Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

970 Charged Particle and Photon Interactions with Matter
where
C
i
is the concentration of species i (mol L
−1
)
G
i
is the G-value of species i (number 100 eV
−1
)
P
is the absorbed energy (100
eV
s
−1
L
−1
)
N
is the Avogadro’s number
k
mn
is the reaction rate constant between species C
m
and C
n
As input data, the G-values, reaction rate constants of the radiolysis products, neutron and gamma
dose rates at each portion of the loop, ow velocity, and residence time are necessary. The BWR
primary loop is divided into 11 sections. The concentration proles of each radiolysis product are
obtained
at a certain hydrogen injection rate.
34.2.4.2.1
G-Values
G-Values are necessary as input data to the radiolysis model. Those at room temperature are known for
both neutrons and gamma-rays, as shown in Table 34.5 (Burns and Moore, 1976). There is still a need
for high-temperature G-value measurements for modeling work on water radiolysis in the BWR primary
system. Because this is a complicated system under high temperature and pressure, very few reports are
currently available, as shown in Table 34.6 (Elliot etal., 1993; Elliot, 1994; Sunaryo etal., 1995).
In general, water radiolysis is enhanced by 10%–30% with temperature increasing up to reactor
temperature for both fast neutrons and gamma-rays (high-energy electrons). The G-values for the
reducing species (e
aq
−
and H) under neutron irradiation, however, are suppressed by about 30% at
high
temperature.
34.2.4.2.2
Reactions and Reaction Rate Constants
In order to perform a water radiolysis simulation, the secondary reactions of the radiolytic products
must also be considered. In the current radiolysis model, 25 secondary reactions and 3 dissociation
equilibrium equations are considered. Reverse reactions are also considered for three key reactions.
Table 34.7 shows the secondary reactions and their rate constants at high temperature (Takagi
and Ichikawa, 2001). At high temperature, the rate constant is estimated by extrapolation from the
room
temperature value assuming Arrhenius behavior and an activation energy.
table 34.5
G-values
of w
ater
d
ecomposition
p
roducts
at r
oom
t
emperature
e
aq
−
h
+ •
h
•
oh h
2
h
2
o
2
ho
2
•
references
n 25°C 0.96 0.96 0.52 1.13 0.91 1.03 0.04 Burns
and Moore (1976)
Gamma 25°C 2.80 2.80 0.63 2.96 0.45 0.63 0.03 Burns and Moore (1976)
Note:
Unit
is (10
−7
mol J
−1
).
table 34.6
G-values
of w
ater
d
ecomposition
p
roducts
at elevated t
emperatures
e
aq
− •
h
+ •
h
•
oh h
2
h
2
o
2
ho
2
•
references
n 250°C 0.70 0.70 0.54 1.66 1.72 1.34 Sunaryo
etal. (1995)
Gamma 250°C 3.67 3.67 0.97 3.61 0.58 1.10 0 Sunaryo etal. (1995)
n 300°C 0.63 0.63 0.35 2.09 1.31 0.67 0.05 Elliot etal. (1993)
Gamma 285°C 3.66 3.66 0.93 4.85 0.65 0.52 0 Elliot (1994), Elliot etal. (1993)
Note:
Unit
is (10
−7
mol J
−1
)
−1
.

