Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

980 Charged Particle and Photon Interactions with Matter
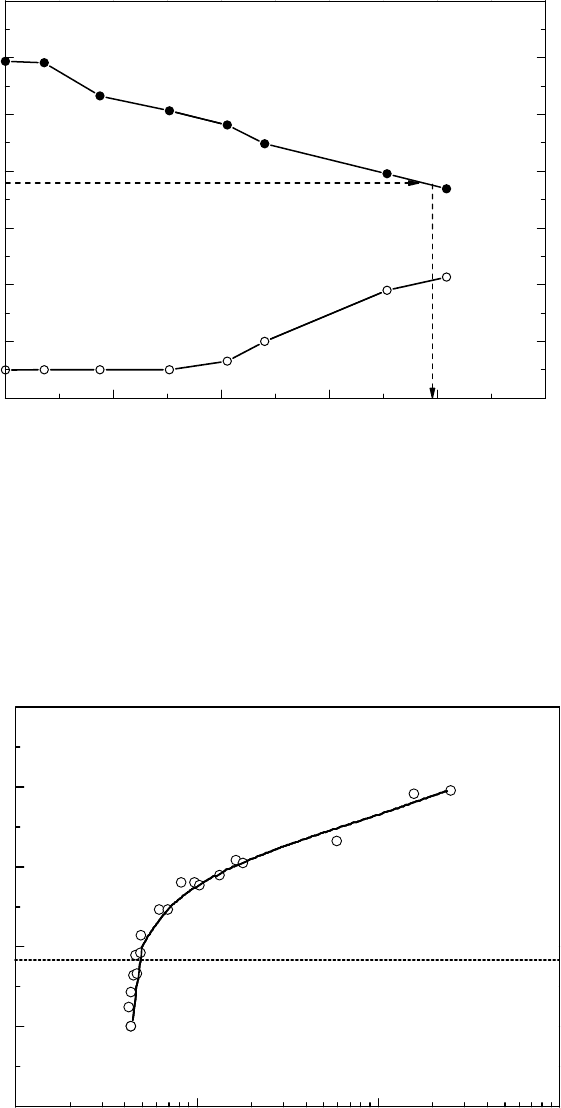
The corrosion potential of the stainless steel at the ECP sensor unit decreases with the decrease
in the dissolved oxygen concentration in the RPV bottom drain line. There is a unique relationship
between the corrosion potential and the oxygen concentration, as shown in Figure 34.13. The cor-
rosion potential decreased sharply in the range of the dissolved oxygen concentration below 10 ppb.
However, it should be noted that the corrosion potential was measured at the ECP sensor unit loca-
tion whereas the oxygen was measured at the end of the sampling line far apart from the sensor
location.
200
400
0
–200
EPRI guidelines –230 mV
SHE
1000100101
–600
–400
Dissolved oxygen in reactor water (ppb)
Corrosion potential (mV
SHE
)
Figure 34.13 Correlation between corrosion potential and dissolved oxygen in bottom drain under hydro-
gen
water chemistry.
400 14
0
200
Corrosion potential
in RPV bottom drain
10
12
Normalized average MSLRM reading
–200
EPRI guidelines –230 mV
SHE
8
–600
–400
Main steam line radiation
4
6
0.0 0.2 0.4 0.6 0.8 1.0
–1000
–800
Dissolved hydrogen in feedwater (ppm)
Corrosion potential (mV
SHE
)
0
2
Figure 34.12 Behavior of bottom drain line corrosion potential against hydrogen injection rate.
Radiation Chemistry inNuclear Engineering 981
The corrosion potential behavior has a strong dependency on the ow rate (Ichikawa etal., 1992,
1994). The relationship between the corrosion potential and the dissolved oxygen (in Figure 34.6)
was obtained with a ow rate of 3.1 m s
−1
. In the slower ow rate region, the corrosion potential
decreases
faster with the decrease of oxygen.
