Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.

944 P. Lin e t al.
the techniques include (i) treatment of silica or silicone surfaces by organosilanes,
(ii) treatment of noble metal surfaces (Au, Pt, Ag, etc.) by thiol or dialklydisulfide
compounds, and (iii) treatment of silicon or silicon nitride surfaces with alkenes.
Silanization of Silica or Silicone Surfaces
Organosilanes are commonly used in MEMS fabrication as adhesion promoters or
antistiction l ayers (see also Section 13.4.2). They can modify substrate surfaces such
as silica (e.g. glass, quartz) or silicone (e.g. PDMS) via the covalent Si-O-Si link-
age between organosilanes and the substrate surface. Although some silanol groups
(-SiOH) may originally exist on the PDMS surfaces, an O
2
plasma treatment on the
PDMS can produce more silanol groups for a more effective silanization reaction.
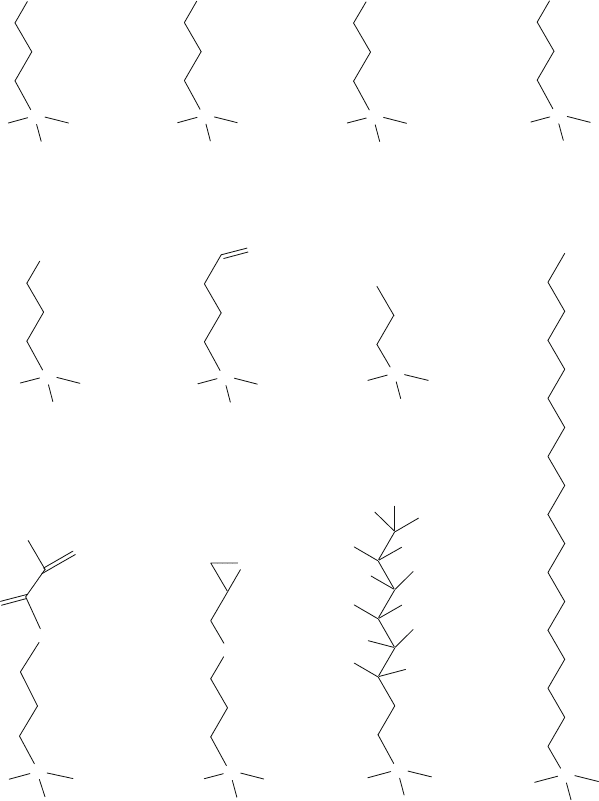
Examples of some commonly used organosilanes are shown in Fig. 13.7, which
include alkyl-substituted alkoxysilanes (1–6, 9) and alkyl-substituted chlorosilanes
(7, 8, 10, 11). Some chemicals in Fig. 13.7 (such as FOTS, No. 10) have similar
characteristics as those mentioned in Tables 13.1 and 13.2 (such as FDTS). FOTS
is commercially available for producing hydrophobic and non-stick surfaces using
a molecular vapor deposition tool (such as MVD-100, by Applied Microstructures).
The same tool can also be used to deposit self-assembled coatings based on APTMS
(No.3inFig.13.7), MAOPTS (No. 8), or MPTMS (No. 5) to enhance adhe-
sion, change hydrophobicity, and/or to create functional groups on the surface for
subsequent reactions. The organosilanes can be monofunctional, bifunctional, or
trifunctional, depending on the number of the silanol groups (Si-OH) that can be
converted upon hydrolysis (Fig. 13.8).
In general, coatings are more robust when organosilanes contain multi-
functional silyl groups. However, silanization involving trifunctional silyl groups
usually results in rougher surfaces because of the complex cross-linking reactions
(Fig. 13.9a). Silanization using organosilanes with monofunctional silyl groups
results in smoother surfaces (Fig. 13.9b), but they are more vulnerable to hydrolytic
desorption in a high-pH alkaline buffer.
Thiolation of Noble Metal Surfaces
As shown in Fig. 13.10, thiols or dialkyldisulfides can be used to modify the surfaces
of noble metals such as Au, Ag, or Pt, to form self-assembled monolayers (SAMs)
[76, 77]. These modifications can yield excellent systems to study the interactions of
proteins with organic surfaces. Some examples of thiol compounds [76] and disul-
fide compounds [77] are shown in Figs. 13.11 and 13.12, respectively. Typically,
to prepare SAMs on the surfaces of noble metals, a thiol or disulfide solution in
an organic solvent (e.g. ethanol or methanol) with a very dilute concentration, i.e.