Radiation Chemistry inNuclear Engineering 971
table 34.7
reactions
and r
eaction
r
ate
Constants at 285°C
rate
Constants a
re
e
xpressed
in a u
nit
of m
−1
s
−1
Forward reaction reverse reaction Forward reverse
H
atom/hydrated electron equilibrium
1 e
aq
−
+ H
2
O
•
H + OH
−
1.69E+02 6.88E+08
2 e
aq
−
+ H
+ •
H 2.54E+11 1.00E+05
Radical–radical
reaction
3
•
H +
•
H H
2
1.06E+11 —
4 e
aq
−
+
•
H + H
2
O OH
−
+ H
2
4.76E+09 —
5 e
aq
−
+ e
aq
−
+ H
2
O + H
2
O OH
−
+ H
2
+ OH
−
1.74E+07 —
6
•
H +
•
OH H
2
O 2.12E+11 —
7 e
aq
−
+
•
OH OH
−
3.17E+11 —
8
•
OH +
•
OH H
2
O
2
4.76E+10 —
Radical–molecule
reaction
9
•
H + H
2
O
2
•
OH + H
2
O 3.07E+09 —
10 e
aq
−
+ H
2
O
2
•
OH + OH
−
1.38E+11 —
11 e
aq
−
+ HO
2
−
+ H
2
O
•
OH + OH
−
+ OH
−
6.67E+08 —
12
•
OH + H
2
•
H + H
2
O 1.27E+09 1.10E+03
13
•
OH + H
2
O
2
H
2
O + HO
2
•
3.91E+08 —
HO
2
reaction
14
•
H + HO
2
•
H
2
O
2
2.11E+11 —
15
•
H + O
2
−•
HO
2
−
2.11E+11 —
16 e
aq
−
+ HO
2
•
HO
2
−
2.12E+11 —
17 e
aq
−
+ O
2
−
•
+ H
2
O HO
2
−
+ OH
−
1.13E+08 —
18
•
OH + HO
2
•
H
2
O + O
2
1.27E+11 —
19
•
OH + O
2
−
•
OH
−
+ O
2
1.27E+11 —
20 HO
2
•
+ HO
2
•
H
2
O
2
+ O
2
9.29E+07 —
21 HO
2
•
+ O
2
−
O
2
+ HO
2
−
5.16E+08 —
22 2
O
2
−
•
+ 2H
2
O O
2
+H
2
O
2
+2OH
−
1.93E+05 —
O
2
reaction
23
•
H + O
2
HO
2
•
2.01E+11 —
24 e
aq
−
+ O
2
O
2
−
•
2.01E+11 —
Dissociation
equilibrium
25 OH
−
+ H
2
O
2
HO
2
−
+ H
2
O 1.72E+10 1.08E+05
26 HO
2
•
H
+
+ O
2
−
•
8.46E+06 5.29E+11
27 H
+
+ OH
−
H
2
O 1.48E+12 1.81E−01
H
2
O
2
decomposition
28 2H
2
O
2
2H
2
O + O
2
— —
Source: Takagi, J. and Ichikawa, N., Water radiolysis modeling and the prediction of cor-
rosion potentials at actual BWRs, in Proceedings of the Seminar on Water
Chemistry of Nuclear Reactor Systems 2001, paper 23, Chung-Hwa Nuclear
Society, Taipei, Taiwan,
2001, pp. 1–6.
With
permission.
972 Charged Particle and Photon Interactions with Matter
k A
E
RT
=
−
exp ,
a
(34.19)
where
k
is the reaction rate constant
A
is the frequency factor
E
a
is the activation energy
R
is the gas constant
T is the absolute temperature
H
2
O
2
, HO
2
, and H
2
O each have dissociation constants and the dominant chemical form depends on
pH.
The backward reaction rate constants are shown in Table 34.7.
The
following two reverse reactions are also added to the reaction scheme, as shown in Table 34.7:
•
H e H
aq
→ +
− +
(34.20)
• •
H H O OH H+ → +
2 2
(34.21)
A parametric study made under gamma-ray irradiation conditions demonstrated the importance of
including
these reverse reactions (Ichikawa and Takagi, 1998).
34.2.4.2.3
Radiolysis Simulation in BWR Primary Circuit
In order to perform the radiolysis simulation in the BWR primary circuit, not only the G-values
and the rate constants but also the various plant parameters are needed. It is common to divide the
coolant loop into several blocks (regions) depending on dose rate, ow rate, and residence time. In
the core region, the void fraction distribution and the hydrogen and oxygen gas stripping rates from
the
liquid to the vapor phase are also necessary to simulate the two-phase ow.