34.2.5.6
hydrogen
w
ater
Chemistry s
ummary
Through the long-term verication program, hydrogen water chemistry control has been veried
as an environmental countermeasure against potential IGSCC issues for BWRs. In order to evalu-
ate the effectiveness of hydrogen water chemistry control on the protection of the vessel bottom
region, a high ow and in situ corrosion potential measurement device was developed and installed
in a branched sampling line from the RPV bottom drain line. In this program, 0.8ppm hydrogen
in feedwater was necessary to achieve a corrosion potential of −230mV (SHE) at the vessel bottom
region. The ow rate effect on the corrosion potential should be taken into account to predict corro-
sion
potential distributions in the BWR primary system.
34.2.6 developMent of an ecp SenSor for bwr applicationS
An experimental approach to directly measure the corrosion potential at BWRs is important to
verify the modeling approach. In order to measure the corrosion potential, ECP sensors are used as
reference
electrodes and working electrodes.
34.2.6.1
reference
electrode
A
reference electrode is absolutely necessary for the ECP measurement. It shows its own potential,
which is not affected by water chemistry or ow changes. In BWR systems, a reference electrode,
such as silver/silver chloride (Ag/AgCl) or iron/iron oxide (Fe/Fe
3
O
4
), has been used in high-
temperature
water.
Under
hydrogen water chemistry control conditions, a platinum electrode is used as a reference
electrode because it works as a theoretical hydrogen electrode. It also has a longer lifetime com-
pared with the conventional reference electrodes. Therefore, it has long been accepted that both
conventional reference and platinum reference electrodes can be used.
34.2.6.2 working
electrode
Stainless
steel or nickel-based alloys are used as working electrodes that behave similar to plant
structural materials. Attention should be paid to the initial value before the electrode is prelmed.
The plant structural material itself can be regarded as a working electrode when the ground poten-
tial
from the plant is measured against the reference electrode.
34.2.7 radiation effectS on corroSion in nuclear reactor SySteMS
The radiation effects on material corrosion in nuclear reactor systems is typically classied as a direct
radiation effect on materials and an indirect effect through the change of the water chemical environ-
ment. The former is often recognized as radiation damage by neutrons or heavy ions, and the latter is
comprehended as enhanced corrosion in liquid underirradiation. In this chapter, the relationshipbetween
the corrosion behavior and water radiolysis is discussed based on laboratory experimental ndings.
34.2.7.1 radiolysis
e
ffects
on the w
ater
e
nvironment
34.2.7.1.1
Production of Oxidant Species by Radiation
Oxidizing radiolytic products enhance material corrosion due to their contributions to the cathodic
reactions.
Anodic reactions M M ne: = +
+ −n
(34.1)
982 Charged Particle and Photon Interactions with Matter
Cathodic reactions O H e H O
2
: + + =
+ −
4 4 2
2
(34.3)
H O H e H O
2 22
2 2 2+ + =
+ −
(34.4)
OH e OH+ =
− −
(34.31)
HO H e H O
22 2
+ + =
+ −
(34.32)
O H e H O
22 2
2
− + −
+ + =
(34.33)
Under irradiation conditions, various kinds of oxidizing radicals or species are produced,
which contribute to the cathodic reactions and enhance the corrosion reaction. The distribution
of the yield of each species is dependent on the quality of radiation known as linear energy
transfer (LET).
When reducing species like hydrogen coexist in the system, the radiation effect does not
always enhance the corrosion but sometimes reduces the oxidizing species concentrations due to
the recombination effect. As a result, the cathodic reactions are suppressed and so is the corro-
sion reaction.
34.2.7.1.2
Corrosion Behavior by Radiolytic Oxidant Species
34.2.7.1.2.1
Stainless Steel Corrosion behavior of materials in reactor systems is due to the
concentrations of the radiolytic oxidizing species such as oxygen, hydrogen peroxide, and radical
species. Those concentrations are inuenced by radiation. The oxidizing power of the system is
described by an ECP on the material surface, which is a function of not only the chemical species
concentrations
but also the oxide lm and hydrodynamic conditions.
