Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.

556 6 Applications
mobility. Hutchinson has pointed out that the computed and measured angu-
lar misorientation distributions about 111 axes are not identical. However,
the computation certainly demonstrates that caution has to be exercised when
interpreting apparently non-random misorientation distributions.
6.2.4.4 Recrystallization in Single Phase Alloys
From innumerable experiments it is known that the addition of solute ele-
ments to a pure metal drastically affects its recrystallization behavior. The
recrystallization kinetics slows down, the recrystallized grain size decreases
and the recrystallization texture may change completely even with small con-
tents of the alloying element.
Evidently, the effect of alloying elements on recrystallization kinetics, grain
size and texture has to be attributed to a change in nucleation rate
˙
N and/or
growth rate v. The deformation microstructure and deformation texture is
usually less dramatically influenced by minor additions of alloying elements;
thus the nucleation rate ought to be less affected or at least not markedly
increased by alloying. For instance, the addition of only 0.1% P to pure Cu
hardly affects the rolling texture but leads to a very different recrystallization
texture upon annealing (Fig. 6.36) [599]. Other prominent examples are the
system Fe in Al or Nb in steel. Therefore, the delay of recrystallization kinet-
ics and the grain refinement upon alloying have to be attributed to a decrease
in the growth rate rather than to an increase in nucleation rate. The effect
can be very drastic, however, e.g. the addition of 0.01%Fe to Al increases the
recrystallization time by orders of magnitude (Fig. 6.37).
A qualitative explanation of these phenomena provides the impurity drag
theory, as presented in Chapter 3. Much more difficult is a quantitative treat-
ment of the impurity drag effect on recrystallization kinetics. This is due to the
fact that impurity drag is a consequence of segregation in grain boundaries,
and the understanding of segregation is still in its infancy. Only recently have
atomistic calculations on segregation in select systems been provided [601] for
a stationary grain boundary, but virtually nothing is known about dynamic
segregation in a moving grain boundary.
Another shortcoming of the impurity drag theory is its phenomenological
character, which does not make use of grain boundary structure. From the
experimental data presented in Chapter 3, in particular the different behavior
of pre-exponential factor and activation enthalpy, respectively, it is very likely
that segregated elements not only exert an impurity drag on the boundary but
also affect grain boundary structure and thus its mobility. An improvement
of the traditional impurity drag theory by also accounting for solute-solute
interaction in the boundary is a first attempt to tackle this problem (see
Chapter 3). Also, it is not improbable that minute precipitates form in the
boundary and move along with the boundary, which would strongly reduce
the growth rate, as discussed in Chapter 3.
The effect of segregation on grain boundary structure is particularly dras-
© 2010 by Taylor and Francis Group, LLC
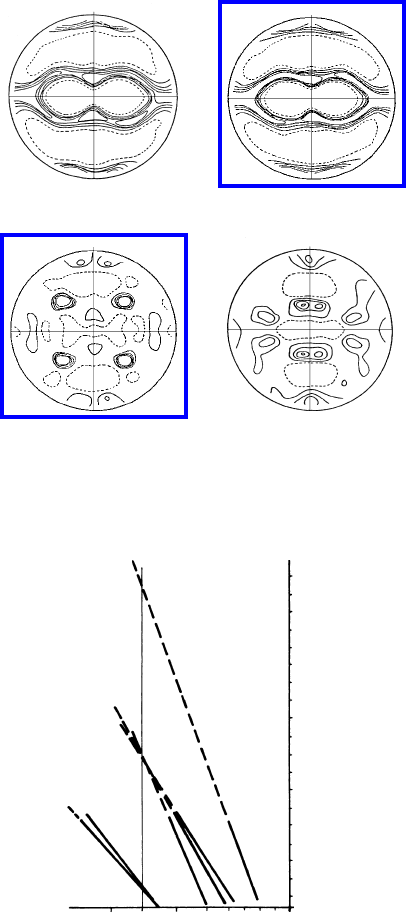
6.2 Recrystallization annd Grain Growth 557
rolling textures
recystallization textures
pure Cu Cu-0.1% P
(a) (b)
(c) (d)
FIGURE 6.36
{111} Pole figures of rolling and recrystallization textures of pure Cu and
Cu-0.1%P (degree of rolling: 95%).
