Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.


574 D.W. Burns
Table 8.21 (continued)
Material
Etch rate
(Å/s) Etchant Remarks and references
32 Polyethylene glycol
(PEG)
H
2
O
undiluted
Room temperature; also dissolves in acetone and alcohol [404]
33 Polyethylene
terephthalate,
(PET), Mylar
R
4200 H
2
SO
4
(96%):
Phenol:H
2
O
115:76:9 by
weight
110
◦
C; nonstandard chemical; DI water rinse [405]
34 Polyimide (PI,
Kapton
R
),
adhesive-backed
tape
Acetone
undiluted
Apply tape with tweezers on top of PR; peel tape off manually or soak
in acetone to remove [406]
35 Polyimide (PI) 150 CF
4
/Ar/O
2
plasma
5:10:30 sccm
Dry etch; 300 mT; 180 W; etches Si
3
N
4
(9 Å/s), oxide-LTO (9 Å/s), Si
(3 Å/s); doesn’t significantly etch Al [45]
36 Polyimide–Kevlar
(PI–Kevlar
R
),
PCB substrates
H
2
SO
4
(96%)
undiluted
185
◦
C; DI water rinse [407]
37 Polyimide (PI) H
2
SO
4
(96%):
H
2
O
2
(30%)
7:1
80
◦
C; Caro’s acid; DI water rinse [45]
38 Polyimide (PI), spun 2800 H
2
SO
4
(96%):
H
2
O
2
(30%)
50:1
120
◦
C; add fresh peroxide; DI water rinse [28]
39 Polyimide (PI), spun 1 HF(49%): NH
4
F
(40%)
1:5
20
◦
C; BHF etchant (5:1); DI water rinse [28]
40 Polyimide (PI) HNO
3
(70%)
undiluted
60
◦
C; DI water rinse [45]

8 MEMS Wet-Etch Processes and Procedures 575
Table 8.21 (continued)
Material
Etch rate
(Å/s) Etchant Remarks and references
41 Polyimide (PI) 650 O
2
plasma Dry etch; lateral etch rate; doesn’t significantly etch SiC [408]
42 Polyimide (PI,
Kapton
R
)
55–295 Proprietary 40–60
◦
C; Transene Kapton polymide film etchant; mask with PR or
Riston [409]
43 Polymer, residues H
2
SO
4
(96%):
H
2
O
2
(30%)
2:1 to 4:1
90–140
◦
C; general organics; DI water rinse [109]
44 Polymer, residues HF(49%):H
2
O
1:10 to 1:200
25
◦
C; DHF etchant; can undercut organics; DI water rinse [109, 410]
45 Polymer, residues NH
4
OH(29%):
H
2
O
2
(30%):H
2
O
1:1:5
40–75
◦
C; general organics; DI water rinse [109]
46 Polymer, sidewall NH
4
OH(29%):
H
2
O
2
(30%)
5:1
120
◦
C; polymer-dependent; can etch Si; DI water rinse [411]
47 Polymer, sidewall H
2
SO
4
(96%):
H
2
O
2
(30%)
4:1
120
◦
C; polymer-dependent; DI water rinse [411]
48 Polymethylmeth-
acrylate
(PMMA)
Acetone
undiluted
Room temperature; may use ultrasonic agitation; DI water or
isopropyl alcohol rinse [412]
49 Polymethylmeth-
acrylate
(PMMA)
Chloroform
undiluted
Room temperature; nonstandard solvent; solvent bonding of
PMMA–PMMA [413]
50 Polymethylmeth-
acrylate
(PMMA)
DCM
undiluted
Room temperature; nonstandard solvent; DI water or isopropyl
alcohol rinse [414]

576 D.W. Burns
Table 8.21 (continued)
Material
Etch rate
(Å/s) Etchant Remarks and references
51 Polymethylmeth-
acrylate
(PMMA)
NMP
undiluted
Room temperature; nonstandard solvent; consider MEK, THF or
others [415]
52 Polyparaxylylene
(Parylene C)
80 O
2
RIE
120 sccm
Dry etch; 400 W; 200 mTorr [416, 417]
53 Polypropylene (PP) 0.3 CrO
3
:H
2
O
600 g:1000 mL
70
◦
C; chromic acid, 6 M solution; nonstandard chemical; DI water
rinse [418]
54 Polypropylene (PP) DCM
undiluted
70
◦
C; DI water or isopropyl alcohol rinse [419]
55 Polypropylene (PP) HCl(38%):
HNO
3
(70%)
3:1
Warm; aqua regia; DI water rinse [420]
56 Polystyrene (PS),
sheet film
Acetone
undiluted
Room temperature; DI water rinse [394]
57 Polystyrene (PS),
spun