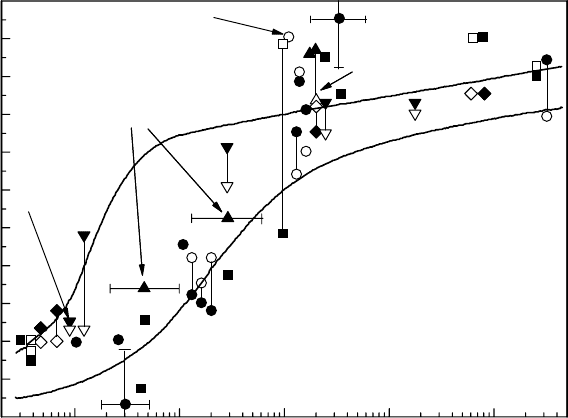
In
the case of stainless steel corrosion in high-temperature water, both the general corrosion rate
and the SCC susceptibility are high at high corrosion potentials. Figure 34.14 shows the radiation
0.2
0.3
0.4
200 ppb H
2
O
2
31 ppb Cu
2+
–0.1
0.0
0.1
200 ppb H
2
H
2
injection conditions
–0.4
–0.3
–0.2
E
corr
(V
SHE
)
1 10 100 1000 10,000
–0.7
–0.6
–0.5
Dissolved oxygen (ppb)
Figure 34.14 The radiation effect on ECP of stainless steel.
Radiation Chemistry inNuclear Engineering 983
effect on the ECP of stainless steel (Andresen, 1992). Figure 34.15 also shows the gamma-ray effect
on the ECP of stainless steel, which increases under excess oxygen conditions due to the production
of hydrogen peroxide, and decreases under excess hydrogen conditions due to the recombination
reaction
of the oxidizing species (Ichikawa etal., 1987).
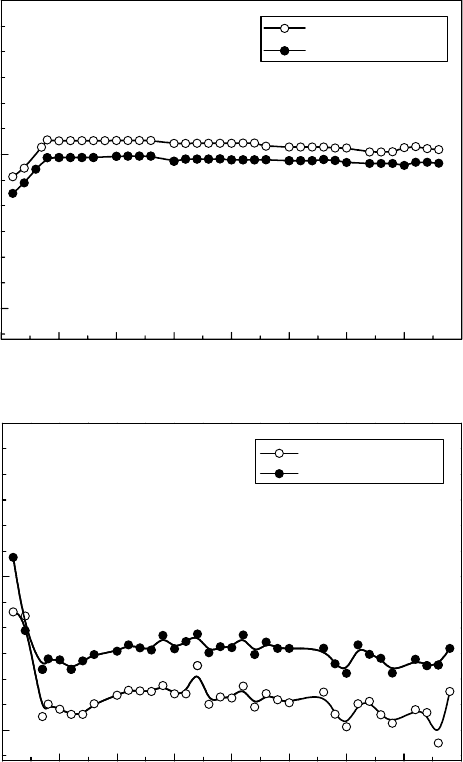
The
same tendency is shown in the case of SSRT (slow strain rate test) results to evaluate the
IGSCC susceptibility of sensitized Type 304 stainless steel, as shown in Figure 34.16 (Saito etal.,
1990). The IGSCC ratio on the fracture surface of the test specimen is reduced by gamma irradia-
tion
under hydrogenated conditions.
Figure
34.17 shows that the critical corrosion potential for IGSCC susceptibility is understood
in relation to ECP and it is almost the same regardless of the gamma irradiation (Saito etal., 1997).
However, for general corrosion, iron dissolution rates from stainless steel in high-temperature water
are accelerated by gamma-ray irradiation, as shown in Figure 34.18 (Ishigure etal., 1980). Figure
34.19 shows that the metal dissolution rate from stainless steel is also accelerated by hydrogen peroxide
addition
and gamma-ray irradiation (Hemmi etal., 1994).
