Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.

566 6 Applications
where n (r
0
) is the number of particles of size r
0
per unit area.
As discussed in Chapter 3 (see Table 3.2), the particle mobility is deter-
mined by its size and the mass transport mechanism. If mass transport is
conducted through the bulk of a particle m
p
(r) ∼
1
r
3
;ifitoperatesbyinter-
facial diffusion m
p
(r) ∼
1
r
4
.
In particular, for rather small particles which are moving by interfacial dif-
fusion (D
S
), their mobility can be derived as (Table 3.2)
m
p
(r)=
Ω
a
D
S
λ
πkTr
4
(6.61)
where Ω
a
, D
S
, λ are the atomic volume, interfacial diffusion coefficient, and
thickness of the interfacial layer in which the diffusion occurs, respectively.
Neglecting the change of grain boundary surface tension due to the depletion
of concentration of the impurity atoms in the bulk of the grains, the condition
for grain growth acceleration can be written (in the case of a uniform size
distribution r = r
0
) as [607]
r
2
0
< Ω
a
λ · Γ(c
0
)
D
S
D
m
·
c
B
− c
A
c
0
− c
A
(6.62)
For D
S
≈ 10
−10
m
2
s
−1
, D
im
≈ 10
−14
m
2
· s
−1
,Ω
a
∼
=
10
−5
m
3
· mol
−1
,Γ(c
0
) ≈
10
−5
mol · m
−2
, λ ≈ 10
−9
m, c
b
=1,c
a
≈ 10
−4
, c
0
∼
=
10
−3
, the particle size
r
0
< 10
−6
m.
Grain boundary motion together with the particles is bound to have a
point of detachment. As shown in [607], the detachment condition can be
represented as
f (r
0
) m
p
(r
0
) <V (c
A
) (6.63)
or
r
3
0
¯
R
>λΩ
a
Γ(c
A
)
D
S
D
im
(6.64)
For the values of the parameters given above Eq. (6.64) is followed for
r
3
0
¯
R
> 10
−15
m
2
, for instance, by r
0
> 10
−7
mand
¯
R<10
−6
m.
Consequently, the predicted effect can actually occur in systems with mo-
bile and immobile particles. From Eqs. (6.55) and (6.56) follows that the effect
in samples with immobile particles is more pronounced in systems with low
solute solubility (c
A
and c
0
are small) and rather small mean grain size
¯
R.By
contrast, for mobile particles the only constraint is the size of the particles:
they must be sufficiently small.
The only experimental data which can be connected with this effect [607]
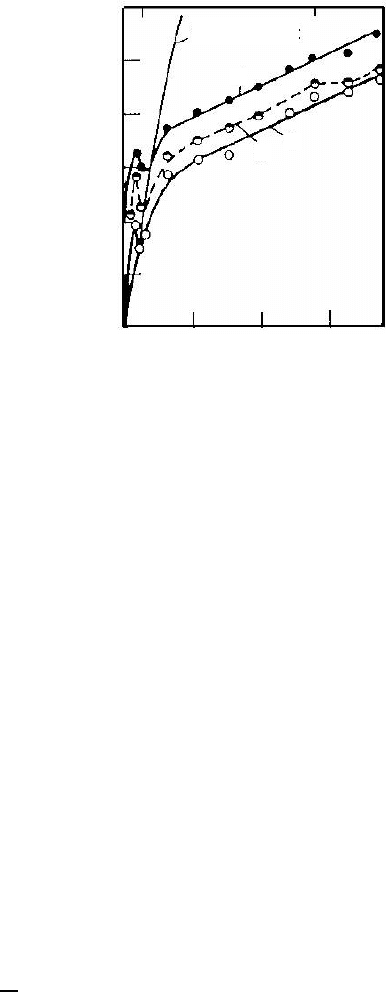
are the recrystallization of Al-Mn alloys [608] (Fig. 6.44). Fig. 6.44 shows the
concentration dependence of recrystallization in Al-Mn alloys for the temper-
ature of different stages of recrystallization — the early stage (1 −t
e
r
), 50%
recrystallized
2 − t
0.5
r
and the final stage of recrystallization
3 − t
f
r
[608]
(Fig. 6.44). In the close vicinity of the solubility limit a sharp minimum can
© 2010 by Taylor and Francis Group, LLC
6.3 On Precipitation-Controlled Grain Size 567
380
220
300
0.0
0.4 0.8
1.2
RX °C
T
solubility limit
3
2
1
380
220
300
0.0
0.4 0.8
1.2
RX °C
T
solubility limit
3
2
1
FIGURE 6.44
Concentration dependence of recrystallization temperature T
recr.