Toluene
undiluted
Room temperature; nonstandard solvent; doesn’t significantly etch
SU-8; DI water rinse [421]
58 Polysulfone (PSF) NMP
undiluted
Room temperature; nonstandard solvent; DI water or isopropyl
alcohol rinse [422]
59 Polytetrafluoro-
ethylene (PTFE,
Teflon), substrate
160 Ar IBE Ion beam etch; Ar
+
ions; 500 eV; 0.5 mA/cm
2
,[423]
60 Polytetrafluoro-
ethylene (PTFE,
Teflon), film
Benzoin dianon:
Potassium tert-
butoxide:Me
2
SO
0.27 g:1.0 g: 35
mL
50
◦
C; nonstandard chemicals; surface modification; strip with KClO
3
in H
2
SO
4
; DI water or isopropyl alcohol rinse [424, 425]

8 MEMS Wet-Etch Processes and Procedures 577
Table 8.21 (continued)
Material
Etch rate
(Å/s) Etchant Remarks and references
61 Polytetrafluoro-
ethylene (PTFE,
Teflon), sheet film
Sodium, naphthalene 50
◦
C; FluoroEtch
R
, Acton Technologies; non-standard chemical;
surface treatment; not for cross-linked fluoropolymers; IPA and DI
water rinse [426, 427]
62 Polytetrafluoro-
ethylene (PTFE,
Teflon), film
THF
undiluted
Warm; nonstandard solvent; surface modification; DI water or
isopropyl alcohol rinse [424]
63 Polyurethane (PU) Acetone Room temperature; causes swelling then weight loss with 65
◦
C
bakeout [428, 429]
64 Polyvinyl chloride
(PVC)
MEK
undiluted
Room temperature; nonstandard solvent; DI water or isopropyl
alcohol rinse [430]
65 Polyvinyl chloride
(PVC)
THF
undiluted
Room temperature; nonstandard solvent; DI water or isopropyl
alcohol rinse [431]
66 Polyvinylidene
fluoride (PVDF)
HNO
3
(70%)
undiluted
Elevated temperature; DI water rinse [432]
67 Polyvinylidene
fluoride (PVDF)
NMP
undiluted
Room temperature; nonstandard solvent; DI water or isopropyl
alcohol rinse [422]
68 Residues, inorganic H
2
O
undiluted
Room temperature; water-soluble inorganics such as salts,
electrolytes, acidic residues, alkaline residues [45]
69 Residues, organic H
2
SO
4
(96%):
H
2
O
2
(30%)
7:3
Elevated temperature; stubborn organics; exothermic; stir and mix
carefully; do not store; consider O
2
plasma; DI water rinse [45]
70 Residues, organic Isopropyl alcohol,
Acetone
undiluted
Room temperature; soluble organics; DI water rinse [45]
71 Silicone, gel Isopropyl alcohol
undiluted
Room temperature; effective for silicone gels; DI water rinse [45]
72 Silicone HNO
3
(70%)
undiluted
Room temperature soak; can heat to boiling for faster removal;
consider commercial strippers; DI water rinse [45]
578 D.W. Burns
Table 8.21 (continued)
Material
Etch rate
(Å/s) Etchant Remarks and references
73 Silicon rubber
(RTV),
encapsulant
530 TMAH (24 wt% in
methanol):IPA
1:10
38
◦
C; IPA rinse [433]
74 SU-8 (PR), spun and
uncured
Acetone
undiluted
Room temperature; DI water rinse [434]
75 SU-8 (PR), spun H
2
SO
4
(96%):
H
2
O
2
(30%)
50:1
60
◦
C; DI water rinse [435]
76 SU-8 (PR), spun Methyl chloride,
phenol, and
organic acids
Room temperature; Miller–Stephenson MS-111 epoxy stripping
agent, nonstandard solvent; DI water rinse [436]
77 SU-8 (PR), spun 165 NMP
undiluted
Hot; nonstandard solvent; DI water rinse [421]
8 MEMS Wet-Etch Processes and Procedures 579
aCF
4
:O
2
(20:80) or SF
6
:O
2
(10:90) plasma, or by using a piranha solution of sul-
furic acid and hydrogen peroxide (4:1) at 120
◦
C followed by a DI-water rinse and
spin dry [376, 437, 438].