0.2–20 mM, is prepared and then used to treat the surfaces of noble metals. SAMs
comprising mixtures of two or more components can also be prepared by chemisorp-
tion from solutions containing mixtures of these components [76]. Consequently, the
surface properties (e.g. hydrophobicity) can be tailored according to the ratio of the
different alkanethiolates on the modified surfaces.
13 Surface Treatment and Planarization 945
Si
H
3
CO OCH
3
NH
2
OCH
3
Si
H
3
COCH
3
NH
2
OCH
3
Si
H
3
CCH
3
NH
2
OCH
3
Si
H
3
CO OCH
3
SH
OCH
3
Si
Cl Cl
Cl
Si
H
3
CO OCH
3
O
OCH
3
O
Si
C
2
H
5
OOC
2
H
5
NH
2
OC
2
H
5
Si
H
3
CCH
3
Cl
Si
C
2
H
5
OOC
2
H
5
OC
2
H
5
O
Si
Cl Cl
Cl
F
F
F
F
FF
F
F
FF
F
F
F
Si
Cl Cl
Cl
O
1234
567
8 91011
O
Fig. 13.7 Examples of organosilanes: (1) 3-aminopropyldimethylmethoxysilane, (2) 3-amino-
propylmethyldimethoxysilane, (3) 3-aminopropyltrimethoxysilane (APTMS), (4) 3-amino-
propyltriethoxysilane (APTES), (5) 3-mercaptotrimethoxysilane (MPTMS), (6) triethoxysi-
lylbutyraldehyde, (7) propyldimethylchlorosilane, (8) 3-methacryloxypropyltrichlorosilane
(MAOPTS), (9) 3-glycidoxypropyltrimethoxysilane (GPTMS), (10) 1H,1H,2H,2H-perfluoro-
octyltrichlorosilane (FOTS), and (11) octadecyltrichlorosilane
946 P. Lin e t al.
Si
H
3
CO OCH
3
OCH
3
R
Si
H
3
CO CH
3
OCH
3
R
Si
H
3
CCH
3
OCH
3
R
Si
Cl Cl
Cl
R
Si
H
3
CCH
3
Cl
R
Si
HO OH
OH
R
Si
HO CH
3
OH
R
Si
H
3
CCH
3
OH
R
Si
HO OH
OH
R
Si
H
3
CCH
3
OH
R
Hydrolysis
Trifunctional
alkyl-substituted alkoxysilane
Bifunctional
alkyl-substituted alkoxysilane
Monofunctional
alkyl-substituted alkoxysilane
Trifunctional
alkyl-substituted chlorosilane
Monofunctional
alk
y
l-substituted chlorosilane
Fig. 13.8 Hydrolysis of organosilanes
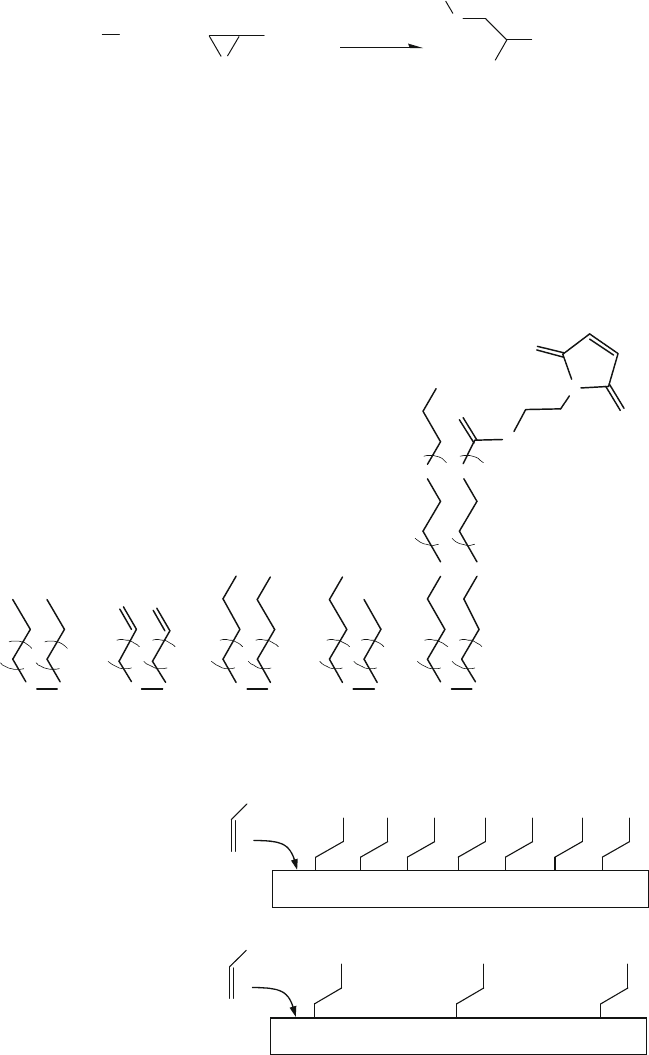
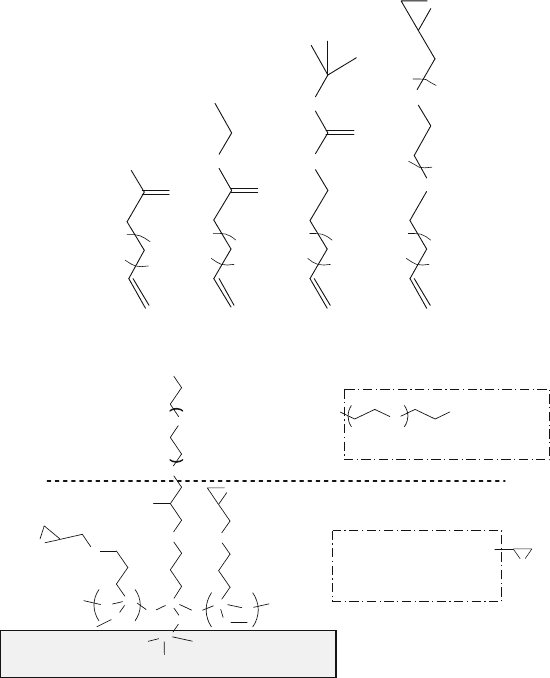
Hydrosilylation of Silicon or Silicon Nitride Surfaces