of Al-Mn al-
loys with different Mn content: 1 — beginning of recrystallization; % recrys-
tallized volume; 2 — 50% recrystallized volume; 3 — terminal recrystallization
[608].
be observed for all t
r
, indicating an acceleration of the process. It is stressed
that the minimum mentioned is also evident for temperatures at the end of
recrystallization, when the major contribution to the microstructure change is
associated with grain growth, as assumed in the theoretical framework given
above. From the analysis given above it is obvious that the necessary condi-
tions for the effect of precipitation accelerated grain growth are not so tough,
and certainly ought to be observed in nanocrystalline materials.
The approach put forward in [607] was developed in [609]. The main idea
of the proposed approach can be easily understood from consideration of the
simplest case: the behavior of a binary system with a solubility limit c
0
of
solute atoms and with zero solubility of the solvent atoms in the pure solute.
The second-phase particles in such a system are particles consisting of pure
solute. For simplicity the density of the solid solution (matrix) and the second-
phase particles is assumed to be equal. The grain boundary is immobile when
the sum of the forces acting on it vanishes
P
gg
− P
drag
= 0 (6.65)
where P
gg
=
2γ
R
is the driving force for grain growth, γ,R are grain boundary
surface tension and radius of curvature, which is proportional to the grain
size D: R = ηD,whereη is in the range of 5 to 10. P
drag
is the drag force
induced by immobile particles, Eq. (6.51). In [609] the situation was considered
© 2010 by Taylor and Francis Group, LLC

568 6 Applications
when the solute atoms are partitioned among the particles, matrix, and grain
boundaries. Both grain boundary adsorption (segregation) and solute drag
are constant since the concentration in the bulk is equal to the solubility
limit during grain growth. However, for rather small particles of radius r the
equilibrium concentration of the second component (impurity) in the bulk
should be different from the solubility limit in accordance with the Gibbs-
Thomson relation
c
0
= c
0
exp
2γΩ
rkT
(6.66)
c
0
Ω+AΩΓ (c
0
)+¯c = const (6.67)
where c
0
is the changed solubility limit, Ω is the atomic volume, Γ (c
0
), A =
3
D
are grain boundary adsorption (segregation) and the grain boundary area per
unit volume of a polycrystal (all grains are supposed to be cuboidal). On the
other hand, the average volume concentration of impurity atoms c,inother
words, the number of the impurity atoms per unit volume of a polycrystal
with mean grain size (radius) D = D
0
,isequalto
c = c
0
+ A · Γ(c
0
)=c
0
+
3
D
0
Γ(c
0
) (6.68)
Therefore
¯c = cΩ − c
Ω − AΩΓ (c
0
)=c
0
Ω+
3Ω
D
0
Γ(c
0
) − c
0
Ω − AΩΓ (c
0
) (6.69)
Eqs. (6.65) and (6.69) yield the size of the particles which arrest grain growth
in a polycrystal. Eqs. (6.65) and (6.67) allow us to find the relation between
grain size and the size of the particles in the form of a differential equation
[609]. Differentiation of Eq. (6.69) yields
4
3
1
ηD
−
4
3
r
ηD
2
dD
dr
=
2γΩ
2
c
0
r
2
kT
exp
2γΩ
rkT
+
3Ω
D
2
Γ(c
0
)
dD
dr
−
3Ω
D
dΓ(c
0
)
dD
dD
dr
(6.70)
where γ is the surface tension of the interface particle-matrix.