An
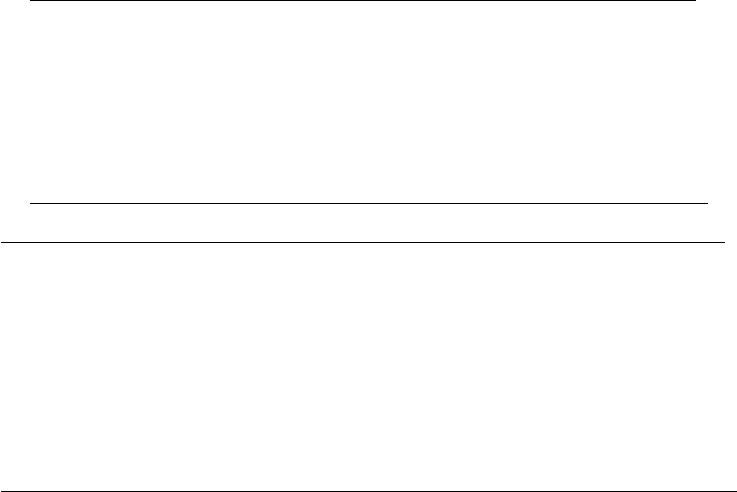
example of a block diagram for a jet-pump type BWR is shown in Figure 34.7 (AESJ, 2000c).
Recently, efforts have been made to divide the loop into more detailed regions or to divide the
Steam
Feed
water
Mixing plenum
Upper downcomer
Lower downcomer
Recirculation line
Jet pump
Lower plenum
Core channel
Core bypath
Fuel surround
Upper plenum
Separator
1
1
1
3
3
3
4
4
4
5
5
5
6
6
6
7
7
7
7
7
8
8
8
8
9
9
9
9
10
10
10
11
11
11
2
2
2
Figure 34.7 Block diagram of the radiolysis model for the BWR primary cooling system. (From AESJ,
Handbook of Water Chemistry of Nuclear Reactor System, K. Ishigure (ed.), Atomic Energy Society of Japan,
Tokyo,
Japan, 2000c. With permission.)
Radiation Chemistry inNuclear Engineering 973
downcomer region into several layers depending on the gamma-ray dose rate because of the large
sensitivity
of the model to recombination effects in the downcomer region.
The
absorbed dose rate to the high-temperature water is important in the simulation as well as
G-values. These dose rates are based on the neutron ux and gamma-ray distribution calculations,
usually
performed for the purpose of shielding calculations.
The
ow rate and the residence time at each region are plant specic and are determined from
the design parameters. Because the radiolytic reaction is fast compared to the residence time of the
coolant
at each region, an equilibrium always holds for each region depending on its dose rate.
Another
parameter to be noted is the gas stripping rate from the liquid to the vapor phase at the
boiling region, especially under hydrogen injection conditions. The distribution between the two
phases is estimated to be largely different from the Henry’s law constant because of the dynamics
of core boiling. Therefore, those parameters are determined by benchmarking of the calculated
hydrogen
and oxygen concentrations in the main steam system to measured concentrations.