500
Gamma-irradiation
Non-irradiation
0
E
corr
(mV
SHE
)
0 5 10 15 20 25 30 35 40
–500
Loop operating days (day)(a)
500
Gamma-irradiation
Non-irradiation
0
–500
0 5 10 15 20 25 30 35 40
Loop operating days (day)
(b)
E
corr
(mV
SHE
)
Figure 34.15 The gamma-rays effect on ECP of stainless steel. (a) Oxygen excess condition and (b) hydro-
gen
excess condition.
984 Charged Particle and Photon Interactions with Matter
34.2.7.1.2.2 Other Materials According to the above discussion, it seems acceptable to simulate
the irradiation effect by adding suitable amounts of hydrogen peroxide to the system based on the
nding that the oxidizing power generated by radiation is in most part due to the accumulated hydro-
gen peroxide concentration. The general corrosion rate of Alloy X750 (Ni-based alloy) and Stellite #6
(Co-based alloy) is reported to be enhanced by four times under the BWR core simulated condition
with the addition of hydrogen peroxide (Hemmi etal., 1994). Zircaloy is used as fuel cladding mate-
rial for both BWR and PWR reactors. It is reported that perhydroxy radical (HO
2
•
or O
2
•−
) produced
in the high radiation elds in the core plays a role in zircaloy corrosion (Hurst and Tyzack, 1974).
100
75
71
77
Gamma-irradiation
80
74
40
60
40
26
37
20
<1 0 0 0
24
IGSCC ratio (%)
0
0.1 0.32 0.56 0.1 0.32 0.56
Conductivity (μS cm
–1
)
(a) (b)
Non‐irradiation
Figure 34.16 SSRT (slow strain rate test) experiment to evaluate IGSCC susceptibility of sensitized Type
304 stainless steel, sensitized at 650°C for 3h. (a) BWR normal water chemistry condition at 250°C. (b) BWR
hydrogen
water chemistry condition at 250°C.
80
Gamma-irradiation
60
Non-irradiation
40
IGSCC ratio (%)
No IGSCC
0
20
–800 –600 –400 –200 0 200 400
E
corr
(mV
SHE
)
Figure 34.17 Critical corrosion potential of sensitized Type 304 stainless steel for IGSCC susceptibility at
the
SSRT speed of 4 × 10
−7
s
−1
. Sensitized condition is 24h at 620°C.
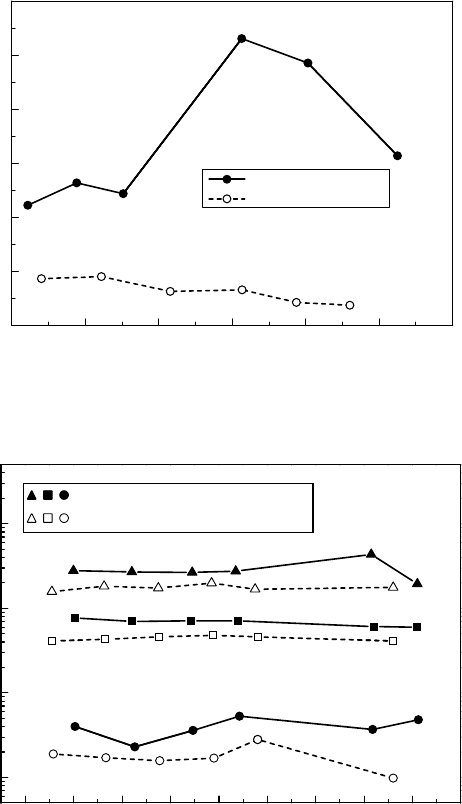
Radiation Chemistry inNuclear Engineering 985
34.2.8 radiation effect on MaterialS
It is widely known that direct neutron irradiation effects to the structural materials should be considered
in the core region. Austenitic stainless steel with solution heat treatment for an IGSCC countermea-
sure sometimes shows IGSCC under neutron irradiation. This is called irradiation-assisted intergranular
stress corrosion cracking (IASCC). Although the mechanism is not yet fully understood, it is speculated
that irradiation-assisted segregation in grain boundaries is a cause of corrosion property changes.