Eq. (6.70) predicts an extremum (minimum) of D(r). The integration of
Eq. (6.70) in the limits of D
0
and D gives the size of the particles which
arrest grain growth in the polycrystal. The equations derived reflect the sit-
uation when the particles are located in the bulk of the grains and the grain
boundaries interact with them in the course of grain growth. However, the
conditions of particle formation suggest that there is a large probability for
the scenario that the particles will nucleate directly at grain boundaries. Let
us consider such a situation where all particles are located at grain bound-
aries. In the framework of the Zener approach the drag force of the particles
at grain boundaries is equal to
P
drag
=2πrγn (6.71)
© 2010 by Taylor and Francis Group, LLC
6.3 On Precipitation-Controlled Grain Size 569
where n is the number of the particles per unit area at the grain boundary,
γ is the grain boundary surface tension. The volume fraction of the particles
canbeexpressedas
¯c = A · n ·
4
3
πr
3
=
4πn
D
r
3
(6.72)
and
P
drag
=
1
2
¯cγD
r
2
(6.73)
With Eqs.(6.65) and (6.73) we arrive at
2γ
ηD
=
1
2
¯cγD
r
2
(6.74)
Eqs. (6.69) and (6.74) yield the size of the particles which arrest grain growth
in a polycrystal for the case when all particles are located at grain boundaries.
The differential equation, derived in [609], predicts an extremum of D(r),
in analogy to Eq. (6.70). The equations considered are somewhat simplified.
In the vicinity of c ≈ c
0
the grain boundaries can be considered as a saturated
by impurity atoms and Γ (c
0
) = const. For rather “large” particles we can
neglect the Gibbs-Thomson relation. In this case Eq. (6.65) has the solution
r =
9
4
ΩΓ (c
0
) η
D − D
0
D
0
(6.75)
where the initial grain size D
0
can be extracted from Eq. (6.68)
D
0
=
3Γ (c
0
)
c − c
0
(6.76)
When all rather “large” particles are located at the grain boundaries we get
from Eqs. (6.69)) and (6.74)
r
2
=
3Γ (c
0
)Ω
1
D
0
−
1
D
D
2
4
(6.77)
The approach and the equations developed were applied to grain growth in
the system Ni-P studied intensively in [610, 611]. For a polycrystal of Ni with
mean grain size D = 100 nm doped with 3.6% of P the critical (i.e. the max-
imum) radius of the particles which arrests grain growth in such a system is
given in Eq. (6.70): r = η · 2.25 nm and in Eq. (6.77) r =1.6η nm. With
η ≈ 5 the critical particle radius is of the order of 10 nm. We would like to
remind the readers that Eq. (6.70) is derived using the Gibbs-Thomson re-
lation, whereas Eq. (6.77) does not take into account the capillary effect of
small particles.
Up to this point the main goal was to find the radius of the particles which
arrest grain growth in the system. This critical radius was treated as a func-
tion of the solute concentration, its limit of solubility, running grain size and
© 2010 by Taylor and Francis Group, LLC
570 6 Applications
grain boundary adsorption. However, the particle pinning of grain boundaries
results from two interdependent processes. On the one hand, solute atoms
are liberated in the course of grain growth, owing to the decrease in grain
boundary area. This gives rise to the nucleation and growth of precipitates.
On the other hand, because particles nucleate and grow, grain growth is hin-
dered and eventually ceases. It is virtually unfeasible to model accurately the
interdependency between grain growth and precipitation. If the bulk solute
concentration corresponds to the solubility limit, grain growth leads to the
formation of precipitates owing to the reduction of grain boundary area. The
concept of grain boundary adsorption in conjunction with particle drag in the
Zener approximation makes it possible to determine both the volume fraction
and the radius of the particles which arrest grain growth.