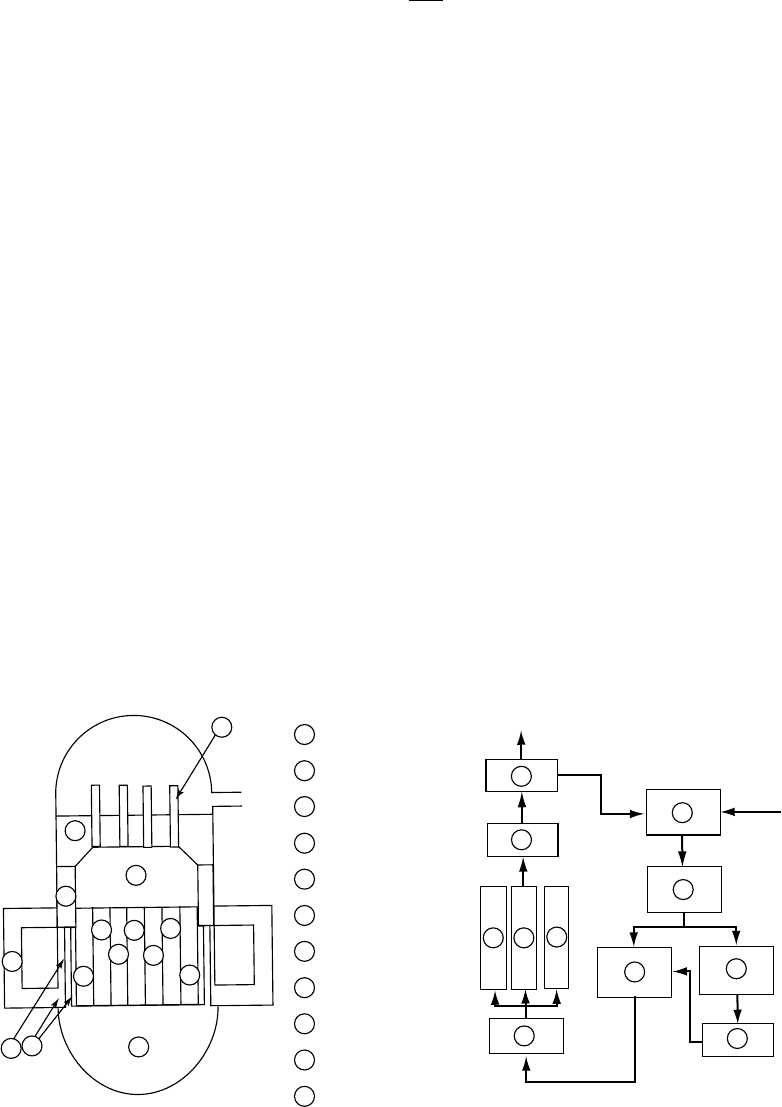
Figure
34.8 shows an example of the radiolysis simulation result for the BWR primary circuit
(AESJ, 2000c). The stable species of hydrogen, oxygen, and hydrogen peroxide are shown. However,
the concentrations of the short-lived species such as radicals are also numerically obtained.
34.2.4.3 eCp modeling
Depending on the radiolysis modeling results, the ECP is calculated using mixed potential theory
by combining the chemistry species distributions inside the BWR primary circuit and the hydrody-
namic
parameters such as ow rate and hydraulic diameter.
The
corrosion potential (E
corr
) is dened as the potential at which the anodic current (i
a
) and
the cathodic current (i
c
) are balanced. The anodic reaction is described as metal dissolution and
hydrogen oxidation, whereas the cathodic reaction is described as reducing reactions of oxygen
and hydrogen peroxide, as shown in Section 34.2.3.2.
In order to reduce the corrosion potential to mitigate IGSCC, the cathodic current must be con-
trolled by hydrogen injection or by controlling the anodic current, as shown in Figures 34.4 and 34.5.
The anodic polarization curve is derived from the measurement with stainless steel at high tem-
perature. Under hydrogenated conditions, the anodic current of hydrogen oxidation will be added
and in such cases it will become dominant. On the other hand, the cathodic polarization curve can
be analytically obtained assuming appropriate parameters such as equilibrium potential, exchange
current
density, Tafel slope, and limiting current density.
>100 ppb
Dissolved oxygen
50–100 ppb
20–50 ppb
0–20 ppb
Figure 34.8 An example of the radiolysis simulation result for the BWR primary circuit under the condi-
tion of 0.8ppm hydrogen injection into the feedwater. (From AESJ, Handbook of Water Chemistry of Nuclear
Reactor System,
K. Ishigure (ed.), Atomic Energy Society of Japan, Tokyo, Japan, 2000c. With permission.)

974 Charged Particle and Photon Interactions with Matter
34.2.4.3.1 Equilibrium Potential
The equilibrium potential of the corrosion equation is calculated using Nernst’s equation. It depends
on the chemical species concentration and the temperature. In the case of the oxygen reduction reac-
tion,
the equilibrium potential is described as shown below (AESJ, 2000c).
E P= + −
= = °
1 02 0 028 0 112
0 289 100 290
2
2
. . log .