120 150 200 300 400 500
1
10
3
10
18
10
15
10
12
10
9
10
6
time [s]
temperature [°C]
pure Al
Ag
Mg
Si
Cu
Fe
FIGURE 6.37
Temperature dependence of the recrystallization time of binary alloys of high
purity aluminum and 1/100 atomic percent of a second metal (after [600]).
© 2010 by Taylor and Francis Group, LLC
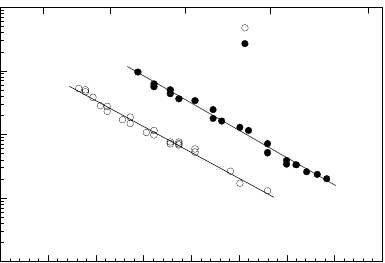
558 6 Applications
5N Al
5N Al+10 ppm Ga
5N Al
5N Al + 10 ppm Ga
600 550 500 450 400
T [°C]
10
-6
10
-7
10
-8
10
-9
10
-10
1.1
A [m
2
/s]
1.15 1.2 1.25 1.3 1.35 1.4 1.45
1.5
1/T [10
3
/K]
FIGURE 6.38
Arrhenius plot of the reduced mobility of a 38.2
◦
111 tilt grain boundary in
pure Al and pure Al doped with 10 ppm Ga.
tic if the segregated solute promotes the tendency for a grain boundary phase
transformation, e.g. in terms of a prewetting or a regular wetting transition,
i.e. essentially the formation of a more or less continuous film of solute in
the boundary. If this film becomes viscous it may drastically change grain
boundary mobility. This is likely for immiscible elements as for the system
Al-Ga. Contrary to expectations, grain boundaries in Al doped with 10 ppm
Ga show a mobility far higher than grain boundaries in pure Al (Fig. 6.38).
This behavior cannot be understood in terms of the impurity drag theory;
rather, it provides unambiguous evidence for the effect of segregated solute
atoms on grain boundary structure (see Chapter 3).
Of course, such behavior has a drastic influence on recrystallization kinet-
ics and microstructure evolution, i.e. small amounts of Ga added to pure Al
considerably speed up recrystallization kinetics and alter the recrystallized
grain size. In fact, most commercial Al alloys contain some amount of Ga.
6.2.5 Grain Growth
After primary recrystallization, when the grains impinge with a random mor-
phology, the grain boundary surface area can be reduced by arranging grain
boundaries in planar positions. Grain growth is basically caused by the fact
that it is impossible to reconcile a completely planar boundary arrangement
and an equilibrium of surface tensions at grain boundary junctions. This is
easy to visualize for a two-dimensional model. Equilibrium of surface tensions
© 2010 by Taylor and Francis Group, LLC
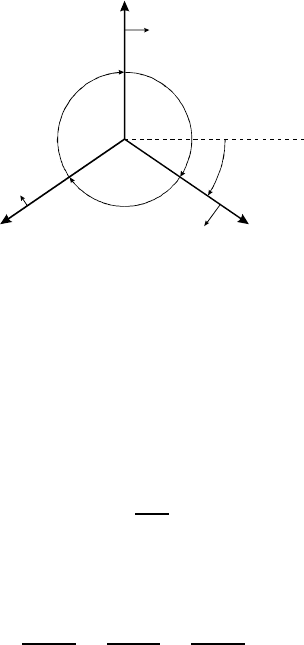
6.2 Recrystallization annd Grain Growth 559
n
2
b
1
b
2
b
3
n
1
n
3
Θ
2
α
1
α
2
α
3
FIGURE 6.39
Equilibrium of grain boundary surface tensions at a triple junction. α
i
—
contact angles; Φ
i
— inclination; n
i
— boundary normal; b
i
—inplane
direction.