For a computational exercise an Al-Cu alloy will be considered in the fol-
lowing, with the nominal composition Al-1at%Cu. The initial grain size is set
to D
0
= 100 nm. The copper adsorption at grain boundaries Γ
Cu
is assumed
to be saturated, i.e. 3.2 ·10
−5
mol/m
2
. These conditions lead to an initial Cu
concentration c
0
in the grain of 4.5 · 10
1
mol/m
3
or 4.4 · 10
−2
at% which cor-
responds to the solubility limit of copper in aluminium at T = 200
◦
Cwhere
tempering is performed. To simplify the calculations, it is also assumed that
the precipitates which arrest grain growth are composed of pure copper and
have a spherical shape.
At the beginning of the heat treatment, there are no particles in the sys-
tem, the average grain size is D
0
, and the concentration in the matrix equals
the solubility limit c
0
. This state is labeled S in Fig. 6.45. In the first stage
of grain growth, all solute liberated from the grain boundaries enriches the
matrix and the solute concentration in the matrix c
M
rises to
c
M
= c
0
+3Γ
c
M
1
D
0
−
1
D
(6.78)
As c
M
increases, the driving force for the phase transformation increases and
the critical radius r
crit
for nucleation shrinks rapidly (dotted line in Fig. 6.45,
[609])
r
crit
=
2γV
m
R
g
T · ln
c
M
c
0
=
2γV
m
R
g
T · ln
1+
3Γ(c
M
)
c
0
1
D
0
−
1
D
(6.79)
where R
g
is the gas constant.
Eventually a state (N in Fig. 6.45) is reached where nucleation can be
thermally activated. Precipitates are not yet present. The process requires an
incubation period τ
nucl
τ
nucl
=
2 · Z
2
· β
∗
−1
(6.80)
where Z is the Zeldovich factor and β
∗
the rate at which atoms are added to
the critical nucleus. After incubation the system reaches position I in Fig. 6.45.
At this point, grain boundary pinning can occur. The first condition for an
© 2010 by Taylor and Francis Group, LLC
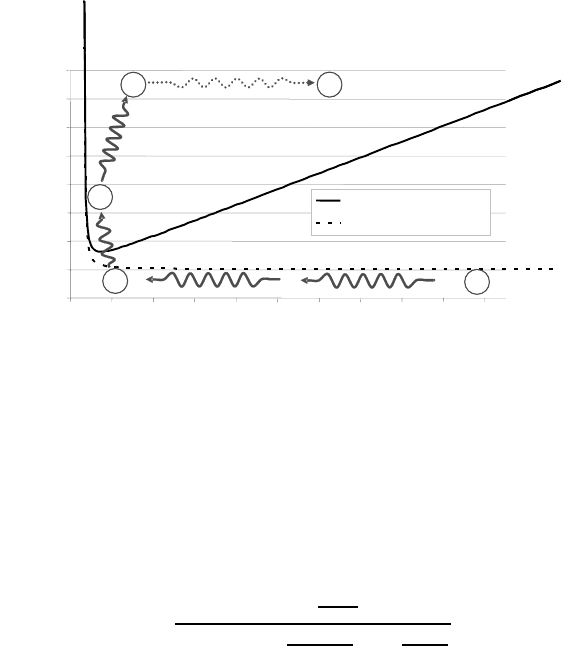
6.3 On Precipitation-Controlled Grain Size 571
0.E+00
1.E-07
2.E-07
3.E-07
4.E-07
5.E-07
6.E-07
7.E-07
8.E-07
0
.
E
+
0
0
1
.
E
-
0
9
2
.
E
-
0
9
3
.
E
-
0
9
4
.
E
-
0
9
5
.
E
-
0
9
6
.
E
-
0
9
7
.