. ( ( )) ( ,
O
pH
V SHE O ppb at CC),
(34.22)
where
E
is the equilibrium potential
P
O
2
is the oxygen partial pressure
pH
is the pH of the solution
34.2.4.3.2
Exchange Current Density and Tafel Slope
Exchange current density is dened as a current density that occurs on the electrode surface under
the equilibrium condition without over potential (MacDonald, 1992). Tafel slope is dened as a slope
of potential against current where over potential exists by polarization. Charge transfer is the limiting
process in this area. A slope of 0.23V decade
−1
at 290°C is reported from laboratory measurements.
34.2.4.3.3
Limiting Current Density
In the case of the ECP Model, a ow rate effect is taken into consideration in the formula of limiting
current
density together with the equivalent hydraulic diameter (Selman and Tobias, 1978).
I
nFDC
i
lim
=
0 0165
0 86 0 33
.
,
. .
Re Sc
d
(34.23)
where
F
is the Faraday constant
D is the diffusion constant
C
i
is the concentration of species i
Re
is the Reynolds number (Vd/v)
Sc is the Schmidt number (v/D)
d
is the equivalent hydraulic diameter
V is the ow velocity
v
is the kinematic viscosity
When considering the BWR primary loop condition, the corrosion potential becomes less under
lower ow conditions due to a smaller limiting current density and higher under high ow condi-
tions due to larger limiting current density. This means that ow velocity is one of the key param-
eters
controlling the corrosion potential evaluation.
34.2.4.4
eCp e
valuation for bwr p
rimary
Circuit
The
same block diagram for the radiolysis modeling can be applied to the ECP simulation for the
BWR primary loop. As input parameters, the concentrations of the chemical species like hydro-
gen, oxygen, and hydrogen peroxide are provided by the radiolysis modeling. The ow rate and
the hydraulic diameter of each region of interest are necessary to carry out the ECP evaluation.
34.2.4.5 advanced
e
Cp e
valuation u
sing
3-
d
Flow d
istribution
The corrosion potential of the structural materials at the jet pump outlet region has been evaluated
by ECP modeling using a mixed potential theory (Ichikawa etal., 1992, 2002). One of the important

Radiation Chemistry inNuclear Engineering 975
parameters in this model is the limiting current density which is strongly affected by the water ow
velocity.
From the principle, it is expressed as
I knFC
jlim
= ,
(34.24)
where
k
is the mass transfer coefcient
n is the number of electrons transferred per mole
F
is the Faraday constant
C
j
is the bulk concentration of species j
The
mass transfer coefcient is expressed as
k
Sh
d
= ,
(34.25)
where
Sh
is the Sherwood number
d is the hydraulic diameter
Two
equations for evaluating Sh have been reported, as given below:
Sh Sc Sc= <0 023 100
0 8 0 4
. ( ) ( , )
. .
Re Macadamas 1954
(34.26)
Sh Sc= < <0 0165 1000 6000
0 86 0 33
. ( ), ( , )
. .
Re Sc Berger and Hau 1977
(34.27)
where
Re
is the Reynolds number (Ud/v)
Sc is the Schmidt number (v/D)
U is the ow velocity
v
is the kinematic viscosity
D is the diffusivity
The
Schmidt numbers for O
2
, H
2
, and H
2
O
2
at 288°C are 0.34, 1.63, and 0.32, respectively. Equation
34.26
is suitable for BWR conditions.
Equations
34.24 and 34.25 make it necessary to have the hydraulic diameter (d) in order
to calculate the limiting current density. A precise evaluation of hydraulic diameter, however,
is impossible because of the very complex structure of the reactor pressure vessel (RPV) bot-
tom region. One method of evaluating the mass transfer coefficient (k) without introducing
the hydraulic diameter is to bring in the Stanton number (St). The Stanton number can be
expressed as
St
Sh
Sc
kU= =
( )Re
(34.28)
St
S
PS
=
+
( )
1 1 2/ Prt
,
(34.29)
976 Charged Particle and Photon Interactions with Matter
where
S
is the coefcient of friction
P is the P function (P = 9.0(Sc/Prt−1)(Sc/Prt)−1/4)
Prt
is the Prandtl number of turbulent ow
In
this study, the Prandtl number was set at 0.9. This number simulates the Sh equation (34.26). On
this
basis, the limiting current density of species j can then be expressed as
I StUnFC
jlim
= .
