )
. (k ll., 2008)
(34.67)
The TBP radical was then postulated to undergo reaction with additional
•
NO
3
to produce nitrated
TBP
(He etal., 2004):
i i
NO C H O C H O PO C H O OC H NO PO
33 4 9 2 4 8 4 9 2 4 8
+ →( ) ( ) ( ) ( ) (34.68)
The
•
NO
2
radical might be expected to add in the same way:
i i
NO C H O C H O PO C H O OC H NO PO
22 4 9 2 4 8 4 9 2 4 8
+ →( ) ( ) ( ) ( ) (34.69)
Methylated, hydroxylated, and nitrated phosphates, resulting from radical addition reactions of
methyl radical, hydroxyl radical, and the nitro-radicals shown above, have been identied in post-
irradiation TBP solutions by numerous investigators (Nowak, 1977; Adamov etal., 1987; Lesage
etal., 1997; Tripathi etal., 2001b). It can be seen that radical addition reactions can create a wide
range
of products, sometimes of high molecular weight.
34.3.2.2
purex diluent
d
egradation
The TBP alkane diluent undergoes similar radiolytic degradation to generate metal complexing
agents that do not wash out in solvent treatment with alkaline solutions (Lane, 1963). The decompo-
sition of diluents in TBP solvent extraction was reviewed by Tahraoui and Morris (1995). Alkanes
undergo radiolytic nitration of their carbon-centered radicals, in analogy with Equations 34.68
and 34.69 above. Stieglitz and Becker (1985) and Tripathi et al. (2001a) identied both nitro-,
and nitrosoalkanes (RNO
2
and RONO
2
) in alkanes irradiated in the presence of nitric acid. These
nitroparafns and their hydroxamic acid reaction products have been implicated in ssion product
complexation in the PUREX process. Nitroparafns are thought to be converted to the complexing
enol form by contact with the alkaline scrub intended to remove the acidic products of TBP decom-
position
(Blake etal., 1963):
RCH N==O O RCH==NO O
2
( ) ( )↔
−
(34.70)
994 Charged Particle and Photon Interactions with Matter
Hydroxamic acids (Lane, 1963; Egorov etal., 2002) are metal complexing and reducing agents
formed
from nitroparafns by the Victor Meyer reaction:
RCH NO RCONHOH
22
→ (34.71)
Although hydroxamic acids rapidly hydrolyze in acidic media, small but steady-state organic phase
concentrations have been identied in irradiated solvent (Ohwada 1968; Zaitsev etal., 1987; Huang
etal., 1989). Additional diluent radiolysis products identied in irradiated TBP–dodecane–nitric
acid include alkane oligomers, aliphatic ketones, and acids (Becker etal., 1983). Thus, stripping
difculties and poor separation factors result from a combination of higher molecular weight acidic
phosphates
from TBP radiolysis and from compounds produced by diluent nitration.
Finally,
it should be noted that the use of more stable TBP diluents results in higher yields of
HDBP. This has been attributed to the diluent ionization potential (Tahraoui and Morris, 1995).
Direct diluent radiolysis produces the diluent radical cation, as shown in Equation 34.53. A common
radical
cation stabilization route would be that of charge transfer by reaction with TBP:
[ ( ) ] ( )CH CH CH TBP CH CH CH TBP
3 2 3 3 2 3
i i+ +
+ → +
n
(34.72)
Thus, the use of very stable diluents with high ionization potential may increase the rate of TBP
degradation. It is not possible to completely eliminate adverse radiation chemical effects in an irra-
diated
solvent extraction system.