E
-
0
9
8
.
E
-
0
9
9
.
E
-
0
9
1
.
E
-
0
8
Particle radius r in [m]
GrainsizeD in [m]
(D_pin , r_pin) [eq. (21)]
r_crit [eq. (19)]
S
N
P
I
E
[Eq. (6.70)]
[Eq. (6.68)]
0.E+00
1.E-07
2.E-07
3.E-07
4.E-07
5.E-07
6.E-07
7.E-07
8.E-07
0
.
E
+
0
0
1
.
E
-
0
9
2
.
E
-
0
9
3
.
E
-
0
9
4
.
E
-
0
9
5
.
E
-
0
9
6
.
E
-
0
9
7
.
E
-
0
9
8
.
E
-
0
9
9
.
E
-
0
9
1
.
E
-
0
8
Particle radius r in [m]
GrainsizeD in [m]
(D_pin , r_pin) [eq. (21)]
r_crit [eq. (19)]
S
N
P
I
E
[Eq. (6.70)]
[Eq. (6.68)]
FIGURE 6.45
Pinning of grain boundaries by second-phase particles [609].
effective pining is a state (D, r) above the limit (D
pin
, r
pin
). This limit can be
obtained by integration of the differential equation (6.70), and is represented
by a solid line in Fig. 6.45 [609]
D
pin
=
3Γ (c
0
)+
4
3V
m
η
r
pin
c
0
1 − exp
2γV
m
R
g
T ·r
pin
+
3Γ(c
0
)
D
0
(6.81)
The second necessary condition is the precipitation of a sufficiently large vol-
ume fraction. This is achieved at P in Fig. 6.45, where an efficient pinning oc-
curs and grain growth ceases. However, grain growth does not become arrested
because of particle coarsening, and it evolves slowly to a point E, the end of
the heat treatment. In order to illustrate the above methodology, the points
N-I-P were estimated by computer simulation using the precipitation kinetics
model ClaNG. This model is based on the classical nucleation and growth
theory for precipitation. It follows the Kampmann and Wagner methodology
to determine the evolution of the precipitate size distributions. A detailed
description of the simulation tool can be found elsewhere [610]. In order to
simplify the calculation, the parabolic grain growth kinetics D
2
(t)−D
2
0
= A·t,
A is kept constant and set to 5.0 · 10
−15
m
2
/s
−1
(see Chapter 3) are consid-
ered to remain constant with time, i.e. not influenced by solute content or
precipitate volume fraction, until it finally ceases. Using the ClaNG model, it
is found that the point N in Fig. 6.45 is obtained very quickly, in less than 1 s.
The first precipitates appear after about 20 s when the grain size has reached
D ≈ 300 nm. After 50 s (D ≈ 500 nm), which corresponds to the calculated
© 2010 by Taylor and Francis Group, LLC
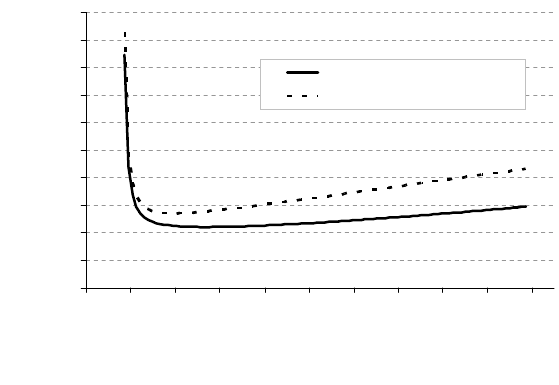
572 6 Applications
0.E+00
5.E-08
1.E-07
2.E-07
2.E-07
3.E-07
3.E-07
4.E-07
4.E-07
5.E-07
5.E-07
0
.
E
+
0
0
1
.
E
-
0
9
2
.
E
-
0
9
3
.
E
-
0
9
4
.
E
-
0
9
5
.
E
-
0
9
6
.