γ
s
i
at a junction is obtained for the angles α
i
according to the Herring equation
[73] (Fig. 6.39)
3
i=1
γ
s
i
b
i
+
∂γ
s
i
∂Θ
i
n
i
= 0 (6.41)
If the grain boundary energy does not depend on the spatial orientation of
the grain boundary plane, then the torque terms ∂γ/∂Θvanishand
γ
1
sin α
1
=
γ
2
sin α
2
=
γ
3
sin α
3
(6.42)
In the absence of an orientation dependence of grain boundary energy α
1
=
α
2
= α
3
≡ α = 120
◦
. Both straight boundaries and α = 120
◦
can be achieved
only for an exclusively hexagonal grain structure. A single five-sided grain has
to bend at least one grain boundary to balance the force at all boundary junc-
tions (Fig. 6.40). The curvature of this boundary, however, results in a driving
force to straighten the boundary. The boundary will move toward the center of
curvature and thus mistune the equilibrium angles at the junctions and cause
repeated rearrangements. As a result, no equilibrium at all can be obtained. It
follows that grains with fewer than six sides have convexly curved boundaries
and thus shrink while grains with more than six sides have concavely curved
boundaries and thus expand. Since large grains are in contact with many small
grains, their boundaries are composed of a large number of concavely curved
segments, even though their macroscopic appearance may suggest a convex
shape (Fig. 6.41); consequently, they will expand. In three dimensions the
situation is similar, although more complex in detail. The balance of surface
© 2010 by Taylor and Francis Group, LLC
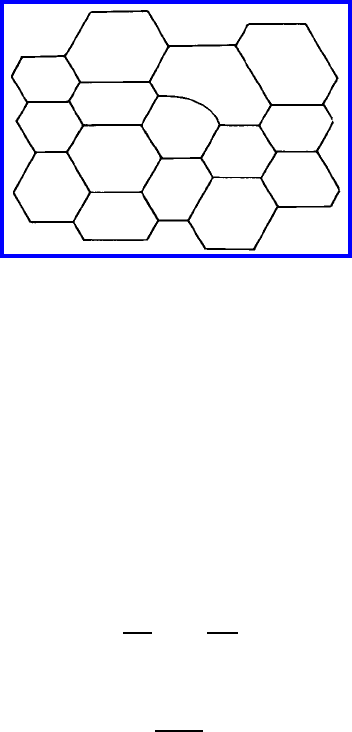
560 6 Applications
FIGURE 6.40
Two-dimensional equilibrium structure that, except for one defect, consists
only of hexagons with 120
◦
contact angles.
tensions requires in this case an angle of 120
◦
at grain boundary junctions
and a tetrahedral angle where four grains are in contact. The impossibility of
reconciling these requirements with planar grain boundaries again causes an
expansion of large grains, while small grains shrink.
The kinetics of normal grain growth can be readily obtained by assuming
that the radius of curvature R is proportional to the average grain diameter
D (R = αD), and the grain diameter varies proportionally to the average
growth rate (v = βdD/dt). Eqs. (6.34b) and (3.6) combine to form
β
dD
dt
= m
b
2γ
αD
(6.43)
which integrates for D(t =0)=D
0
to
D
2
− D
2
0
2m
b
γ
(αβ)
t = K
1
t (6.44)
The kinetic constant K
1
has the temperature dependence of the grain bound-
ary mobility m
b
. For a small initial (primary) grain size D
0
, the grain diameter
is expected to grow proportionally to the square root of annealing time, which
is observed only for ultrapure materials. Normally, grain growth progresses
less rapidly (Fig. 6.42), so that the results can be better approximated by the
relation
D = K
2
t
n
(6.45)
where n<1/2andK
2
is a constant. Frequently, grain coarsening even ceases
completely, which may be due to a variety of reasons.