Alkenes can be used to modify substrate materials based on single crystalline sil-
icon, porous silicon, or silicon nitride via Si-C bonding (Fig. 13.13)[78]. Prior
to hydrosilylation, the native SiO
2
layer on silicon must be removed (e.g. by
HF). Dilute (1–2%) HF solution treatment of a single crystalline Si (100) wafer
yields dihydride (-SiH
2
) on the surface, and a 40% NH
4
F solution treatment on Si
(111) results in monohydride (–SiH) groups. Similar etching treatments can also
be applied to silicon nitride substrates. For porous Si (created by electrochemical
HF etching), etching yields a –SiH
x
terminated silicon surface with x ranging from
1 to 3. The freshly prepared silicon hydride terminated surface is chemically homo-
geneous (>99% H terminated) and is a useful precursor because the Si-H bonds can
be further functionalized.
13 Surface Treatment and Planarization 947
Si
OO
O
R
Si
O
Si
O
Si
O
R
O
Si
O
R
HO
Si
R
O
O
Si
O
Si
Si
O
Si
O
Si
O
OH
OH
R
O
Si
OH
O
Si
Si
R
O
OH
Si
O
R
O
Si
O
R
O
Si
R
R
O
Si
HO
OH
R
Si
O
Si
HO
O
O
Si
Si
O
R
HO
R
Si
HO
OH
O
OH
HO
Si
O
HO
R
Si
O
R
OH
Si
O
Si
O
Si
O
Si
O
Si
O
Si
O
Si
O
O
Si
R
H
3
CCH
3
OH O
Si
H
3
CCH
3
R
OH O OH O
Si
Si
H
3
CCH
3
R
H
3
CCH
3
R
Si
O
Si
OH
O
O
Si
H
3
C
CH
3
R
Si
HO
(A)
(B)
(
)
(
)
a
b
c
d
e
f
g
Fig. 13.9 A silica surface subjected to silanization treatment by (a) a trifunctional organosilane
and (b) a monofunctional organosilane
Hydrosilylation between C=C and Si-H can be started using radical initiators
such as diacyl peroxide at ~100
◦
C. However, thermally induced hydrosilyla-
tion of porous silicon at 110–180
◦
C, without initiators, has also been reported.
Alternatively, UV irradiation (185 and 253.7 nm wavelength UV light; 2 h) can
also be used to start hydrosilylation on the Si-H surface at room temperature. Some
examples of alkenes [78] are shown in Fig. 13.14.