34.3.3 radiation cheMiStry in the future fuel cycle
In addition to uranium recovery by the PUREX process, future fuel cycle designs include solvent
extraction steps for the recovery of americium and curium, the so-called minor actinides. The sepa-
ration of the trivalent actinides from the trivalent lanthanides has long been one of the signicant
challenges in radiochemistry. The organophosphorous compound octylphenyldiisobutylcarbamoyl-
methylphosphine oxide (CMPO) has been proposed for the group separation of the trivalent actinides
and lanthanides in the United States (Kalina et al., 1981), whereas in European work tetraalkyl-
diamides have been proposed for the co-extraction of the actinides and lanthanides. The DIAMEX
process uses N,N′-dimethyl-N,N′-dioctyl-hexylethoxy-malonamide (DMDOHEMA) in an alkane
diluent for this purpose (Sorel etal., 2008). Similarly, the tridentate amide N,N,N′,N′-tetraoctyl-3-
diglycolamide (TODGA) has been developed primarily in Japanese work. A 0.1M dodecane solution
of TODGA is capable of extracting U and Pu, as well as Am from nitric acid solution (Sasaki etal.,
2008). Dialkylmonoamides, such as N,N-di-(2-ethylhexyl)isobutyramide (DEHiBA) have also been
developed as suggested alternatives for TBP in U and Pu extraction (Miguirditchian etal., 2008). The
potential advantages and uses of amides as actinide extractants have been reviewed by Gasparini and
Grossi (1986). While the radiation chemistry of these newer solvent extraction processes has not been
characterized as well as for TBP, a picture of their behavior is beginning to emerge.
34.3.3.1 amide
and d
iamide
r
adiolysis
The general structures of dialkylmonoamides and tetralkyldiamides are shown in Figure 34.21. The
effects of radiolysis on the solvent extraction behavior of these compounds have been investigated
by numerous researchers. For example, Ruikar etal. (1993) reported increasing D
Pu
and decreasing
D
U
over the absorbed dose range 0–1800kGy for dialkylamides irradiated in dodecane solution
after preequilibration with 3.6M nitric acid. The decrease in amide concentration and ingrowth of
the corresponding acids and amines were monitored by IR spectroscopy (Ruikar etal., 1991). The
decrease in amide concentration satisfactorily explained the decrease in uranium extraction ef-
ciency while increases in D
Pu
were postulated to result from the participation of acidic degradation
products in plutonium complexation. The distribution ratio for ssion product zirconium, D
Zr
, was also

Radiation Chemistry inNuclear Engineering 995
elevated by irradiation. Increased distribution ratios for zirconium and plutonium may also be due
to the nitrated products of diluent radiolysis, as has been reported for the PUREX solvent (Mincher
etal., 2009a,b,c). The irradiation of 0.5 M of 11 different dialkylamides in benzene solution after
pre-equilibration with 3.6M nitric acid also resulted in degradation to the corresponding amines
and carboxylic acids, with symmetrically substituted dialkylamides being more stable (Mowafy,
2004). In this work also, uranium extraction efciency decreased with increasing absorbed dose,
while
D
Zr
increased.
Mowafy (2007) concluded that within a class of compounds, stability decreased in the order:
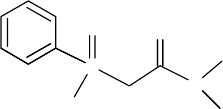
symmetrical amides > unsymmetrical amides > branched amides. The pentaalkylpropane
diamides (malonamides) have the general structure (RR′NCO)
2
CHR″, an example of which is
shown in Figure 34.22. For the malonamide with R = CH
3
, R′ = C
4
H
9
and R″ = C
2
H
4
OC
2
H
4
OC
6
H
13
more than 30% was decomposed at an absorbed dose of 500 kGy for a 1M t-butylbenzene solution
irradiated in contact with 5 M nitric acid. There was a corresponding decrease in D
Am
, although
D
U
and D
Pu
were not adversely affected. Cuillerdier etal. (1991) concluded that stability as a func-
tion of R″ increased in the order: H < C
2
H
5
< C
2
H
4
OC
6
H
13
< C
2
H
4
OC
2
H
4
OC
6
H
13
. Long oxyalky
chains appear to protect extraction efciency. Inclusion of a sacricial ether linkage in the mol-
ecule may result in the generation of relatively harmless radiolysis products while maintaining
diamide extraction capability. The radiolytic decomposition of a malonamide to the correspond-
ing acid and amide is shown in Figure 34.23. A review of amide radiolysis is provided in reference
(Mincher etal., 2009c).
34.3.3.2 diamex
and todga p
rocess
r
adiolysis
The DIAMEX solvent extraction process for recovery of
the minor actinides was developed using DMDOHEMA,
shown in Figure 34.22. The gamma-radiolysis of this com-
pound in alkane solution has been investigated by Berthon
et al. (2001) using a suite of analytical techniques includ-
ing gas chromatography, potentiometry, and mass spec-
trometry. Organic acids were a major diamide radiolysis
product, and alcohols were also created by rupture of the
ether linkages in the R″ group. No alcohols were detected in
compoundswithout an oxygen atom in that chain. Products
R΄
R
΄
O
O
N OH
R
΄
O
O
R΄
H
2
O
N N
CH
3
H
3
C
C
2
H
4
O
N
H
3
C
C
2
H
4
O
HN
CH
3
+
O
R
΄
R΄
Figure 34.23 Radiolytic decomposition of a malonamide into the corresponding amine and carboxylic acid.