E
-
0
9
7
.
E
-
0
9
8
.
E
-
0
9
9
.
E
-
0
9
1
.
E
-
0
8
Particle radius r in [m]
Grain size D in [m]
prec. at grain boundaries
prec. in bulk
FIGURE 6.46
Pinning region (D
pin
, r
pin
) in case of preferred precipitation at grain bound-
ary. The dotted line indicates the case of homogeneous nucleation in the bulk
[609].
incubation period τ
nucl
, a high density of particles, 10
20
m
−3
is present but
the precipitated volume fraction ¯c =2.2 · 10
−7
is still too low to allow an
efficient pinning of the grain boundaries. The critical condition P
drag
= P
gg
is finally achieved after 150 s. At this time the precipitate density reached
4.3 ·10
23
m
−3
for an average radius of 0.8 nm. The grains have grown to their
final size D
crit
=0.9 μm and are efficiently pinned by second-phase particles.
It is emphasized that the simulations are based on several strong assump-
tions (spherical shape, constant grain growth kinetic) and thus will not repre-
sent a real case, but they render important information on the interplay of the
various concurrent atomistic processes. For instance, it can be learned that
the incubation period τ
nucl
plays an important role [609]. This also holds for a
scenario where pinning originates from particles heterogeneously precipitated
at grain boundaries. In such case the pinning region (D
pin
,R
pin
) is also larger
(Fig. 6.46).
A sensitive parameter is, of course, the initial grain size since it controls
both the amount of liberated solute and the driving force for grain growth
(Fig. 6.47). The interaction of grain growth and precipitation is most effective
for small grain sizes, in particular for nanocrystalline material. If the initial
grain size is changed from D
0
= 100 nm to D
0
= 50 nm grain growth will
cease after 43 s at a final grain size of D
pin
=0.5 μm and a precipitate size of
r
pin
=1.4nm(¯c = 0.45%) compared to 150 s, D
pin
=0.9 μm, r
pin
=0.8nm,
© 2010 by Taylor and Francis Group, LLC
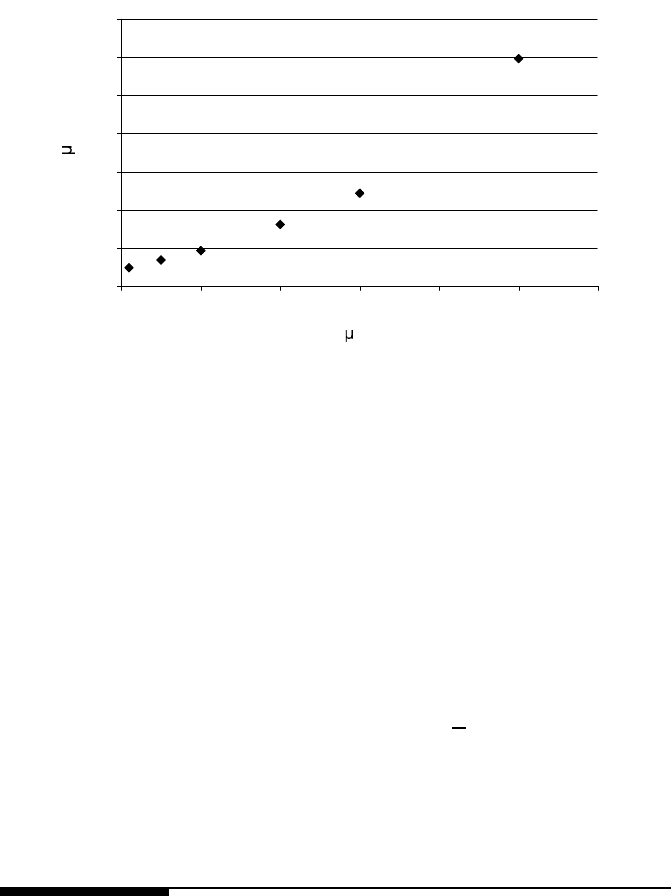
6.4 Mechanisms on Retardation of Grain Growth 573
0.0
1.0
2.0
3.0
4.0
5.0
6.0
7.0
0.00.10.20.30.40.50.6
D
0
[ m]
D
pin
[ m]
FIGURE 6.47
Dependency of the pinned grain size D
pin
on the initial grain sized D
0
[609].
