Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.

954 P. Lin e t al.
EXAMPLE 8:
It is increasingly important to build BioMEMS devices with the capability of
detecting antigens or viruses. A two-step surface modification process was used by
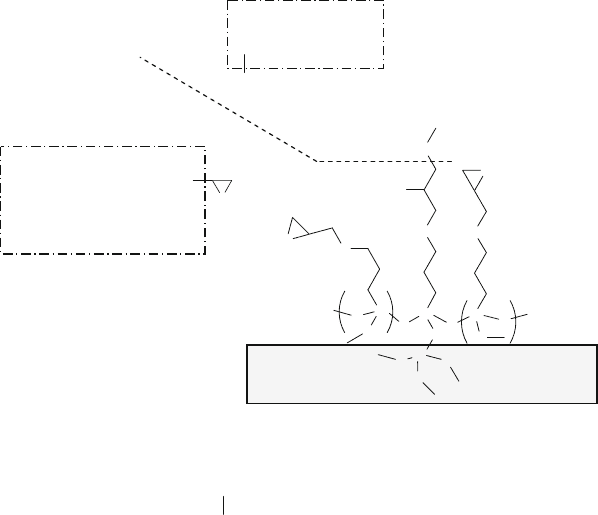
Li et al. to fabricate antibody chips for the detection of HIV-1 antigen [84]. The first
step was to bond GPTMS (No. 9 in Fig. 13.7 and Example 4) to the glass slides.
After cleaning in ethanol, the slides were immersed in a 2.5 vol% GPTMS ethanol
solution containing 10 mM acetic acid for 1 h at room temperature. In the second
step of the surface modification process (Fig. 13.19), the reaction (13.7) occurred
between the epoxy group on a silanized glass substrate and the amino group of a
goat anti-HIV-1 p24 polyclonal antibody molecule [84].
Si
O
O
O
O
Si
Si
Si
O
O
O
O
O
O
O
O
O
HO
HN
O
O
a
b
Glass
Y
NH
2
Y
Goat Anti-HIV-1 p24 Polyclonal Antibody
R
NH
2
Y
R'
(Epoxysilanized Glass)–
O
Fig. 13.19 Reaction between the amino group of a goat anti-HIV-1 p24 polyclonal antibody
molecule and the epoxy group on an epoxysilanized glass-based substrate, as described in Example
8 and reaction (13.7)
13.5.3.3 Chemistry of Carboxyl Groups (R–COOH: Carboxylic Acids)
The carbodiimide-facilitated condensation shown in (13.8) allows a carboxylic acid
to form a covalent amide bond with an amino group. Examples of carbodiimides
are dicyclohexyl carbodiimide (DCC; H
11
C
6
−N=C=N−C
6
H
11
) and diisopropyl
carbodiimide (DIC; H
7
C
3
−N=C=N−C
3
H
7
).
13 Surface Treatment and Planarization 955
CNNR1 R
2
R'H
2
NCR
O
OH
+
CR
O
N
H
R'
Carbodiimide
(13.8)
EXAMPLE 9:
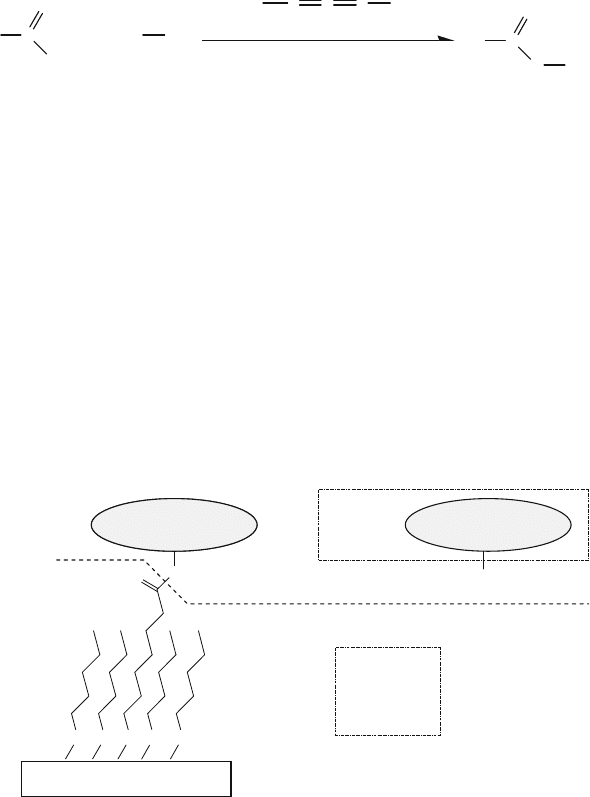
As illustrated in Fig. 13.10, thiols can react with noble metals such as gold.