8.5.3.2 Example 2: COC Patterning and Solvent Bonding
A homogeneous, trilaminate microfluidic device for DNA sequencing is formed
from three layers of cyclo–olefin copolymer (COC) by hot-embossing (above the
glass-transition temperature) 50 µm-deep features from a 50 µm-thick, negative-
tone dry-resist pattern on a 200 µm-thick PET backing sheet into a middle layer
of 130 µm-thick COC. An upper layer of COC is solvent-bonded to the middle
layer by pretreating the bonding surface of the upper COC film with a 1:12 mix-
ture of hexadecane in isopropyl alcohol (IPA), removing the excess liquid, allowing
the solvent mixture to dry while penetrating and plasticizing the COC surface, then
warm-laminating (below the glass-transition temperature) the two layers t ogether
to form covered channels. After punching features through the two layers to access
the channels, a lower layer of COC is treated with the solvent mixture in a similar
manner and warm-laminated to the previously laminated middle layer to seal off
one side of the access ports. A blade may be used to trim the tri-layer laminate to
size, and fittings may be epoxied over the ports [378].
8.5.3.3 Example 3: LIGA Mold Removal
A 120 µm-thick layer of PMMA is cast and polymerized on a silicon wafer hav-
ing a 500 Å deposited nickel seed layer on top of a 3.0 µm deposited oxide layer.
The PMMA is exposed with an X-ray source using an X-ray mask, developed in a
commercial developer, and plated with nickel from a nickel sulfamate plating bath.
The PMMA mold is stripped in acetone, re-exposed briefly from the X-ray source
without a mask, and redeveloped to remove residual PMMA. The nickel seed layer
is removed in the open areas with a dilute solution of nitric acid and water (1:20) for
1 min. The sacrificial oxide layer is removed under narrower nickel features by etch-
ing in BHF etchant (5:1) for 15 min and the wafer with freestanding nickel actuator
features is rinsed carefully in DI water [414, 439].
8.6 Anisotropic Silicon Etching and Silicon Etch Stops
Anisotropic etching is direction-dependent etching, stemming from crystalline char-
acteristics of the material being etched or from induced anisotropies in the etch
process itself. The effect has been studied extensively and used for a variety of
MEMS devices including pressure sensors, accelerometers, angular rate sensors,
microphones, microfluidic devices, inkjet nozzles, through-wafer vias, and cap
wafers. Crystallographically dependent anisotropic etchants have etch rates that are
dependent on the crystal orientation of the substrate or thin film. Isotropic etchants
are blind to the crystallography and etch in all directions at approximately the same
580 D.W. Burns
rate, resulting in appreciable undercutting of a selective masking layer with a lateral
distance close to the etched depth.
Anisotropic etches, with carefully aligned masking patterns, can result in min-
imal undercutting although the etches generally produce pronounced flanks. A
single-crystal silicon substrate, with its diamond crystal lattice, provides certain
planes that etch quickly and other planes that etch slowly in anisotropic etchants
such as potassium hydroxide (KOH), tetramethyl ammonium hydroxide (TMAH),
ethylene-diamine pyrocatecol (EDP), ammonium hydroxide (NH
4
OH), sodium
hydroxide (NaOH), hydrazine (N
2
H
4
), and others. Surfaces with exposed {100}
planes such as the topside or bottomside of a (100) wafer etch quickly in these
etchants, whereas (111) sidewalls at 54.74
◦
(the sidewalls are closer to the vertical
than 45
◦
) etch appreciably more slowly. For example, a large open-square feature
in a hard mask on the wafer surface will result in a cavity with a square bottom
and tapered sides whereas a small open feature r esult in a pointed, self-stopping
inverted pyramidal cavity. Rectangular patterns produce rectangular cavity bottoms
with tapered sidewalls or a v-groove trench for small geometries. Silicon wafers
with (111) surfaces etch minimally and are not often used for anisotropic etching.
Wafers with (110) and more exotic orientations allow trenches with nearly verti-
cal sidewalls, however, they don’t allow rectangular-bottomed cavities. Geometrical
features such as exterior (concave) corners result in oddly faceted structures that
may require specially configured undercutting features placed on the mask or
etchant additives to achieve the desired etched shape with minimal loss of pattern
fidelity. Deeply etched cavities may show pillowing effects where the anisotropic
etchant etches slightly more quickly near the sides of the cavity than in the middle,
resulting in a somewhat thicker center region.