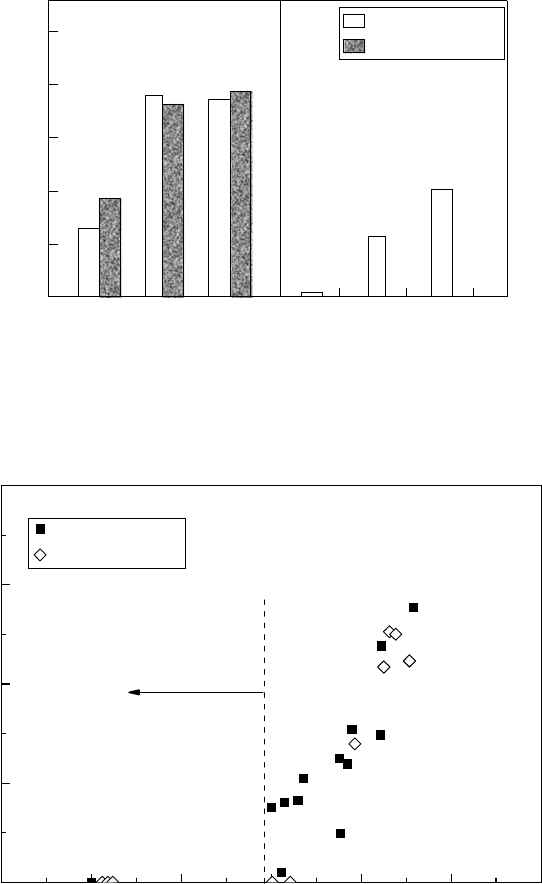
SCC susceptibility of austenitic stainless steel under neutron irradiation is shown in Figure
34.20 (Andresen etal., 1989). From the high-temperature water SSRT result with 32ppm of dis-
solved oxygen, the IASCC susceptibility increases with more than 5 × 10
24
nm
−2
(E > 1 MeV) of
neutron uence and becomes signicant with more than 2 × 10
25
nm
−2
(E > 1 MeV) of neutron
uence.
250
300
200
100
150
Gamma-irradiation
Non-irradiation
0
50
Crud concentration of Fe (ppb)
0 50 100 150 200 250 300
Time (h)
Figure 34.18 Acceleration of iron dissolution rate from Type 304 stainless steel in high-temperature water
at
250°C under 200
ppb
O
2
.
10
H
2
O
2
addition, gamma-irradiation
No H
2
O
2
, non-irradiation
1
Cr
Ni
0.1
4 6 8 10 12 14 16 18 20 22
0.01
Fe, Ni, Cr concentration (ppb)
Fe
Loop operating days (day)
Figure 34.19 Effect of gamma-rays and H
2
O
2
addition to the acceleration of iron dissolution rate from
Type
316 stainless steel in high-temperature water containing 400 ppb of O
2
and 60 ppb of H
2
at 270°C.
986 Charged Particle and Photon Interactions with Matter
The essential countermeasure to this level of radiation damage must be through material improve-
ment. However, research may eventually provide chemical control mitigations that will also work
to
increase corrosion resistance.
34.2.9 concluSion
The evaluation of the corrosion environment in BWRs is discussed from the viewpoint of both
modeling and experimental approaches. The comparison between measurement and calculation is
of
signicance for establishing the countermeasures for plant aging issues.
Corrosion
potential control is essential in reducing the IGSCC susceptibility of BWR primary
loop components. Two basic approaches for reducing the corrosion potential are reviewed. One is
cathodic reaction control and the other is anodic reaction control.
By applying the latest radiolysis and ECP models, normal water chemistry and hydrogen water
chemistry
control can be well evaluated.