O
R
R
R
R
˝
N
R
R
˝
N
R
΄
N
R
΄
R΄
O
O
Figure 34.21 The general structure of dialkylmonamides (left), and tetraalkyldiamides (right).
C
6
H
13
O
CH
3
N
N
CH
3
H
17
C
8
C
8
H
17
O O
Figure 34.22 The pentaalk-
ylpropane diamide (malonamide)
dimethyl dioctyl hexylethoxymalon-
amide
(DMDOHEMA).
996 Charged Particle and Photon Interactions with Matter
detected byBerthon etal. (2001, 2004) for DMDOHEMA radiolysis included the monoamide
methyloctylhexyloxybutanamide, the acid amide methoxyoctylcarbamoyl 4-hexyloxybutanoic
acid, the malonamides dimethyloctyl 2-hexyloxyethyl malonamide (DMOHEMA), and methyl-
dioctyl 2-hexyloxyethyl malonamide (MDOHEMA) and the amine methyloctylamine. The loss
of one amide function occurs by rupture of the carbamoyl C–N bond resulting in the amine and
an acidic amide product (Berthon etal., 2001, 2004). This occurs only in the presence of acid,
and is promoted by higher acid concentrations. Although signicant products, monoamides
are rapidly degraded in irradiated solution and the concentrations detected there are low, or
sometimes undetectable.
For DMDOHEMA, with its eight carbon R′ group, the radiolysis products are soluble in the
organic phase. A dose of 690 kGy to 1M DMDOHEMA in dodecane in contact with 4 M nitric acid
resulted in a nal malonamide concentration of 0.59M. The decrease in malonamide concentration
was accompanied by a decrease in D
Am
, D
Eu
, and D
Nd
. When solutions containing varying amounts
of the major degradation products were prepared, all products were found to interfere with Am
and Nd extraction, with the amine being the most harmful. An acidic solvent wash quantitatively
removed the amine degradation product while an alkaline wash removed about 80% of the acidic
amide (Nicol etal., 2000). The combination of an acid wash, followed by water scrubbing and then
an alkaline wash restored the solvent’s extraction capabilities to that expected for the decreased
malonamide
concentration (Berthon etal., 2004; Bisel etal., 2007).
Modolo
etal. (2008) investigated the post-irradiation solvent extraction performance of 0.2 M
TODGA in hydrocarbon diluent. The D
Am
gradually decreased with absorbed dose for TODGA
and TODGA/TBP mixtures irradiated in the presence and absence of nitric acid, while the D
Eu
remained unchanged over the absorbed dose range 0–1000 kGy. The products of TODGA radi-
olysis include dioctylamine, dioctylacetamide, dioctylglycolamide, and dioctylformamide (Sugo
etal., 2002, 2007). The presence of nitric acid did not enhance radiolytic degradation but did
favor cleavage of the C–N bond to form the amine and acidic products over ether bond cleavage
to form acetamide and glycolamide. These results are analogous to those described above for
diamide radiolysis.
34.3.3.3 truex process
r
adiolysis
The compound octylphenyldiisobutylcarbamoylmethylphosphine oxide (CMPO), used in combina-
tion with TBP in an alkane diluent in the TRUEX (TRansUranic EXtraction) process, is a phos-
phorous-containing amide, and is shown in Figure 34.24. The irradiation of this solvent system in
contact with an acidic aqueous phase results in decreased forward extraction distribution ratios for
Am (D
Am
), but not to the extent predicted by loss in CMPO concentration (Chiarizia and Horwitz,
1986). Stripping distribution ratios exceeded those of the forward extraction following irradiation to
an absorbed gamma-dose of 195kGy. Similar effects were encountered for only 36kGy delivered
from an alpha-source (Buchholz etal., 1996). Cleavage of the C–N bond was proposed to result in
decomposition of CMPO to a carboxylic acid and an amine, followed by decarboxylation of the car-
boxylic acid to a phosphine oxide, in analogy with the reactions for DMDOHEMA discussed above.