13.5.3 Modification of Pre-treated Substrate Surfaces
After the pristine surface has been modified using one of the methods described in
Section 13.5.2, the surface is either ready for use or requires further modification. To
perform further modification, the methods described in the previous section can still
be used. The modified surfaces may have exposed functional groups such as –OH,
-NH2, -COOH, -SH, or −CHO that are ready for further modification. Therefore,
it is advantageous and convenient to exploit those chemical reactions for further
surface modification in a multi-step procedure. The commonly employed chemical
reactions involving −OH, −NH
2,
−COOH, −SH, and −CHO functional groups on
MEMS device surfaces for BioMEMS applications are summarized and elaborated
on in the sections that follow.
948 P. Lin e t al.
R
(A)
R
M
S
SH
R
(B)
S
R
S
(C)
S
R
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
M
S
R
S
R
S
R’
R
M
R
M
S
R
M
S
M
S
R
M
S
R
M
S
M
R
M
SS
M
SS
M
SS
M
SS
M
SS
M
SS
M
SS
M
SS
M
R
M
R’
M M
R’
M
R
M
R’
M
R
M
R’
Fig. 13.10 A noble metal
surface subjected to a
thiolation treatment by (a)a
thiol, (b)a
homodialkyldisulfide, and (c)
a heterodialkyldisulfide
SH
OH
O
SH
NH
2
SH SH
OH
O
O
SH
OH
O
O
SH
OCH
3
SH
OCH
3
SH
CF
3
SH
CN
9
5
5
9
9
9
9
9
9
9
9
Fig. 13.11 Examples of thiols
13.5.3.1 Chemistry of Hydroxyl Groups (R-OH: Alcohols)
The chemical reaction shown in (13.3) allows an alcohol to form a covalent linkage
with another chemical compound containing an epoxy group.
13 Surface Treatment and Planarization 949
ROH
R’
O
+
R’
HO
O
R
(13.3)
EXAMPLE 4:
A three-step surface modification process was used by Moorcroft et al. to modify
the PDMS substrate for in situ oligonucleotide synthesis on the surface of PDMS
microchannels [79]. First, PDMS channels were prepared by soft lithography using
SU-8 as a mold [80]. The PDMS was cured at room temperature overnight, and
then at 70
◦
C for 1 h. The PDMS channels were initially oxidized by an ozone
cleaner (UVO Cleaner
R
, by Jelight Co.). Secondly, vapor-phase silanization was
O
S
O
OH
S S S
NH
O
O
O
S S
OH
OH
S S
OH
9
3
S
O
S
O
9
9
9
9
9
9
9
9
9
O
O
6
N
Fig. 13.12 Examples of dialkyldisulfides
R
(A)
RRR R R R R
Si Si Si Si Si Si Si
R
(B)
RRR
Si N N Si N N Si
Fig. 13.13 Hydrosilylation
of (a) a silicon surface and
(b) a silicon nitride surface
950 P. Lin e t al.
HO
O
O
O
CF
3
HN
O
O
O
O
O
7
3
7
7
7
Fig. 13.14 Examples of alkenes
Si
OO
O
O
Si
Si
Si
O
O
O
O
O
O
HO
O
O
HO
O
O
a
b
PDMS
R'
(epoxysilanized PDMS)
O
R
O
OHHO
5
5
Fig. 13.15 Reaction of the hydroxyl group of HO-(C
2
H
4
O)
5
-C
2
H
4
-OH with the epoxy group of
an epoxysilanized PDMS substrate, as described in Example 4 and reaction (13.3)
carried out in a vacuum oven with 3-glycidoxypropyltrimethoxysilane (GPTMS,
No. 9 in Fig. 13.7) to functionalize the substrate surface with epoxy groups. In
the third step of the surface modification process (Fig. 13.15), the reaction (13.3)
was involved to create the covalent linkage between the surface-bound epoxy group
on the silanized PDMS substrate and the hydroxyl group of PEG spacer (HO-
(CH
2
CH
2
O)
5
CH
2
CH
2
OH). The oligonucleotides can be in situ synthesized on
the PEG surface using the conventional phosphoramidite chemistry with a DNA
synthesizer [79].