(34.30)
This approach has an advantage that, when the coefcient of friction (S) and the water ow velocity
(U) are given and the bulk concentration of species j(C
j
) is calculated using the radiolysis model, the
Stanton number (St) and then the limiting current density at any location inside the primary loop can
be
estimated without the need for the hydraulic diameter.
34.2.5 hydrogen water cheMiStry application to actual bwrS
34.2.5.1 introduction
Hydrogen water chemistry (HWC) is a chemistry control technology used to reduce oxidizing spe-
cies in reactor water. When hydrogen is injected from the feedwater and is introduced to the core, a
recombination reaction of hydrogen with oxygen and hydrogen peroxide is enhanced under irradia-
tion, resulting in the mitigation of corrosion by the scavenging of oxidizing species.
In Japan, the rst hydrogen water chemistry verication for commercial BWRs was carried out
in 1992 (Ashida etal., 1992). It was a short-term program to identify the effectiveness of hydrogen
water chemistry control and to measure the plant parameter responses including main steam system
radiation level increases. That program was successfully done and the next verication program for
a longer period was planned.
In 1995, a long-term verication program on hydrogen water chemistry was started at another
BWR (Takagi etal., 1998). In this program, it was planned to measure corrosion potentials at the
bottom drain line of the RPV and to perform crack growth measurements in the out-of-core auto-
claves
for demonstration. The outline of the above verication program is described below.
34.2.5.2
environmental
m
itigation
of igs
CC
by h
ydrogen
w
ater
Chemistry
In
order to mitigate IGSCC of the primary system components in BWR plants, hydrogen water
chemistry control has been implemented in several BWRs, especially in overseas plants. In those
plants, it has been veried that reactor water oxygen and hydrogen peroxide are well suppressed
by hydrogen addition. Efforts have also been made to measure corrosion potential directly in the
primary system of some plants. The locations of the sensors are out-of-core piping, upper and lower
core
regions, and the bottom region of the reactor pressure vessel.
When
hydrogen is injected from the feedwater line, the recombination reaction of injected hydro-
gen and core-produced oxygen and hydrogen peroxide occurs in the so-called downcomer region,
that is, the region between the core shroud and pressure vessel wall through which water ows
downward with a rather moderate velocity. The gamma dose rate at that region enhances the radio-
lytic recombination reactions to produce water, which results in higher efciency of oxygen and/or
hydrogen peroxide suppression in the downcomer region and the out-of-core piping system con-
nected to the downcomer. On the other hand, comparatively more hydrogen is required to protect
core
internal components.
According
to the in-plant autoclave type material testing results, it has been accepted that there is a
threshold potential of −230mV (SHE) (standard hydrogen electrode) for IGSCC occurrence. The target
of hydrogen water chemistry is to reduce ECP of the key components below this threshold potential.
Radiation Chemistry inNuclear Engineering 977
To evaluate the effectiveness of hydrogen injection, ECP is a useful indicator for the predic-
tion of IGSCC prevention. The reduction of the concentration of oxidizing species such as oxygen
and hydrogen peroxide will cause the reduction of ECP of the materials. In the case of austenitic
stainless steels or nickel-based alloys, IGSCC initiation or propagation is mitigated under a certain
threshold of corrosion potential. Therefore, estimation of the corrosion potential of the structural
materials in the BWR primary system is needed to determine the required amount of hydrogen to
be
added to the system.
34.2.5.3
program
d
escription
34.2.5.3.1
Objectives
The main objective of this program is to verify the effectiveness of hydrogen water chemistry con-
trol as an IGSCC countermeasure and its applicability to BWRs. The goal is to evaluate IGSCC
behavior of plant structural materials under hydrogen water chemistry. The program consists of
three
parts in order to accomplish this goal.