A two-step surface modification process was used by Davis et al. to construct
gold electrodes covalently attached with cytochrome c molecules [85]. First, as
shown in Fig. 13.20, the gold surface was coated with self-assembled monolay-
ers (SAMs) having a mixture of HS−(CH
2
)
6
−COOH and HS−(CH
2
)
4
−OH. The
ratio of COOH/OH was adjusted t o control the surface reactivity, since the hydroxyl
groups were relatively non-reactive with the amines in the next reaction. The
SAMswereformedbysubmergingthegoldsurfaceina2mMthiolsolutionfor
12–16 h at room temperature. In the second step of the surface modification process
(Fig. 13.20), the reaction (13.8) was involved to generate the covalent amide link-
age between the carboxyl group of Au−S−(CH
2
)
6
−COOH and the amino group
of cytochrome c molecules. The biochips containing immobilized cytochrome c
molecules were used for electron-transfer studies.
SS
OH
S
OH
S
OH
S
OH
NH
O
cytochrome ccytochrome c
Au
Au-S-(CH
R
Au-S-(CH
2
)
6
-COOH
R'
cytochrome c
NH
2
Fig. 13.20 Reaction of the amino group of a cytochrome c molecule with the carboxyl group on
an Au-based substrate, as described in Example 9 and reaction (13.8)
13.5.3.4 Chemistry of Mercapto Groups (R–SH; Thiols)
The addition reaction shown in (13.9) allows a thiol to react with a maleimide
to form a covalent carbon-sulfur (thioether) linkage. Maleimides are excellent
reagents for thiol-selective modification, and the reaction is useful for the fabri-
cation and development of biosensing s urfaces and microarrays. Maleimides linked
to polyethylene glycol (PEG) chains are often used as flexible crosslinkers to attach
proteins or oligonucleotides (DNA) to surfaces.
956 P. Lin e t al.
RSH
N
O
O
+
R'
N
O
O
R'
SR
(13.9)
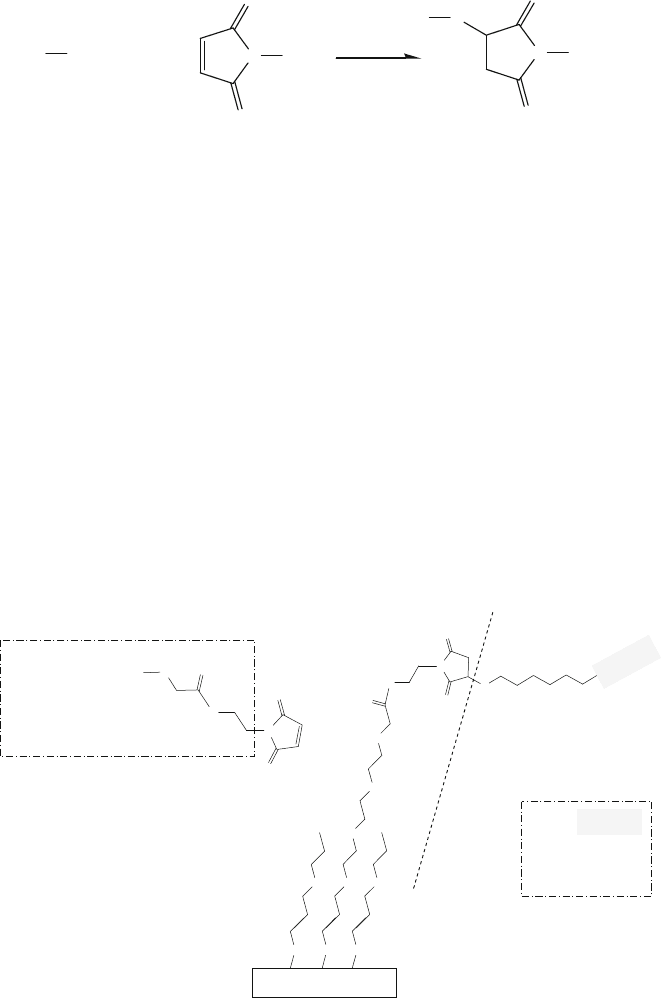
EXAMPLE 10:
DNA immobilization on maleimide-ethylene glycol self-assembled monolayers
was performed by Lee et al. [86]. A two-step surface modification process was
used to construct DNA chips for the studies on DNA probe surface orientation
and target hybridization efficiency. First, 0.1 mM of maleimide-ethylene glycol-
terminated disulfide [HO(C
2
H
4
O)
4
(CH
2
)
11
-SS-(CH
2
)
11
(OC
2
H
4
)
6
-OCH
2
-CONH-
(CH
2
)
2
-C
4
H
2
NO
2
] (MEG) was prepared in an ethanolic solution. The gold-coated
surface was then immersed in the MEG solution at room temperature for 1 h. As
shown in Fig. 13.10c, heterodialkyldisulfide reacts with the gold surface and can
create two different functional groups (one hydroxyl group and one maleimide
group, as shown in this example). In the second step of the surface modifica-
tion process (Fig. 13.21), the reaction (13.9) took place, generating the covalent
linkage between the surface-bound maleimide group of Au-S-(CH
2
)
11
-(OC
2
H
4
)
6
-
OCH
2
-CONH-C
2
H
4
-maleimide and the thiol group of thiolated single-strand DNA
(HS-ssDNA) molecules. The HS-ssDNA solution was prepared in pH 7 buffer at
concentrations from 5 to 500 μM to study the effect on immobilized DNA density
using XPS and TOF-SIMS surface analysis tools.
O
OH
O
O
HN
N
O
O
S
OH
O
O
O
O
Au
ssDNA
R
HS—(CH
2
)
6
—
(
)
4
(
)
4
(
)
4
ssDNA
R'
Au-S-(CH
2
)
11
-(OC
2
H
4
)
6
O
O
N
N
O
H
O
S
S
(
)
9
(
)
9
(
)
9
S
Fig. 13.21 Reaction of the thiol group of a thiolated ssDNA molecule with the maleimide group
on an Au-based substrate, as described in Example 10 and reaction (13.9)
13 Surface Treatment and Planarization 957
The exchange reaction shown in (13.10) allows a thiol to react with a pyridyl
disulfide to form a covalent linkage. The exchange reaction can be performed at
physiologic pH, but the optimum reaction rate is at pH 4–5.