34.3 radiation-induCed proCesses in the
spent
n
uClear
Fuel repro
Cessing
Increased human populations and industrialization are causing increased energy demands at the
same time that concerns about rising carbon dioxide concentrations in the atmosphere have made
fossil fuels less attractive. Opposition to nuclear power has decreased with a renewed interest being
expressed in many countries (Boullis, 2008). However, among the reasons cited by opponents against
nuclear energy are difculties with the disposition of nuclear waste. About 10,500t of spent fuel are
already discharged yearly from more than 400 nuclear reactors (IAEA, 2004). The rate of spent fuel
generation could reach 15,000t by 2050 (Laidler, 2008), and this spent fuel contains appreciable
quantities of actinides and ssion products, some with very long half-lives. For example, 1t of UO
2
fuel, after 30GW days of burnup, contains 950kg U, 9kg Pu, 75 g Np, 140g Am, 47 g Cm, and 31kg
ssion products (Benndict etal., 1981). With half-lives ranging from 433 years for
241
Am to 2.4 mil-
lion years for
237
Np, the alpha-emitting actinide nuclides determine the radiotoxicity of the waste
80
100
IASCC threshold
60
High stress
Low stress
O
2
(ppm)
γ (Rh
–1
)
40
32 —
0.2
20
×
0.2 —
0.005 —
1E19 1E20 1E21 1E22
0
Neutron ux E > 1 MeV (n cm
–2
)
Sensitivity of IASCC (IGSCC ratio (%))
2×10
6
Figure 34.20 SCC susceptibility of Type 304 stainless steel under neutron irradiation at 288°C.
Radiation Chemistry inNuclear Engineering 987
for periods of time on a geological scale. To address this, research programs in several countries are
investigating the partitioning of these radionuclides and their transmutation to short-lived isotopes
to
signicantly reduce the long-term hazard (OECD-NEA, 1999).
Aqueous
solvent extraction is the most mature of these partitioning strategies, beneting from
more than 60 years of research and experience at the industrial scale (Mathur etal., 2001). As cur-
rently performed, an alkane solution of tributyl phosphate (TBP) is used to complex and extract
U, Np, and/or Pu from nitric acid solutions of dissolved spent fuel. The process is called PUREX
(Plutonium Uranium Rening by EXtraction) and remains successful because it provides high
yields of the desired elements and high separation factors from the undesired elements, while pro-
ducing
only a small amount of secondary waste.
Looking
to the future, many countries are currently investigating solvent extraction processes
for the separation of the minor actinides Am and Cm from dissolved nuclear fuel. This separation
would both decrease the radiotoxicity of waste prior to deep geological repository disposal and
produce additional energy by burning these elements in a reactor. While none of the advanced pro-
cesses have yet been implemented, their development has reached demonstration tests at the labora-
tory scale (Nash etal., 2006). The eventual application of such a partitioning strategy will result in
natural uranium savings, uranium enrichment cost savings, simpler and safer waste disposal, and
provide
the potential for the recovery of other useful elements from spent fuel (Boullis, 2008).
The
new extraction schemes vary in their details, but following the traditional PUREX extraction
all would extract the minor actinides using hard donor (oxygen containing) ligands. Unfortunately,
undesirable short-lived lanthanide ssion products are also extracted and they are neutron poisons
that must then be separated from Am and Cm, prior to incorporating the actinides in fuel. This
second extraction employs soft donor ligands (usually nitrogen containing) capable of perform-
ing this difcult separation. Since these new complexing agents will be used in highly radioactive
applications, the effects of radiation chemistry on their performance must be understood prior to
their reliable application. Based on PUREX experience, the main effects may include decrease in
extraction efciency due to decomposition of the ligand, decrease in separation factors due to the
accumulation of radiolysis products that are also complexing agents, and deteriorated phase separa-
tion performance due to ligand or diluent radiolysis products (Pikaev etal., 1988). In addition, the
irradiation of aqueous nitric acid produces reactive species that can alter the oxidation states of the
metals to be complexed, affecting their extraction efciency (Pikaev etal., 1988). This chapter will
examine the conditions and reactive species to be expected in the irradiated biphasic organic/aque-
ous environment of the solvent extraction process, and review what is known about radiolysis effects
on
selected ligands and metals during solvent extraction for nuclear reprocessing applications.