f =0.1%.ForD
0
= 700 nm grain growth would not be arrested even after 10
hours. Therefore, for conventional material with a typical grain size of 10 μm,
a stagnation of grain growth will not be obtained by a redistribution of solute
from boundaries to the bulk.
In spite of several strong assumptions made in the course of the calcu-
lations (spherical shape of the particles, constant grain growth kinetic, the
neglecting of the change of the volume by vanishing grain boundaries: in the
relations discussed the strict Gibbs approach was used, i.e. the volume of the
grain boundaries is neglected; since the volume fraction S/V of grain bound-
aries changes in proportion to 1/D such approximation is reasonable when
D ≥ 50 nm because for cuboidal grain shape S/V=
3
D
δ which amounts to 6%
for D = 50 nm and a boundary thickness of 1 nm) computer simulations re-
veal that the respective precipitation kinetics that eventually terminate grain
growth sensitively depend on solute supersaturation and solute diffusivity.
6.4 Mechanisms on Retardation of Grain Growth
The design of materials with given requirements for microstructure stability is
connected inseparably with an essential engineering problem: a comparison of
the efficiency of the various drag effects on grain growth. Actually, to produce
a fine-grained or nanocrystalline material is part of the problem only. It is of
equal importance to slow down the process of grain growth since the small
© 2010 by Taylor and Francis Group, LLC
574 6 Applications
grain size generates a large driving force for grain growth. In [612] an attempt
is undertaken to assess the efficiency of drag effects by different structural
elements of a polycrystal on grain growth. The rate of grain area change is
chosen as a measure of stability of a grain structure and the inhibition of
grain growth is pairwise evaluated among all drag effects considered. The
rate of grain area change dS/dt is chosen as a measure for the stability of
a 2D grain structure. The change of grain area characterizes grain growth
to a greater extent than the change of grain size and both can be distinctly
different even for the same grain. The consideration of the problem is restricted
by 2D polycrystalline structures. The concepts of grain growth on the basis of
boundary and junction migration best for 2D systems were elaborated. Since
the issue considered is the relative efficiency for grain growth retardation a
2D approach is physically adequate. To define the relative efficiency of grain
growth drag due to different drag effects the authors of [612] construct a
hierarchy of pairwise dimensionless criteria λ
i,k
λ
i,k
=
dS
dt
i
dS
dt
k
(6.82)
which constitutes the ratio of the rate of grain area change for a pair of differ-
ent drag mechanisms. When λ
i,k
< 1 grain growth is controlled by mechanism
(i), and the magnitude of λ
i,k
< 1 denotes the drag efficiency.
The rate of grain area change
dS
dt
is expressed by the Von Neumann-Mullins
relation (4.55). The influence of impurities is reflected basically by the grain
boundary mobility m
b
(c), where c is the concentration of the impurities. The
criterion λ
imp,0
in this case is equal to
λ
imp,0
=
dS
dt
imp
dS
dt
c=0
=
m
b
(c)γπ
3
(n − 6)
m
b
γπ
3
(n − 6)
=
m
b
(c)
m
b
(6.83)
where
dS
dt
c=0
and
dS
dt
imp
are the rate of grain area change in “pure” metal
and in a metal with impurities, respectively. As the mobility depends strongly
on the crystallographic parameters of a grain boundary the most correct way
to define λ
imp,0
is to use data for specific and defined grain boundaries with
different amount of impurities. For a 111 tilt grain boundary (misorientation
angle 38.2
◦
(Σ7)) in Al with total impurity content 0.4 ppm and 7.0 ppm,
respectively, at 200
◦
C, λ
imp,0
=5· 10
−4
. For a non-special 111 tilt grain
boundary (misorientation angle 40.5
◦
) at the same temperature λ
imp, 0
=
1.7 · 10
−4
. The estimation made in [612] for random grain boundary in Al
dopedwithFe(c ≈ 10
−5
) at 200
◦
Cgivesλ
imp,0
≈ 3.6 · 10
−3
.