1. Water
chemistry measurement
2. Corrosion
potential measurement
3. Crack
growth measurement
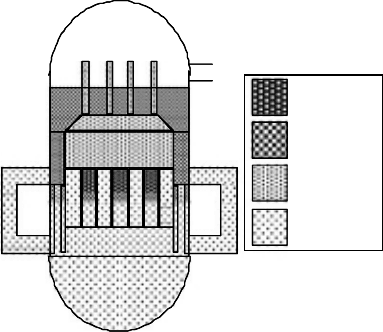
Figure
34.9 shows the evaluation ow for the IGSCC behavior of the materials under HWC condi-
tions. Decreased dissolved oxygen and hydrogen peroxide concentrations due to hydrogen addition
results in decreased corrosion potential. Therefore, the ECP measurement is regarded as a verica-
tion of mitigation. Corrosion potential is measured at the RPV bottom drain line to evaluate the
vessel
bottom corrosion environment.
Secondly,
a decrease in corrosion potential results in a decrease in crack growth rate of the
materials. Out-of-core in-plant material testing is performed as verication of IGSCC mitigation.
Type 304 stainless steel and Inconel 182 are used as test specimens. The results are discussed
elsewhere (Takagi etal., 1996).
Hydrogen injection
Decrease
in DO, H
2
O
2
DH
Decrease in
ECP
ECP
DO
H
2
O
2
DO
Decrease in
SCC
Crack growth
ECP
Verification of
environmental
mitigation
Verification of
SCC mitigation
(material testing)
Figure 34.9 Evaluation ow of IGSCC behavior under hydrogen water chemistry in the verication program.
978 Charged Particle and Photon Interactions with Matter
34.2.5.3.2 Plant Features
The
features of the plant chosen for this program are summarized as follows:
•
BWR
Type-4
•
Thermal
power: 2381
MWth
• Rated
power: 784
MWe
• Power
density: 40
kW
L
−1
• Commercial operation: since April 1978
34.2.5.3.3
Hydrogen and Oxygen Injection System
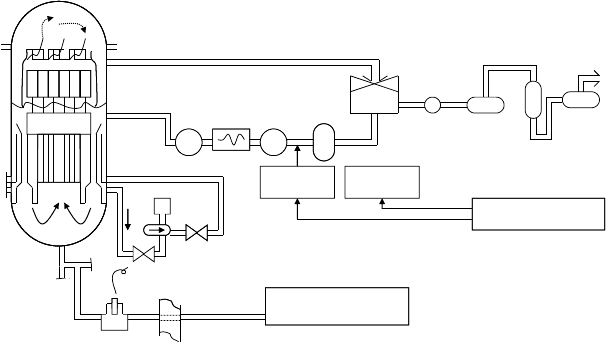
The schematic of the hydrogen water chemistry system used in this program is shown in Figure 34.10.
Hydrogen gas is supplied from a gas loader system with an initial pressure of about 200kg cm
−2
, which
is depressurized to about 8kg cm
−2
at the inlet of the hydrogen injection module. The injection point
is at the outlet of the condensate demineralizer (at the suction header of the high-pressure condensate
pumps) where the system pressure is the lowest at about 4kg cm
−2
. The maximum hydrogen injection
rate was about 100N m
3
h
−1
(2ppm in feedwater) and the injection rate for permanent injection is ten-
tatively set at 25N m
3
h
−1
(0.5ppm in feedwater).
Oxygen
gas is supplied from a liqueed oxygen tank. As the injection point is at the inlet of off-
gas
preheater (upstream of the off-gas recombiner), high pressure is not required for the system. The
oxygen
injection rate is half of the hydrogen injection rate.
34.2.5.4
experimental
34.2.5.4.1
Water Chemistry Measurement
Water chemistry measurements were conducted using existing plant sampling locations such as
oxygen and hydrogen in reactor water, feedwater, condensate, and main steam; conductivity and pH
in
reactor water and feedwater; and other parameters taken by grab sampling.