If the grain size becomes comparable to the smallest specimen dimen-
sions, grain growth is substantially reduced. This is immediately evident for a
bamboo structure in wires. In sheets, a two-dimensional growth ought to pro-
ceed after a columnar grain structure perpendicular to the sheet surface has
© 2010 by Taylor and Francis Group, LLC
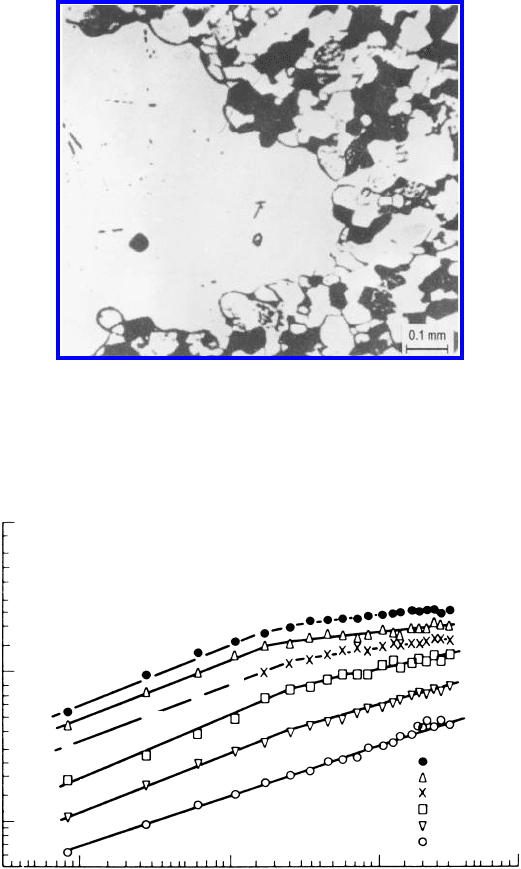
6.2 Recrystallization annd Grain Growth 561
FIGURE 6.41
Abnormal grain growth in Zn. Note that grain boundary is macroscopically
convex but microscopically concave [605].
1000 10000
10
1.0
0.1
average grain diameter [mm]
3 10 100
corrected annealing time [s]
=
10000 ppm
=
3000 ppm
=
1000 ppm
=
500 ppm
=
100 ppm
=
zone refined lead
tin added
n = 0.41
n = 0.40
n = 0.39
n = 0.42
n = 0.34
n = 0.103
n = 0,109
n = 0.140
n = 0.221
n = 0.228
n = 0.345
n = 0.40
FIGURE 6.42
Graingrowthinzonerefinedleadandleadwithdifferenttincontents.The
deviation of the exponent from the ideal value of 0.5, and the increase of this
deviation with increasing grain size are apparent (after [606]).
© 2010 by Taylor and Francis Group, LLC

562 6 Applications
been established. This is, however, prevented by thermal grooving of grain
boundaries at surfaces (see Chapter 3). The final grain size is usually well
approximated by about twice the sheet thickness. For secondary recrystalliza-
tion, however, the driving forces are generally sufficient to overcome grooving,
and growth continues in two dimensions.
In the presence of inclusions of a second phase, grain boundary motion is
hindered (see Chapter 3). Grain coarsening will cease, once the driving force
(Eq. (6.34b)), which decreases with increasing grain size, is balanced by the
Zener drag P
R
=3fγ/2r
p
,wheref and r
p
represent volume fraction and
average size of the particles. Assuming R = αD, the final grain size D
f
is
D
f
=
4r
p
3αf
(6.46)
Even in solid solutions grain growth may tend to cease in the course of time.
Because of the decrease in driving force with increasing grain size, the growth
rate decreases and the boundary may change from a free to a loaded state (see
Chapter 3). This can reduce the growth rate by several orders of magnitude.
Grain coarsening can be substantially restricted in strongly textured mi-
crostructures. Since the grain boundary migration rate is very small for small
orientation differences, grain growth is absent, if the recrystallization texture
consists of only one component such as the cube texture in fcc metals, but
major texture changes can result if minor amounts of other orientations exist
in a sharply textured microstructure.
Thus far we have considered only the effect of grain boundaries on the
evolution of microstructure during recrystallization and grain growth. As ev-
ident from Chapter 4, however, triple junctions may play an essential role in
the dynamics and kinetics of grain boundary assemblies. In this context the
criterion
Λ=
m
tj
· λ
m
b
(6.47)
plays a role, where λ is the characteristic length, i.e. the grain size D in poly-
crystals or the subgrain size d in cellular deformed structures. Owing to the
almost complete neglect of triple junction properties on microstructure evo-
lution there is little quantitative data on the behavior of boundary-junction
systems. In Chapter 4 we already noted the influence of triple junctions on the
Von Neumann-Mullins relation and its drag effect on grain boundary motion.