N
S
S
R'
+
RSH
R
S
S
R'
H
N
S
(13.10)
EXAMPLE 11:
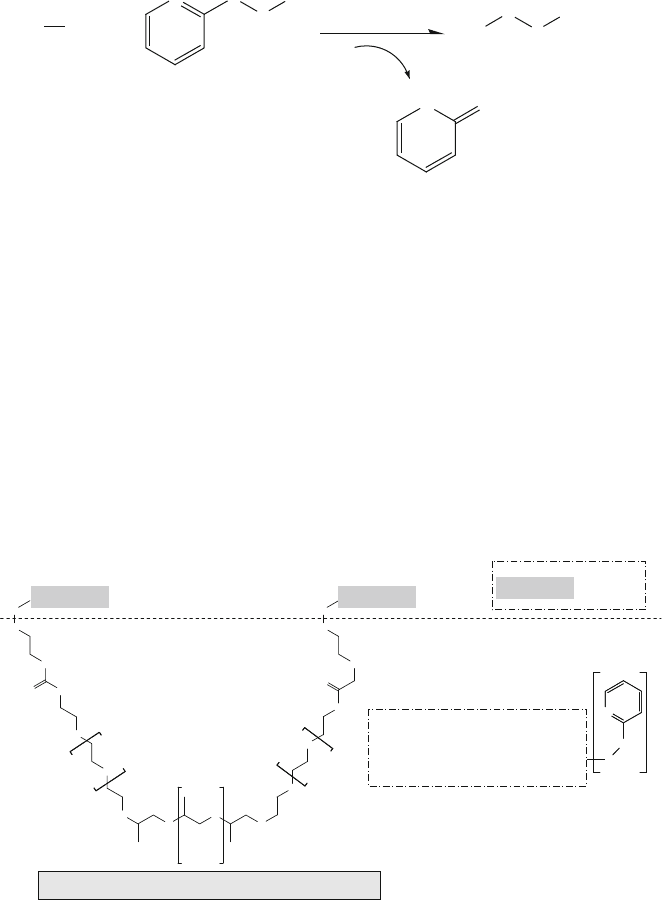
A two-step surface modification process was used by Neff et al. to control sur-
face peptide density and protein adsorption on polystyrene (PS) substrates for cell
adhesion studies [87]. First, a 2-pyridyl disulfide derivative of a PEO/PPO/PEO tri-
block copolymer (Pluronic
TM
F108 [HO-(C
2
H
4
O)
129
-(C
3
H
6
O)
56
-(C
2
H
4
O)
129
-H])
was prepared. The modified F108 was then adsorbed onto the PS substrate surface
by soaking PS in a diluted aqueous solution (0.4 w/v%) for 24 h. The adsorbed
F108 2-pyridyl disulfide derivative formed stable and strong hydrophobic interac-
tions between PPO segments and PS. In the next step of the surface modification
process (Fig. 13.22), the reaction (13.10) was used to generate the covalent linkage
via the exchange reaction between the surface-bound pyridyl disulfide group of the
modified F-108 and the thiol group of the peptides [87]. The peptide surface density
O
OO
O
O
O
O
NH
O
S
S
O
O
O
O
NH
S
S
54
1
2
7
1
2
7
Peptide Peptide
polystyrene substrate
R'
R
HS-
PeptidePeptide
(polystyrene):(F–108 triblock polymer)
S
S
N
2
S
S
N
2
Fig. 13.22 Reaction of the thiol group of an RGD analogue peptide molecule with the pyridyl
disulfide group on a polystyrene-based substrate, as described in Example 11 and reaction (13.10)