The criterion λ for triple junction drag compares the rate of area change
as affected by triple junction with the rate for free uniform boundary motion
© 2010 by Taylor and Francis Group, LLC
6.4 Mechanisms on Retardation of Grain Growth 575
[612]
λ
tj,b
=
−m
tj
γ
¯
Rnsin
2π
n
2sin
π
n
− 1
m
b
γπ
3
(n − 6)
=
λ
tj,b
= lim
n→∞
d
dn
dS
tj
dt
d
dn
dS
b
dt
=
3
4
m
tj
¯
R
m
b
=
3
4
Λ (6.84)
where
¯
R is the grain size, Λ is defined by this equation, n is a topological class
of a grain. A distinctive property of Eq. (6.84) is the explicit dependency of
λ
tj,b
on the grain size. The values of the criterion λ
tj,b
were evaluated in [612]
on the basis of experimental data for a triple junction mobility in pure Al. It
was shown that for Al polycrystal with the grain size
¯
R =10
−8
m at 200
◦
C
the criterion λ
tj,b
is in the range of λ
tj,b
≈ 10
−12
− 10
−11
.
To define the criterion λ
qp,b
, which describes the retardation of grain growth
by quadruple junctions, the approach put forward in [619] can be utilized. As
shown in [447, 619] the grain growth kinetics in polycrystals can be described
by Eq. (4.96)
V =
m
b
γκ
1+
1
Λ
+
1
¯
Λ
qp
where Λ =
m
tj
¯
R
m
b
and
¯
Λ
qp
)
m
qp
¯
R
2
m
b
are the criteria which determine the triple
junction drag and the quadruple junction drag of grain growth, respectively.
Then the desired criterion λ
qp,b
can be expressed as a ratio of grain growth
kinetics where the influence of the junctions is neglected and the grain growth
kinetics influenced by boundary junction(s)
λ
junctions,b
=
V
junctions
V
b
=
1
1+
1
Λ
+
1
Λ
qp
(6.85)
Using the value of the quadruple junction mobility in Al evaluated in [447]
(at 600
◦
C m
qp
≈ 2 · 10
−4
s
−1
) and the experimental data for grain boundary
mobility presented in Chapter 3 for the criterion λ
qp,b
at 600
◦
Cweobtain
λ
qp,b
≈ 10
−13
. The criterion λ
tj,b
at 600
◦
CinAlfor
¯
R ≈ 10
−8
m [436, 437] is
equal to 10
−8
.Atfirstglancetheresults(λ
tj,b
λ
qp,b
) contradict both com-
puter simulation data (see Chapter 4) and our previous calculations. However,
we would like to remind the reader that the latter ones were carried out for
200
◦
C. To recalculate the criterion λ
qo,b
from 600
◦
C to 200
◦
C the quadruple
junction mobility at 200
◦
C should be known. Unfortunately, our knowledge
of quadruple junction mobility is extremely poor. Nevertheless, it can be as-
sumed that the enthalpy of activation of quadruple junction motion is rather
small. It follows both on general grounds (a quadruple junction is a structural
element of zero dimension, similar to a small particle) and the evaluation
of quadruple junction mobility for nanocrystalline Pd at room temperature:
m
qp
≈ 10
−5
s
−1
, a value which is very akin to the quadruple junction mo-
bility in nanocrystalline Al at 600
◦
C, in spite of a huge difference in nature
© 2010 by Taylor and Francis Group, LLC