Oxidation of the phosphine oxide would produce a phosphinic
acid. Products of this nature have been measured (Chiarizia and
Horwitz, 1986; Nash etal., 1988, 1989; Buchholz etal., 1996).
The occurrence of degradation product phosphine oxides may
explain the reasonably high forward D
Am
despite a loss in CMPO
concentration, while the phosphinic acids would complex Am
to prevent adequate stripping under low aqueous phase acidity
conditions. The kinetic constants for the reactions of the major
radicals produced by diluent radiolysis have not been measured
for CMPO, TODGA, or DMDOHEMA.
O
O
C
4
H
9
P
N
H
17
C
8
C
4
H
9
Figure 34.24 The structure of
octylphenyldiisobutylcarbamoyl-
methylphosphine
oxide (CMPO).
Radiation Chemistry inNuclear Engineering 997
34.3.4 radiation cheMiStry and actinide oxidation StateS
34.3.4.1 radiolytic production of nitrous acid
The radiolytic production of nitrous acid in irradiated nitric acid has consequences for nuclear solvent
extraction because of its affect on metal valence. Probably, the most important actinide affected is Np.
Highly extractable by the PUREX process in the tetra-, (Np
4+
) and hexavalent (NpO
2
2+
) states, Np is
inextractable in the pentavalent (NpO
2
+
) state. Attempts to co-extract Np with U and Pu, or to prevent
Np extraction during the PUREX process are often confounded by changes in Np valency during the
extraction. The oxidation state of neptunium in aqueous nitric acid depends on the concentration of
nitrous acid according to Equation 34.73 (Siddall and Dukes, 1959):
NpO / H / NO NpO / HNO H O
22 3 2
2
2
3 2 1 2 1 2
+ + − +
+ + ↔ + + (34.73)
Neptunium(V) is stable in the absence of nitrous acid, however, it is oxidized to NpO
2
2+
with small
amounts of added nitrous acid, at increasing rates with increasing nitrous acid concentration. For
example, 5 × 10
−5
M HNO
2
was found to oxidize neptunium with a half-time of 22min in 3M HNO
3
(Siddall and Dukes, 1959). However, very high concentrations will shift the equilibrium of Equation
34.73
back to the left, favoring NpO
2
+
.
The direct radiolytic production of nitrous acid was shown in Equations 34.47 and 34.48. Indirect
sources were shown in Equations 34.49 through 34.51. The reaction of the hydroxyl radical with
•
NO
3
radical, shown in Equation 34.74 is an additional source (Matthews etal., 1972):
i i
OH NO HNO O HNO
2
+ → → +
3 4 2
(34.74)
Sources of the
•
NO
2
radical include the reaction of nitrate anion with the hydrogen atom in Equation
34.75 (Buxton etal., 1988), the hydrolysis of the
•
NO
3
radical, shown in Equation 34.76 (Vladimirova
and Milovanova, 1972), and the
•
NO
3
radical addition reaction shown in Equation 34.77 (Matthews
etal.,
1972):
NO H NO OH M s
3 2
7 1 1
1 10
− − − −
+ → + = ×
i i
k
(34.75)
i i
NO H O NO H O
3 2
+ → +
2 2 2
(34.76)
i
i
i
NO NO N O O NO
3 3 2
+ → → +
6 2 2
2
(34.77)
Production of the
•
NO
2
radical by any of the above routes is followed by the addition reaction to
produce N
2
O
4
, ultimately resulting in nitrous acid (Sworski etal., 1968; Grätzel etal., 1969): This
was
shown in Equations 34.50 and 34.51.
Radiolytic
reactions also deplete the nitrous acid concentration in irradiated nitric acid.
The hydroxyl radical acts as a sink for nitrous acid produced during radiolysis (Bugaenko and
Roshchektaev,
1971):
i i
OH HNO H O NO M s
2
+ → + = ×
− −
2 2
9 1 1
2 6 10k .