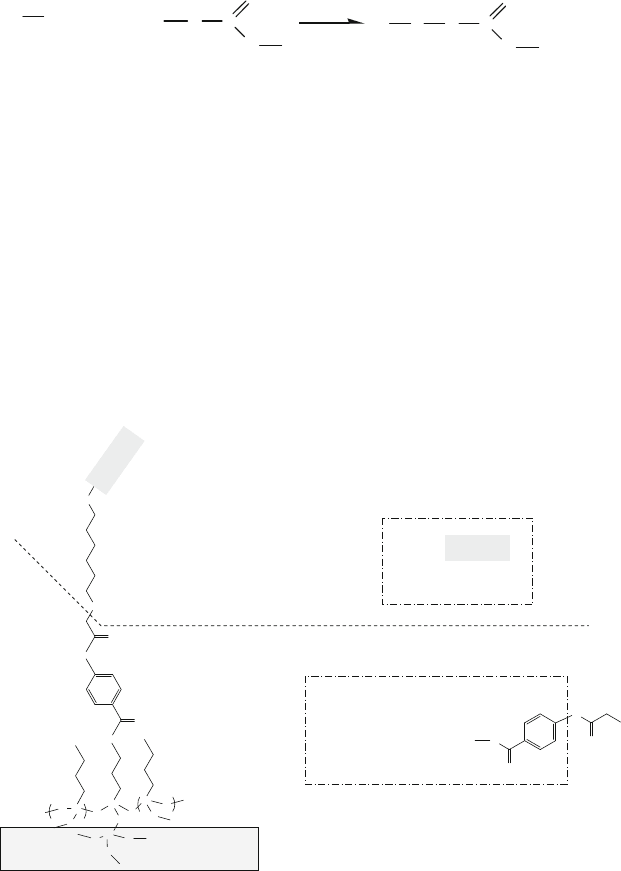
958 P. Lin e t al.
on the PS surface could be varied by mixing the unmodified hydroxyl-terminated
F108 with the disulfide-derived F108 during the adsorption process.
The chemical reaction shown in (13.11) allows a thiol to form a covalent linkage
with an iodoacetyl group in alkaline conditions.
RSH
C
O
C
H
2
I
HN R'
+
C
O
C
H
2
S
HN R'
R
(13.11)
EXAMPLE 12:
The example shown in Fig. 13.23 illustrates a technique for the construction of
high-density DNA chips for nucleic acid hybridization and detection by MALDI-
TOF mass spectrometry [88]. A three-step surface modification process was carried
out for SiO
2
surface modification on silicon wafers [88]. First, the SiO
2
surface was
immersed in a 25 vol% anhydrous toluene solution of 3-aminopropyltriethoxysilane
(APTES,No.4inFig.13.7) for 3 h. This step was similar to the one described
in Examples 5 and 6. Secondly, after washing with toluene and dimethyl sulfoxide
(DMSO), the wafer was soaked in a 10 mM DMSO solution of N-succinimidyl(4-
iodoacetyl)aminobenzoate [SIAB, (C
4
H
4
O
2
N)-O-CO-C
6
H
4
-NH-CO-CH
2
I]. The
reaction i s the same as reaction (13.6) shown in Example 7. In the third step
Si
O
O
O
Si
Si
O
O
O
NH
O
O
S
O
NH
NH
2
NH
2
O
a
b
O
Si
O
O
SiO
2
ssDNA
R
HS-(CH
2
)
6
-O-
R'
(Aminosilanized SiO
2
)
H
N
O
O
I
H
N
ssDNA
Fig. 13.23 Reaction of the iodoacetyl group on a SiO
2
/Si-based surface with the thiol group of a
thiolated ssDNA molecule, as described in Example 12 and reaction (13.11)

13 Surface Treatment and Planarization 959
(Fig. 13.23), the reaction (13.11) took place to immobilize DNA probes via the cou-
pling of the surface-bound iodoacetyl group and the thiol group of thiolated single
strand DNA (ssDNA). This coupling was carried out in 100 mM phosphate buffer
of pH 8 at room temperature for 5 h.
The chemical reaction in (13.12) shows that a thiol group forms a covalent
linkage with an epoxy group.
RSH
R'
O
+
R'
HO
S
R
(13.12)
EXAMPLE 13:
DNA chips were fabricated by Böcking et al. for hybridization efficiency stud-
ies [89]. First, Si (111) wafers were cleaned in concentrated H
2
SO
4
/30% H
2
O
2
(3:1, v/v) for 30 min at 90
◦
C. To prepare for a hydrogen-terminated Si surface,
the wafers were then etched in deoxygenated 40% solution of NH
4
F for 20 min.
The Si surface was then immersed in the solution of epoxy-terminated alkene in
1,3,5-triethylbenzene under an inert atmosphere at 200
◦
C for 2–6 h (the alkene-Si
reaction is illustrated in Fig. 13.13). In the next step of the surface modification
process (Fig. 13.24), the reaction (13.12) was carried out by placing drops of the
DNA solution (10 μMin0.1MNaHCO
3
buffer at pH 8.3) on the epoxy-terminated
surface for 12 h at 40
◦
C. This reaction generated the covalent linkage between the
surface-bound epoxy group on the silicon substrates and the thiol group of thiolated
single strand DNA (HS-ssDNA).