The primary use of anisotropic etching is to form diaphragms, cavities, or
trenches that may require significant removal of the substrate top or bottom. Etched
structures such as diaphragms fabricated using a timed etch can be difficult to pro-
duce repeatedly, particularly when the final diaphragm thickness is a small fraction
of the total wafer thickness. For example, a small 1% variation in the etch rate can
result in a nearly 10% variation in the feature thickness when the target diaphragm
thickness is 1/10 of the substrate thickness. Automatic etch stops that allow signif-
icant overetching to accommodate etch rate variations are often used to surmount
this problem. Etch stops such as heavily boron-doped silicon layers or a buried oxide
layer in a silicon-on-insulator (SOI) wafer are built in, and are more robust compared
to electrochemical etch stops that require externally connected voltage supplies.
Etch-resistant layers such as patterned oxide or silicon nitride are commonly used
to transfer patterns into the substrate and to protect active devices and peripheral
portions of each die during the etch sequence. Anisotropic wet etchants generally
require elevated etch-bath temperatures with careful control over the etchant con-
centration and a watchful eye for etchant exhaustion. Although anisotropic etching
remains prevalent for many devices, surface micromachining with thin-film sac-
rificial layers or dry etching of the substrate with high-speed deep reactive ion
etchers (D-RIE) are used for many contemporary designs. The following sections
8 MEMS Wet-Etch Processes and Procedures 581
and tables provide more detailed information on etch rates for silicon with vari-
ous anisotropic etchants, important types of passive and active etch stops, and a
few examples.
8.6.1 Anisotropic Etching of Silicon
Anisotropic etching of silicon strongly depends upon wafer orientation and pat-
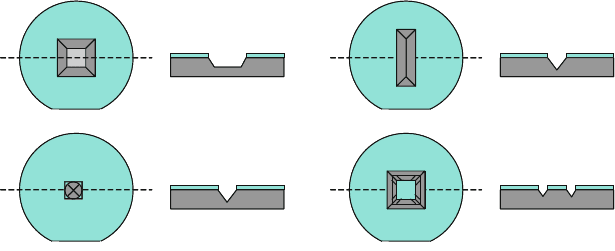
terned features including shape, size, orientation, and corner positions. Figure 8.11
depicts several representative geometries, including a large square, a long rectan-
gle, a small circle, and an annular square to illustrate typical cavity geometries that
result in anisotropic etching of a (100) silicon wafer. Long rectangular features form
trenches with elongated bottoms or self-terminating v-grooves for narrow features
when aligned along a <110> direction. Features such as small squares, circles, and
small mask defects tend to form self-limiting pyramidal pits. Features with exterior
corners such as a square in a square result in undercutting of the internal corners
as higher-order crystalline planes become exposed, each with its own chemistry-
dependent etch rate that can be higher than the (100) etch rate. Area permitting,
enlarged corner-compensation features can be placed on the exterior corners to pro-
duce the desired feature as the etch process reaches completion, although these
approaches place additional sensitivity on the etch rate and etch time. Certain addi-
tives may also slow the etching of the higher-order planes and eliminate the need
for corner compensation features. One may notice that rotational misalignment of
the mask features can result in excessive sidewall undercutting. Manufactured ori-
entation flats and notches can identify the crystal orientation to about 0.5
◦
or so,
b)
c)
a)
d)
Fig. 8.11 Basic patterns in a masking layer on the surface of a (100) silicon wafer, followed
by anisotropic etching into the substrate. (a) A large square pattern results in a smaller square-
bottomed cavity with 54.736
◦
(cos
–1
(1/sqrt(3))) flanks. (b) A long rectangular feature results in a
self-limiting trench or v-groove. (c) A small circular feature or mask defect results in a self-limiting
pyramidal pit. (d) A patterned square within a square results in flanks along the outer walls and
near the centers of the inner walls, whereas the corners of the inside square will preferentially
undercut as higher-order planes become exposed
582 D.W. Burns
however, devices requiring more concise alignment may benefit from initially form-
ing one or more long, narrow v-grooves on each wafer, then aligning to the groove
edges. Devices with deep cavities may have significant projected-area loss due to the
sloped sidewall flanks and the wafers may become quite fragile as they are etched,
prompting special handling protocols and fixtures.
The etch rates of silicon with anisotropic etchants depend primarily upon the
exposed crystal faces, etchant type, etchant temperature, and etchant concentration.