According to Eq. (6.47) a large impact of triple junction mobility may be ex-
pected if the characteristic length λ is very small, i.e. for ultrafine microstruc-
tures or even nanocrystalline material. Owing to the experimental difficulties
to properly characterize nanocrystalline microstructures and in particular to
account for impurities, second-phase particles produced during consolidation,
as well as a potentially altered grain boundary structure at large surface to
volume ratios, it is not possible to accurately compare the grain growth dy-
namics of nano- and microsized grain structures.
However, there are indications that triple junctions substantially slow down
© 2010 by Taylor and Francis Group, LLC

6.3 On Precipitation-Controlled Grain Size 563
grain growth kinetics in ultrafine grain structures, and they may even give rise
to the suppression of recrystallization and set off abnormal grain growth. One
“suspicious” example is mechanically alloyed ODS superalloys, which are very
fine grained (D ∼ 100nm) after hot extrusion and exhibit conspicuous resis-
tance to recrystallization and grain growth, which may be set off only at very
high homologous temperatures (T>0.8T
m
). Although not yet beyond the
state of speculation, this behavior may be due to triple junctions, since all
hypotheses that try to account for the observed effect by second-phase parti-
cles, cannot yet be confirmed. It is in particular noteworthy that intermediate
anneals at not so high temperature can set off abnormal grain growth at even
more elevated temperatures, i.e. small changes in grain size may render the
microstructure unstable [603, 604].
6.3 On Precipitation-Controlled Grain Size
It is textbook knowledge that second-phase particles slow down the process
of grain growth (see Chapter 3). However, in [601, 602, 607] the question
was risen whether the formation of a second phase always slows down grain
growth in polycrystals, in particular in nanocrystals. The authors of [607]
considered the grain boundary motion in an alloy with a miscibility gap. When
the sample (polycrystal) is single phase, grain boundary motion or the rate
of grain growth is described by the theories of impurity drag (see Chapter 3).
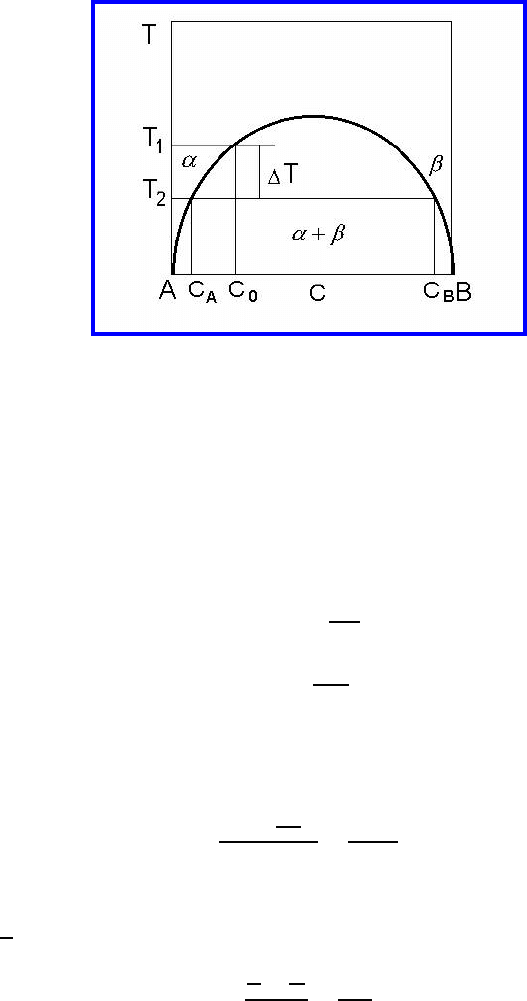
However, if we lower the temperature by ΔT or change the concentration
by Δc (Fig. 6.43) the systems changes to a two-phase field. This causes two
changes, namely the formation of precipitates of a second phase and depletion
of solute atoms in the bulk of a grain. Decreasing the temperature by ΔT will
offset the system to the two-phase field and the mass W
p
of the precipitates
with respect to the solid solution W
sol.sol.
will be according to the lever rule
(Fig. 6.43)
W
p
W
sol.sol.
=
c
0
− c
A
c
B
− c
0
(6.48)
V (c
0
= c
A
, precipitates) >V (c
0
) (6.49)
where V is the boundary migration rate.