13.5.3.5 Chemistry of Formyl Groups (R–CHO: Aldehydes)
The chemical reaction in (13.13) shows that an aldehyde forms a covalent linkage
(-C=N-) with an amino group. To make the covalent linkage more stable, a reducing
agent such as NaBH
3
CN can be used to turn the Schiff base formed via reaction
(13.13) into a secondary amine (13.14).
R
O
H
H
2
NR'
+
RC
NR'
(13.13)
R
O
H
H
2
NR'
RC
NR'
NaBH
3
CN
R
HN R'
+
Schiff base Secondar
y
amine
(13.14)
960 P. Lin e t al.
Si
O
O
O
Si
O
O
O
O
Si
O
O
O
O
O
O
HO
S
O
ssDNA
R
HS-(CH
2
)
6
-
R'
Si-(CH
2
)
11
-(OC
2
H
4
)
3
-OCH
2
O
ssDNA
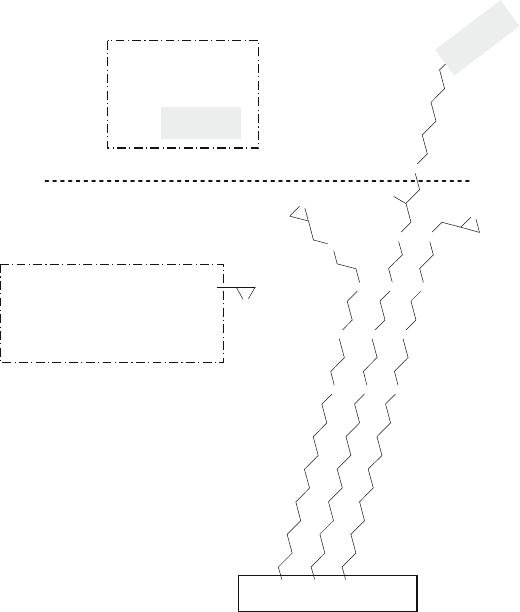
Fig. 13.24 Reaction of the thiol group of a thiolated ssDNA molecule with the epoxy group on a
Si-based substrate, as described in Example 13 and reaction (13.12)
EXAMPLE 14:
A four-step surface modification process was used by Eteshola et al. to construct
a PDMS microfluidic device for enzyme-linked immunosorbent assay (ELISA)
with improved sensitivity [90]. ELISA (also called enzyme-linked immunoassay,
or EIA) is an analytical technique used in immunology for the detection of an
antibody or an antigen. First, PDMS microchannels made from SU-8 molds were
coated with bovine serum albumin (BSA) solution (1 mg/ml in sodium phosphate
buffer) for 3.5 h. In the second step of the modification process (Fig. 13.25), the
reaction (13.13) occurred between glutaraldehyde and the amino group of BSA
on the surface of the PDMS microchannels, i.e., the BSA-coated microchannels
were allowed to react with 0.1% glutaraldehyde aqueous solution for 1 h. In the
third step of the modification process, the same reaction (13.13) occurred again
between the “glutaraldehyde-activated BSA” and the amino group of protein A
molecules (Fig. 13.25). The reaction (13.13) was used twice for this surface modi-
fication. Protein A solution (20 μg/ml in sodium phosphate buffer) was prepared
13 Surface Treatment and Planarization 961
NH
2
N
N
N
N
O
N
BSA
BSA
NH
2
A
A
PDMS
A
Y
+
R
O
O
+
glutaraldehyde
R'
R
(PDMS substrate): (BSA)–
NO
STEP 2
STEP 3
Y
protein A
STEP 1: Non-covalent Adsorption of BSA to the PDMS Surface
NH
2
A
OO
R' (PDMS substrate): (BSA)– NH
2
NH
2
A
rabbit anti-sheep IgM
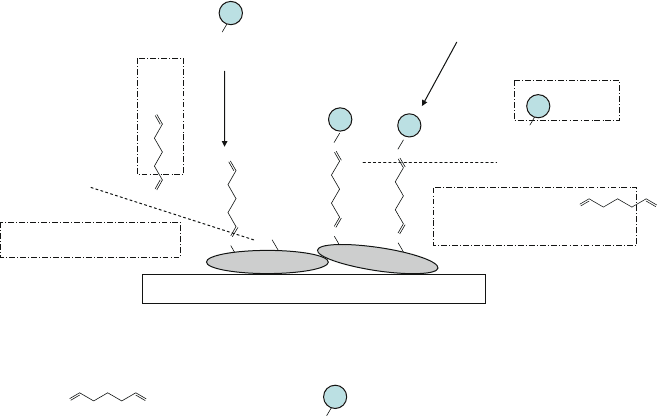
Fig. 13.25 Reaction of the amino groups of proteins (BSA and protein A) with the formyl groups
of glutaraldehyde, as described in Example 14 and reaction (13.13)
for this reaction and glutaraldehyde acted as a crosslinker and spacer for pro-
tein A. The protein A molecules immobilized on the wall of the microchannel