(34.78)
Hydroxyl radical addition reactions also oxidize
•
NO
2
radical to nitrate anion in Equation 34.79
(Vione etal., 2003), and add to generate hydrogen peroxide in Equation 34.80 (Buxton etal., 1988):
i
i
OH NO HOONO H NO M s
2
+ → → + = ×
+ − − −
3
9 1 1
4 5 10k . (34.79)
998 Charged Particle and Photon Interactions with Matter
i
i
OH OH H O M s
2 2
+ → = ×
− −
k 5 5 10
9 1 1
.
(34.80)
The reaction of hydrogen peroxide with nitrous acid oxidizes nitrous acid back to nitric acid, as was
shown in Equation 34.52 (Bhattacharyya and Veeraraghavan, 1977; Vione etal., 2003). Similarly,
•
NO
3
radical can oxidize nitrous acid (Bugaenko and Roshchektaev, 1971):
HNO NO NO H NO
2 3 3 2
+ → + +
− +
i i
(34.81)
Maximum achievable concentrations of nitrous acid are limited by these oxidation reactions, and by the
reversible decomposition to simple oxides of nitrogen, as shown in Equation 34.82 (Park and Lee, 1988):
2HNO NO NO H O
2 2 2
↔ + +
i
i
(34.82)
Park and Lee (1988) modeled that nitrous acid decomposition by the route in Equation 34.82 becomes
signicant at concentrations >10
−5
M. Additional loss of nitrous acid in open systems occurs through
the volatilization of
•
NO and
•
NO
2
, which have limited nitric acid solubility (Andreichuk etal.,
1984). Andreichuk etal. (1984) found that the loss of nitrous acid was reduced by limiting the vol-
ume of air in contact with the system. Thus, a complicated suite of reactions compete to produce
and remove nitrous acid in irradiated aqueous nitric acid. The ability to model and predict its con-
centration under various solution conditions is limited by the lack of the fundamental bimolecular
rate constants for many of these reactions.
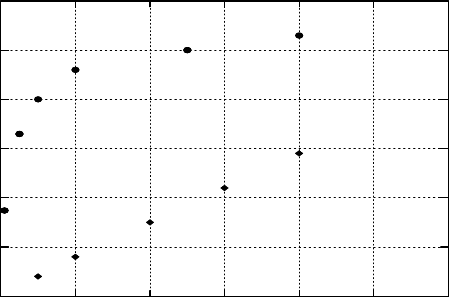
However,
empirical nitrous acid radiolysis yields, G
(HNO
2
)
, have been measured by several authors.
The yield is affected by total absorbed dose, dose rate, radiation LET, and nitric acid concentration.
Bhattacharyya and Saini (1973) reported that G
(HNO
2
)
varied linearly with absorbed gamma-dose using
a
60
Co source with a dose rate of 3kGy h
−1
. This resulted in a value of ∼0.24μmol J
−1
in 3M HNO
3
.
When G
(HNO2)
was evaluated versus nitric acid concentration, it was found to increase with increasing
concentration, as shown in Figure 34.25. The yield was found to be the same in air-free and air-saturated
solution. These data are considered maximum yields, as the produced nitrous acid was reacted in situ
with the colorimetric reagent sulfanilamide for quantitation (Bhattacharyya and Saini, 1973). This
eliminated competition from oxidizing agents that might limit the HNO
2
concentration and, therefore,
the true yield will be lower due to the reactions shown in Equations 34.52, 34.78, and 34.81.
0.25
0.3
0.2
0.1
0.15
G
HNO
2
(μmol J
–1
)
0.05
0
0 2 4 6 8 10 12
Nitric acid concentration (M)
Figure 34.25 Nitrous acid yield in gamma-irradiated nitric acid. Curve a is data of Bhattacharyya and
Saini (1973) (dose rate = 3kGy h
−1
)in the presence of sulfanilamide. Curve b is data of Vladimirova etal.
(1969)
(Dose rate = 6
kGy
h
−1
) without scavenger.