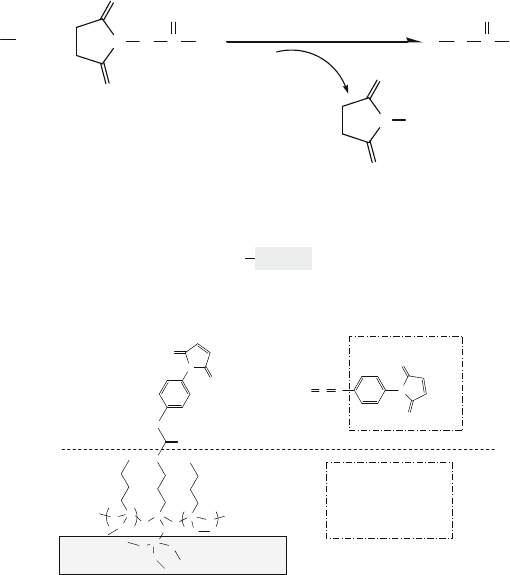
13.5.3.2 Chemistry of Amino Groups (R–NH
2
: Amines)
In this section, four different types of amine reactions are described, and each reac-
tion is accompanied by an example. The chemical reaction shown in (13.4) allows
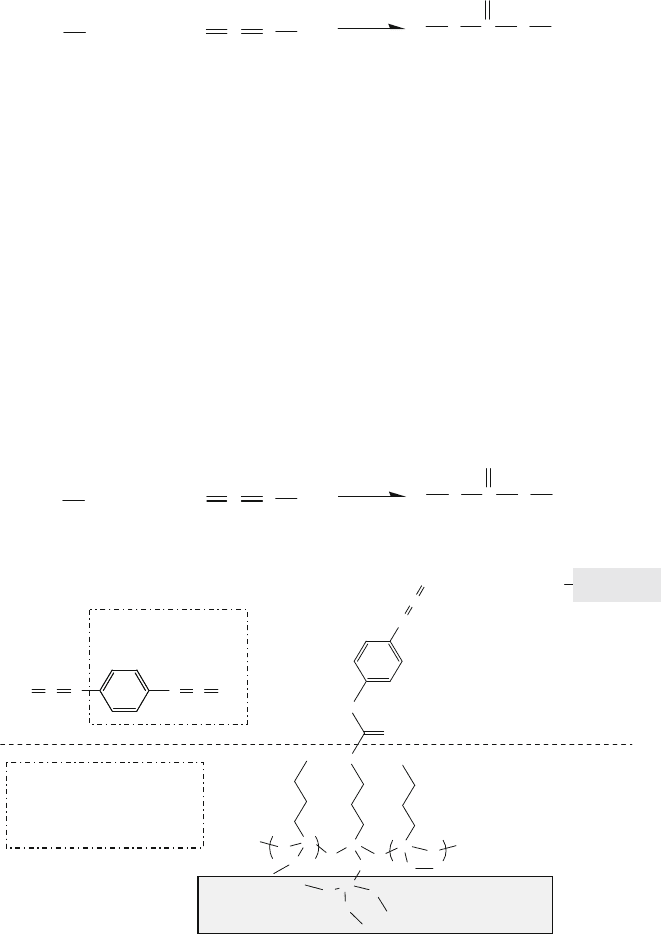
13 Surface Treatment and Planarization 951
an amine to form a covalent linkage with another chemical species containing an
isothiocyanate group.
CSNR'
RNH
2
+
CN
H
R'
S
N
H
R
(13.4)
EXAMPLE 5:
A three-step surface modification process was used by Charles et al. to fab-
ricate DNA chips for DNA hybridization efficiency studies [81]. First, the glass
substrates were acid-cleaned, and then the substrate was treated with a 2% acidic-
methanol solution of 3-aminopropyltriethoxysilane (APTES, No. 4 in Fig. 13.7).
In the second step of the surface modification process (Fig. 13.16), the r eaction
(13.4) was used to generate the covalent linkage between the surface-bound amino
group (on APTES) and the isothiocyanate group of the bifunctional cross-linker,
1,4-phenylene diisothiocyanate (PDC). In the third step, the modified surfaces
(isothiocyanate groups) reacted with amine-modified single strand DNA molecules
(also shown in Fig. 13.16)[81].
A more popular chemical reaction (13.5) for an amine is to form a covalent
linkage with an isocyanate group. Aromatic isocyanates are more reactive than the
aliphatic isocyanates, and thus are more commonly used.