could be functionalized with antibodies (e.g. rabbit anti-sheep IgM) for ELISA
experiments.
EXAMPLE 15:
Microarray technology has been broadly used for a variety of purposes, from
oligonucleotides and proteins to living cells to study gene activity, protein expres-
sion, and interactions between cell surface receptors and ligands [91]. In this
example, cell chips were fabricated using aldehyde-terminated self-assembled
monolayers (SAMs) on gold-coated glass substrates to study the interactions
between bFGF proteins and bFGF receptors on the membranes of BHK-21 cells
[92]. First, thin Au/Cr layers (25 nm/1 nm) were deposited on glass, and were still
transparent enough for optical microscopy. As shown in step 1 (Fig. 13.26), self-
assembled monolayers were formed on the gold surface by immersing it in 1–10 mM
di(10-decanal) disulfide [(CHO-(CH
2
)
9
-S-)
2
] in ethanol for 24 h. In step 2, the reac-
tion (13.14) was used to generate the covalent linkage between the surface-bound
aldehyde group and the amino group of bFGF proteins. For protein immobiliza-
tion, bFGF solution (1 mg/mL in 10 mM HEPES buffer, pH 8.5) was placed on
the aldehyde-terminated surface and incubated overnight at room temperature. In
962 P. Lin e t al.
Au-S-(CH
2
)
9
-CHO
SS S
S
HN
OO
HN
Au
SS S
S
N
OO
N
Au
NaBH
3
CN
Basic Fibroblast Growth Factor
Step 1 Thiolation of Au Surface
Step 2
Step 3
R
R'
NH
2
bFGF
bFGF bFGF bFGF bFGF
NH
2
bFGF
Fig. 13.26 Reaction of the amino group of the bFGF molecule with the formyl group on an
Au-based substrate, followed by the treatment with a reducing agent, NaBH
3
CN, as described
in Example 15 and reaction (13.14)
step 3, a reducing agent, NaBH
3
CN, was used to reduce the formed imine (a Schiff
base) into a more stable secondary amine.
13.5.4 Case Studies
Sections 13.5.2 and 13.5.3 describe most of the commonly used surface modifica-
tion techniques in BioMEMS. However, in view of the broad scope and diversity of
chemical reactions, it must be emphasized that the contents of Sections 13.5.2 and
13.5.3 should only be considered to be a general introduction, covering the basic
concepts necessary to understand surface modification techniques by bottom-up
molecular engineering in BioMEMS. In other words, wherever the laws of chem-
istry allow, alternative plausible reaction schemes can also be developed. Moreover,
when a surface modification scheme is selected, other factors such as reaction kinet-
ics, molecular orientation, reactivity of functional groups etc, should be taken into
consideration as well.