To a lesser extent, the etch rate depends upon the amount of agitation or stirring,
amount of exposed area, etchant exhaustion, substrate doping, and etchant addi-
tives. Approximate etch rates of several standard anisotropic etchants including
KOH, TMAH, NH
4
OH, EDP, and hydrazine are listed in Table 8.22. Ammonium
hydroxide, although much slower than KOH or EDP etchants, is generally a standard
cleanroom chemical and should be considered, particularly for etching shallower
topside features. Sodium hydroxide etchants and others may present contamination
concerns. Wafers etched in KOH, TMAH, or NH
4
OH etchants can be cleaned suf-
ficiently to allow continued processing in standard IC processing equipment. EDP
etchants stop better than KOH on abrupt heavily doped p
++
layers and have an appre-
ciably higher etch selectivity to oxide, although they present some handling and
discarding concerns. Most of the anisotropic silicon etchants will attack standard
aluminum pads and traces, however, TMAH and NH
4
OH etchants can be predoped
with silicon powder or silicic acid to provide an anisotropic etchant with high resis-
tance to standard aluminum metallurgy. Many excellent articles and summaries have
been written on anisotropic etching of silicon, and the reader is encouraged to con-
sult these publications [440–448] and books [78, 449–455] on the subject for more
detailed information.
8.6.2 Heavily Doped Silicon Etch Stops
Silicon with high concentrations of substitutional elemental boron has a substan-
tially slower etch rate in most anisotropic etchants and can be used as an effective
etch stop, as illustrated in Fig. 8.12. Alkaline anisotropic etchants such as CsOH,
KOH, LiOH, NaOH, and RbOH exhibit a strong reduction in the etch rate for high
boron concentrations in silicon exceeding about 2 ×10
19
cm
−3
. Organic anisotropic
etchants such as EDP and TMAH, ammonium-hydroxide etchants, and hydrazine
etchants show similar effects. Boron concentrations in excess of 1 × 10
20
cm
−3
can
cause an etch rate reduction of 100 or more [473].
Highly doped p
++
silicon can be formed in a silicon wafer using solid source dif-
fusion of boron from a boron–nitride wafer at elevated temperatures, from gaseous
deposition and diff usion of boron, from incorporation of high levels of boron dopant
during epitaxial silicon growth, during crystal pulling of silicon from a melt with a
high concentration of boron, or from a high dosage of ion-implanted boron. The
heavily boron-doped silicon is under high tensile stress that may result in the for-
mation of slip planes visible to the eye as plaid-type patterns, although germanium
may be codoped with the boron to provide strain compensation and reduce slippage.

8 MEMS Wet-Etch Processes and Procedures 583
Table 8.22 Anisotropic silicon etchants and etch processes
Material
Etch rate
(Å/s) Etchant Remarks and references
1 Silicon (Si), (100) varies Aqueous alkali
(CsOH, KOH,
LiOH, NaOH,
RbOH)
Less commonly used anisotropic e tchants except for KOH
2 Silicon (Si), (100) 140 Ethylenediamine:
pyrocatechol:H
2
O
680 mL:120 g: 320
mL
110
◦
C; EPW etchant; mask with Ag, Au, Cr, Cu, Si
3
N
4
,SiO
2
,Ta;
Etches Si(110) 83 Å/s; Si(111) 8 Å/s;
Doesn’t significantly etch Ge, SiO
2
(0.05 Å/s); reduced etch rate for
SiGe (>5% Ge); use quartz reflux system; sensitive to oxygen;
reduced etch rate with high p-type doping; DI water rinse [442]
3 Silicon (Si), (100) 225 Ethylenediamine:
pyrocatechol:
pyrazine:H
2
O
750 mL:240 g:
4.5 g:240 mL
115
◦
C; EDP etchant, type F; less sensitive to oxygen; residues at
lower temperatures; DI water rinse [456]
4 Silicon (Si), (100) 40 Ethylenediamine:
pyrocatechol:
pyrazine:H
2
O
880 mL:140 g:
5.3 g:120 mL
75
◦
C; EDP etchant, type S;
Etches Si(110) 53 Å/s, Si(111) 0.6 Å/s, SiO
2
(0.001 Å/s); etches
Si(100) 125 Å/s at 115
◦
C, 90 Å/s at 105
◦
C, 70 Å/s at 95
◦
C, 13 Å/s
at 50
◦
C;
Fewer residues at lower temperatures; low oxygen sensitivity; reduced
etch rate with high p-type doping; stir continuously; DI water rinse
[447, 456]
5 Silicon (Si), (100) 70 Ethylenediamine-
based
100
◦
C; Transene PSE-300 etchant; preferential <100> directions;
negligible <111> plane; mask with SiO
2
; etches Al, Cu;
Doesn’t significantly etch Ag, Au or Ta; use quartz flask with reflux
condenser; initial dip in DHF recommended; DI water rinse [472]