Two situations have to be distinguished, namely, large immobile and small
mobile precipitates.
For large immobile particles the grain growth rate can be represented as
[607]
V = m
b
P − P
tp
im
(V ) − P
p
>m
b
P − P
SS
im
(V )
(6.50)
where P =
αγ
¯
R
is the driving force for grain growth, γ is the grain boundary
surface tension, P
SS
im
and P
tp
are dragging forces in the solid solution and
© 2010 by Taylor and Francis Group, LLC
564 6 Applications
FIGURE 6.43
Binary phase diagram with miscibility gap (see text for details) [607].
the two-phase regime, respectively. P
p
is the dragging force by immobile par-
ticles, and α is a geometrical parameter that correlates grain size and grain
boundary curvature and will be taken to be α = 3 in the following. To assess
Eq. (6.50) the Zener- and the L¨ucke-Detert approximations for particle drag
and impurity drag, respectively
P
drag
=
3¯cγ
2r
(6.51)
P
im
=Γ
V
D
im
kT (6.52)
¯c — the volume fraction of the particles, r — particle radius, Γ — adsorption
at the grain boundary, D
im
— bulk diffusion coefficient of the solute atoms.
The next inequality expresses the acceleration of grain growth caused by
precipitation [607]
P −
3¯cγ
2r
Γ(c
0
= c
A
)
>
P
Γ(c
0
)
(6.53)
Eq. (6.53) is valid under the assumption that the impurity diffusion coefficient
is independent of concentration. With the driving force for grain growth P ≈
3 ·
γ
¯
R
, and the Henry adsorption isotherm Γ(c)=Bc, Eq. (6.53) reads
1
¯
R
−
¯c
2r
c
A
>
1
¯
Rc
0
(6.54)
It is pointed out that, strictly speaking, for solids the isotherms should be used
which take into consideration the interaction between species (see Chapter 1).
© 2010 by Taylor and Francis Group, LLC
6.3 On Precipitation-Controlled Grain Size 565
The Henry isotherm was used in [607] for a rough estimate to establish the
essential physical link between the parameters of the problem.
The next relation follows from Eqs. (6.48), (6.49) and (6.54) [607]
1 −
c
0
−c
A
c
B
−c
A
·
¯
R
r
c
a
>
1
c
0
(6.55)
or, using evident relationships between c
A
, c
0
, c
B
¯
R
r
<
c
B
c
0
(6.56)
Eqs. (6.55) and (6.56) determine the conditions under which precipitation ac-
celerates grain growth in the alloy. Since the relations (6.55) and (6.56) are
derived under the assumption that the second-phase particles are immobile,
the larger r (particle radius) and the smaller
¯
R (mean grain size), the better
the approach. For c
B
∼ 1, c
0
∼ 10
−3
, c
A
∼ 10
−4
Eq. (6.56) yields
¯
R
r
< 10
3
.
However, the phenomenon discussed holds as well for particles moving to-
gether with the grain boundary, in other words, for small, mobile particles.
The theory of grain boundary motion dragged by mobile particles was com-
prehensively considered in Chapter 3. In particular, it was shown that the
velocity of joint motion of a grain boundary and the particles with a contin-
uous size distribution ¯n(r) can be expressed as
V =
Pm
b
1+
,
∞
0
¯n(r)m
b
m
p
(r)
dr
(6.57)
where m
p
(r) is the mobility of a particle of size r. The total number of particles
per unit area n is as follows:
n =
∞
0
¯n(r)dr (6.58)
The border between free grain boundary motion and the joint motion of the
grain boundary with the particles is established by dimensionless criterion ρ:
ρ =
∞
0
¯n(r)m
b
m
p
(r)
dr (6.59)
If ρ 1thenV
∼
=
m
b
P , and the grain boundary velocity is determined by
the mobility of the boundary. If ρ 1 grain boundary motion is determined
by the mobility of the particles and their distribution function, which in the
simple case of a uniform size distribution r = r
0
can be represented as
¯n(r)=n
0
δ (r − r
0
)
V =
Pm
p
(r
0
)
n
0
(6.60)
ρ =
n
0
m
b
m
p
(r
0
)
© 2010 by Taylor and Francis Group, LLC