In this section, several case studies are used as examples to address broader
issues in the modification processes. Issues related to surface blocking/passivation
of biochips will be discussed in case studies 1 and 2. A series of studies aimed at
improving AFM tip coatings for protein interaction studies will be summarized in
case study 3. The use of a specialized cross-linking scheme for peptide immobi-
lization and surface patterning by microcontact printing will be described in case
studies 4 and 5, respectively.
13 Surface Treatment and Planarization 963
13.5.4.1 Case Study 1: Promotion of Immobilized Bioactive Proteins’
Biological Activity
Some key requirements for biochip fabrication are to ensure that the sensing
molecules survive the processing steps, remain in the patterned location, and main-
tain maximum sensitivity compared to background noise. After immobilizing the
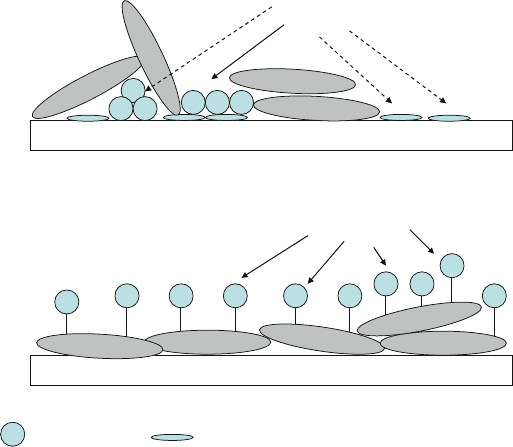
bioactive molecules (e.g. protein A in Fig. 13.27a) on specific areas, bovine serum
albumin (BSA) is commonly used to cover the non-sensing substrate surfaces to
reduce undesired adsorption and background noise. For example, the desired protein
A molecules are immobilized to the substrate surface by non-covalent adsorption;
then BSA is applied, also by non-specific adsorption, to block the surface not occu-
pied by the bioactive protein molecules (Fig. 13.27a). Although this method is easy
to apply, the BSA can also block the active protein A sites, and protein A can be
severely denatured when they are adsorbed directly to a hydrophobic surface. These
drawbacks will significantly reduce the reactivity of the protein A with the antibody
(the Y-shaped molecule).
To increase the reactivity between protein A and the antibody (Y), a better block-
ing strategy was demonstrated by Eteshola et al. [90], as shown in Fig. 13.27b.In
this study, the BSA layer was first deposited by adsorption on the substrate surface.
BSA
BSA
PDMS
Y
protein A
rabbit anti-sheep IgM
BSA
BSA
BSA
PDMS
BSA
BSA
BSA
BSA
Y
Y
x
x
x
Severely Denatured
Blocked
(A)
(B)
denatured protein A
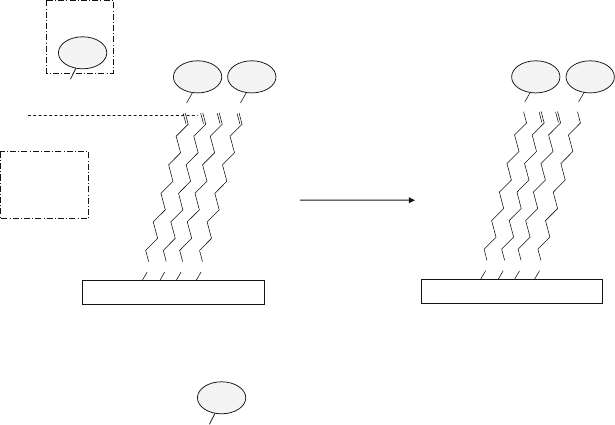
Fig. 13.27 Immobilization of protein A molecules by (a) non-covalent adsorption on a PDMS sub-
strate followed by non-covalent BSA blocking, and (b) covalent immobilization via glutaraldehyde
on the PDMS substrate coated with non-covalently adsorbed BSA