34.3.1 radiolytically produced reactive SpecieS in the biphaSic SySteM
34.3.1.1 produced species in the aqueous phase
The direct radiolysis of ligands in a solvent extraction solution occurs in proportion to their abun-
dance as constituents of that solution. Since ligands are normally present in millimolar concentra-
tions, the diluent absorbs most of the radiation energy. Reactive species created by diluent radiolysis
are largely responsible for the extent and nature of the degradation of ligands and changes the physi-
cal characteristics of the solvent. The direct radiolysis of alkanes and aqueous nitric acid results in
the formation of electronically excited states, free radicals, and ions in spurs along the track of the
incident particle. These reactive species undergo recombination to create molecular species, or they
diffuse away from their point of origin to react with solutes in the bulk solution. The probability
of recombination or diffusion depends on the LET (eV nm
−1
) of the incident particle, which for x,
γ, and β
−
is much lower than for alpha particles. Reactive species that escape these spurs are the
source of the secondary radiolysis reactions that degrade ligands or react with metal ions, and this
phenomenon
begins to occur at about 100
ns
after the initial event.

988 Charged Particle and Photon Interactions with Matter
For water irradiated by low LET particles such as x-rays, gamma-rays, or electrons (β
−
particles)
generated mainly by ssion product decay, the species in Equation 34.34 are produced with the
yields
(G-values in μmol J
−1
) shown in brackets (Buxton etal., 1988):
H
2
O [ . ] [ . ] [ . ] [ . ] [ . ] [ . ]0 28 0 27 0 06 0 07 0 27 0 05
2 2 3
i i
OH e H H O H O
aq
+ + + + +
− +
HH
2
(34.34)
The most reactive species produced are the oxidizing hydroxyl radical (
•
OH) and hydrogen peroxide
(H
2
O
2
), and the reducing aqueous electron (e
aq
−
) and hydrogen atom (H
•
). Massive, highly charged
particles such as alpha-particles (He
2+
) generated mainly by actinide decay have short ranges and
high LET (156eV nm
−1
for 5MeV He
2+
) (Pastina and LaVerne, 1999) and therefore deposit energy
in closely spaced overlapping spurs. This results in high localized concentrations of reactive spe-
cies, many of which undergo recombination before they can diffuse into the bulk solution. Thus,
higher yields of molecular species and lower yields of radicals are found. Using the data of Lefort
and Tarrago (1959) for 5.3MeV
210
Po alpha-particles, the water radiolysis equation may be rewritten:
H
2
O [ . ] [ ] [ . ] [ . ] [ ] [ . ]0 05 0 06 0 15 0 16
2 2 3 2
i i
OH x e H H O x H O H
aq
+ + + + +
− +
(34.35)
The yields for the aqueous electron and hydronium ion are not given because the measurements
were made in acidic solution. It is seen that the yield of the molecular product hydrogen peroxide
due to alpha radiolysis of water is double that for gamma-radiation. Comparatively, few studies have
been
done using alpha-radiolysis, and this chapter will cover mainly low LET effects.
Despite
an initially equal production of oxidizing and reducing species, under the conditions of
nuclear solvent extraction, the aerated, nitric acid system will be predominantly oxidizing due to
fast
scavenger reactions. The aqueous electrons and hydrogen atoms are scavenged according to
e H H M s Buxton et al
aq
− + − −
+ → = ×
i
k 2 3 10 1988
10 1 1
. ( ., )
(34.36)
e O O M s Buxton et al
aq
− − − −
+ → = ×
2 2
9 1 1
1 9 10 1988
i
k . ( ., )
(34.37)
e NO NO M s El liot,
aq
− − − − −
+ → = ×
3 3
2 9 1 1
9 7 10 1989
i
k . ( )
(34.38)
i
H O HO Gordon et al+ → = ×
2 2
10
2 1 10 1964
•
k . ( ., ) (34.39)
i i
HO H O p Bielski et al
a2 2
4 8 1985↔ + =
+ −
K . ( ., )
(34.40)
This leaves H
2
O
2
and the strongly oxidizing
•
OH radical as the most important species in solution.