CONR'
RNH
2
+
CN
H
R'
O
N
H
R
(13.5)
Si
OO
O
Si
Si
Si
O
O
O
NH
S
NH
NH
2
O
O
O
O
NH
2
N
C
S
a
b
Glass
ssDNA
H
2
N
+
NCS
R'
NCS
R
(Aminosilanized Glass)–NH
2
Fig. 13.16 Reaction of the isothiocyanate group of 1,4-phenylene diisothiocyanate with the amino
group of an aminosilanized glass substrate, as described in Example 5 and reaction (13.4)
952 P. Lin e t al.
EXAMPLE 6:
A three-step surface modification process was used by Jin et al. to fabricate
DNA chips for DNA hybridization efficiency studies [82]. Similarly to Example 5,
the silica surface was first modified with 3-aminopropyltriethoxysilane (APTES, in
Fig. 13.7). This was performed by soaking the silica in 1 wt% (40 mM) APTES
solution in anhydrous toluene at room temperature for 30 min. In the second step
of the surface modification process (Fig. 13.17), a maleimide-presenting surface
was generated using the reaction in (13.5). That is, the isocyanate group of the het-
erobifunctional cross-linker, p-maleimidophenyl isocyanate (PMPI), formed a urea
linkage with the APTES amines. The reaction was carried out in a 70 mM solution
of PMPI in anhydrous acetonitrile at room temperature for 30 min. The outermost
functional group became the maleimide group after this PMPI-APTES reaction.
The final step was the immobilization of the thiol terminated DNA strands with
the surface maleimides.
The next example is the chemical reaction shown in (13.6), in which an amine
reacts with an N-hydroxysuccinimide ester (NHS-ester) to form an amide linkage.
RNH
2
N
O
OC
O
O
R' R'
+
N
H
C
O
R
N
O
O
OH
(13.6)
Si
OO
O
Si
Si
Si
O
O
O
NH
O
NH
NH
2
O
O
O
O
NH
2
N
O
O
a
b
Glass
R
(Aminosilanized Glass)–NH
2
ssDNA
HS–(CH
2
)
3
R'
NNCO
O
O
+
Fig. 13.17 Reaction of the amino group on an aminosilanized glass substrate with the isocyanate
group of p-maleimidophenyl isocyanate, as described in Example 6 and reaction (13.5).
13 Surface Treatment and Planarization 953
EXAMPLE 7:
Microfluidic channels made with PDMS (from SU-8 molds) and glass slides
were used to study the amyloid formation and fibril growth [83]. Prior to bond-
ing, the glass slides were cleaned in piranha solution of 70% H
2
SO
4
/30% H
2
O
2
(v/v) at 60
◦
C for 15 min. A three-step surface modification process was used
to covalently attach the insulin molecules to the glass and PDMS walls of the
microchannels. First, the microchannels were injected with a 3% solution of APTES
in ethanol/water (95/5 by volume) for 1 h; then they were washed with ethanol and
cured at 100
◦
C. To activate the microchannel with N-hydroxysuccinimide (NHS)
ester, a solution of 20 mM N,N’-disuccinimidyl carbonate (DSC) in a sodium
bicarbonate buffer (50 mM, pH 8.5) was injected for 3 h at room temperature [83].
In the third step of the surface modification process (Fig. 13.18), the reaction
(13.6) was involved to create covalent attachments between the amino group of
insulin molecules and the microchannel walls containing surface-bound NHS ester
groups. The insulin monomer solution was prepared by dissolving fresh insulin
(1 mg/mL) in 40 mM HCl solution to dissociate hexamers into monomers, and the
solution was flushed through the microchannels for 10 min to covalently bond the
insulin monomers.
Si
Si
O
O
O
Si
Si
O
O
O
NH
O
NH
O
NH
O
NH
2
O
O
NH
O
NH
2
O
a
b
Glass
insulin
R
insulin
NH
2
R'
(Aminosilanized SiO
2
)
H
N
O
O
N
O
O
Fig. 13.18 Reaction of the amino group of an insulin molecule with the N-hydroxysuccinimide
ester group on an aminosilanized glass-based substrate, as described in Example 7 and reaction
(13.6)
The chemical reaction (13.7) allows an amine to form a covalent linkage with
another chemical species containing an epoxy group. This reaction is similar to reac-
tion (13.3), except that an amino group replaces the hydroxyl group in the reaction.
Both groups are commonly used for the ring opening reaction with the epoxy group
.
RNH
2
R'
O
+
R'
HO
HN
R
(13.7)