Radiation Chemistry inNuclear Engineering 999
Also shown in Figure 34.25 are the data of Vladimirova etal. (1969), collected at a dose rate of
6 kGy h
−1
. They irradiated nitric acid in the absence of sulfanilamide, and the actual G
(HNO
2
)
was ∼0.06μmol J
−1
, in 3M HNO
3
, about one-fourth of the value reported by Bhattacharyya and
Saini (1973). The relationship between the yield of nitrous acid and the nitric acid concentration was
linear. Bugaenko and Roshchektaev (1971) reported a linear relationship between total absorbed
dose and nitrite ion concentration. They reported a G
NO
2
−
of 0.07μmol J
−1
for 3M HNO
3
at a dose
rate of 36kGy h
−1
. Similar results were reported by Jiang etal. (1994) also using gamma-irradia-
tion. Production of nitrous acid was proportional to absorbed dose over the investigated range of
250–750Gy, and to nitric acid concentration in the range 2–8 M. The maximum measured value
for G
(HNO
2
)
at a dose rate of 84 Gy h
−1
was 0.1 μmol J
−1
in 8M nitric acid. These authors calculated
a maximum possible yield of 0.32μmol J
−1
. As the concentration of nitric acid increases, the direct
radiolysis of nitric acid becomes more important, resulting in the production of additional nitrous
acid. The nitrous acid yield may be increased in the presence of
•
OH radical scavengers (Vladimirova
and Milovanova, 1972), which would suppress the reactions shown in Equations 34.52 and 34.78
through 34.80. Such scavengers would include the organic diluents and complexing agents used in
solvent extraction. Using the data of Vladimirova etal. (1969), it may be calculated that an absorbed
dose
of 10
kGy
would generate about 7 × 10
−4
M HNO
2
in 3M HNO
3
.
High
LET alpha-radiolysis is of obvious concern in neptunium solvent extraction, and a number
of studies have been performed using
244
Cm irradiation. Bibler (1974) concluded that the direct
radiolysis of nitric acid proceeded in the same fashion for alpha-radiolysis as for gamma-radiolysis,
resulting in the same oxygen (and therefore presumably the same nitrous acid according to Equation
34.47) yield. However, Vladimirova and Milovanova (1972) reported that the production of HNO
2
was greater for alpha-radiolysis. It depended positively on nitrate ion concentration and dose rate,
but the yield decreased with increasing absorbed dose. The increase in hydrogen peroxide con-
centration with absorbed dose, for which the yield is greater for alpha-radiolysis (Equation 34.35),
probably explains the decrease in nitrous acid generation. Andreichuk etal. (1984) reported similar
results. Nitrous acid concentrations increased quickly with absorbed dose, but then leveled out to
reach an equilibrium concentration that was positively dependent on the nitric acid concentration
and the dose rate. Taking into account the higher hydrogen peroxide yield of high LET radiation,
they calculated equilibrium concentrations of nitrous acid under various conditions. At a dose rate
of 11.5 kGy h
−1
, for example, this varied from 1 × 10
−3
M HNO
2
in 1M HNO
3
to 8.7 × 10
−3
M HNO
2
in 7.5 M HNO
3
. A value of 6.8 × 10
−3
M HNO
2
was reported for 3.7 M HNO
3
. According to Siddall
and Dukes (1959), this concentration of nitrous acid is sufcient to oxidize Np(V) to Np(VI) with a
half-time
of only 6
min
in 4.0
M
HNO
3
.
34.3.4.2 actinide
r
eactions
with Free r
adicals
The reaction of neptunium with the primary oxidizing and reducing agents generated by water and
nitric acid radiolysis may also alter neptunium oxidation states. The fundamental bimolecular rate
constants for many neptunium (and the other actinide) reactions with radiolytically produced radicals
have been measured (Table 34.8). Using Np as the example, those of interest in irradiated aqueous
nitric
acid are shown in Table 34.8 with a comprehensive collection provided by Mincher and Mezyk
(2009). It can be seen that the aqueous electron is a powerful reducing agent with fast kinetics for
reaction with neptunium cations. However, in the strongly acidic solutions used in solvent extraction,
the reactions of the aqueous electron may be discounted due to its fast reactions shown in Equations
34.34. The produced hydrogen atom is less reactive, but is likely to be a reducing agent for neptunium.
However, its ability to react with neptunium ions in solution will be limited by competition from
oxygen to produce the less reactive hydroperoxyl radical, as shown in Equation 34.39. The hydrogen
atom will also be scavenged by other dissolved metal ions such as uranium, which may be present at
higher concentrations. Hydroxyl radical oxidizes Np
4+
and NpO
2
+
. However, its affects on the solvent
extraction system will depend on the extent to which it is scavenged by organic compounds, for which