34.2.5.4.2
Corrosion Potential Measurement
An in situ and high-ow corrosion potential measurement device was developed. A corrosion poten-
tial sensor was installed in a new sampling line branched from the existing RPV bottom drain line
(see Figure 34.10), which contained the reactor water of the same water chemistry as that of the RPV
bottom region. The decomposition of hydrogen peroxide between the vessel bottom and the sensor
location
is estimated to be about 10%.
Tb
Preheater
OG cond.
HPCP C/DRFP
SJA
E
H
2
/O
2
supply
O
2
injec.H
2
injec.
Recombiner
FWHx
O
2
PCV
H
2
Material testing
Bottom drain
ECP sensor
Figure 34.10 Description of the hydrogen water chemistry system in the verication program.
Radiation Chemistry inNuclear Engineering 979
The sensor unit consists of two kinds of electrodes: a silver/silver chloride reference electrode
and a platinum electrode. The working electrode of this unit is the stainless steel piping (Type
316L) itself. The temperature and the pressure at the monitoring location are 275°C and 70kg cm
−2
,
respectively. The ow velocity inside the piping is 3.1m s
−1
, which represents the highest ow condi-
tion
inside the vessel along the bottom region.
34.2.5.5
results
and d
iscussion
34.2.5.5.1
Water Chemistry Results
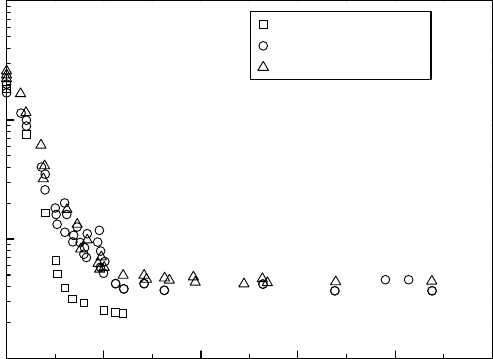
The effect of hydrogen water chemistry is also measured by the decrease in reactor water dissolved
oxygen, which is measured in a conventional sampling manner in the RPV bottom drain line, reac-
tor water cleanup line and primary loop recirculation line. The results are shown in Figure 34.11.
In general, the dissolved oxygen content in the recirculation line is lower than in the RPV bottom
region because of effective recombination in the downcomer region. The dissolved oxygen in the
RWCU
line is the mixture of the recirculation line and the bottom drain line oxygen contents.
It
is shown in Figure 34.11 that with 0.5 ppm hydrogen in feedwater, the reactor water dissolved
oxygen is decreased to ca. 7ppb. Note that at the end of the sampling line all the hydrogen peroxide
thermally decomposes to oxygen and water, and the measured concentration of the dissolved oxy-
gen
is the sum of [O
2
] and [H
2
O
2
]/2.
34.2.5.5.2
Water Chemistry Measurement Results
Figure 34.12 shows the relationship between the RPV bottom drain line corrosion potential and
dissolved hydrogen in feedwater obtained in this program. Under normal water chemistry condi-
tions, the corrosion potential at the bottom drain sensor unit shows almost +200 mV (SHE), which is
slightly higher than conventional autoclave results. Then it decreases with an increase in feedwater
hydrogen
and reaches −230
mV
(SHE) at a feedwater hydrogen concentration of 0.8
ppm.
At the hydrogen injection rate of 0.8ppm in feedwater, the main steam system radiation level
increase is by almost four times, as shown in Figure 34.12. To minimize the radiological impact
due to N-16 carryover, low hydrogen water chemistry becomes an option. For example, 0.4–0.5ppm
hydrogen injection to feedwater will give a bottom drain line corrosion potential of −50 to −100mV
(SHE). It is expected that IGSCC crack growth behavior will be suppressed to some extent accord-
ing
to the degree of the potential reduction.
1000
Recirculation line
RWCU line
RPV bottom drain line
100
10
0.0 0.5 1.0 1.5 2.0 2.5
1
Dissolved oxygen in reactor water (ppb)
Dissolved hydrogen in feedwater (ppm)
Figure 34.11 Behavior of dissolved oxygen in reactor water against hydrogen injection rate.