The
•
OH radical reacts with organic solutes by hydrogen abstraction, addition to unsaturated carbon
bonds, or with organic and inorganic solutes by electron transfer reactions. Rate constants for
•
OH
radical reactions have been tabulated (Dorfman and Adams, 1973; Buxton etal., 1988). The
•
HO
2
radical produced in Equation 34.39 is also an oxidizing agent, although its reactions have not been
well
studied.
Additional
reactive species are created by nitric acid radiolysis. A thorough review of the radia-
tion chemistry of nitric acid is given by Katsumura (1998). Among the most important species, the
oxidizing
•
NO
3
radical is produced indirectly by the reaction of the
•
OH radical with undissoci-
ated nitric acid and directly by radiolysis of the nitrate anion dissociation product of nitric acid
(Katsumura
etal., 1991):

Radiation Chemistry inNuclear Engineering 989
i i
OH HNO NO H O M s+ → + = ×
− −
3 3 2
7 1 1
5 3 10k .
(34.41)
NO NO
aq3 3
− −
+e
i
(34.42)
The reactions of the
•
NO
3
radical are similar to those of the
•
OH radical, including hydrogen atom
abstraction, addition, and electron transfer reactions, although with decreased electrophilic charac-
ter and lower reaction rates (Neta and Huie, 1986; Shastri and Huie, 1990). Rate constants for its
reaction
with numerous species have been compiled (Neta etal., 1988).
In
acidic solution, the NO
3
2−•
radical product of Equation 34.38 will protonate (Grätzel etal., 1970):
NO HNO H NO p a p a
3
2
3 2 3 1 2
4 8 7 5
− −
↔ ↔ = =
i i i
K K. , .
(34.43)
The
product H
2
NO
3
•
decays to produce the
•
NO
2
radical (Løgager and Sehested, 1993):
H NO NO H O s
2 3 2 2
5 1
7 10
i i
→ + = ×
−
k
(34.44)
This species is less reactive than the
•
NO
3
radical, but has been shown to add to unsaturated carbon
bonds, or to carbon-centered radicals to produce nitrated derivatives of the original compound
(Eberhardt, 1975; Olah etal., 1978; Dzengel etal., 1999; Ershov etal., 2007). Perhaps more impor-
tantly, the addition product of these two nitrogen-centered radicals decays to produce nitronium ion,
a
powerful nitrating species (Mincher etal., 2009b):
i i
NO NO N O M s Katsumura et al
2 3 2 5
9 1 1
1 7 10 1991+ → = ×
− −
k . ( ., ) (34.45)
N O NO NO Sworski et al
2 5 2 3
1968→
+ −
+ ( ., ) (34.46)
Another important product of nitric acid radiolysis is nitrous acid. It is produced by direct and
indirect
effects:
HNO O HNO Nagaishi et al
3 2
1994
i
+ ( ., ) (34.47)
NO O NO Daniels,
3 2
1968
−
−
+
i
( ) (34.48)
NO H HNO p Lammel et al
a2 2
3 2 1990
− +
+ ↔ =K . ( ., )
(34.49)
i i
NO NO N O M s Gr tzel et al
f2 2 2 4
8 1 1
4 5 10 1969+ → = ×
− −
k . ( ., )ä (34.50f)
k
reverse
s Gr tzel et al= ×
−
6 0 10 1969
3 1
. ( ., )ä
(34.50r)
N O H O HNO HNO M s Olah et al
2 4 2 2 3
1 1
18 1978+ → + =
− −
k ( ., )
(34.51)
This species is important because of its affect on actinide oxidation states in irradiated aqueous
nitric acid solution. This topic is discussed in more detail in Section 34.3.4.1. Its maximum concen-
tration is limited by radiolytically produced H
2
O
2
, as shown (Bhattacharyya and Veeraraghavan,
1977;
Vione etal., 2003):
