Ghodssi R., Lin P., MEMS Materials and Processes Handbook
Подождите немного. Документ загружается.

224 E. Meng et al.
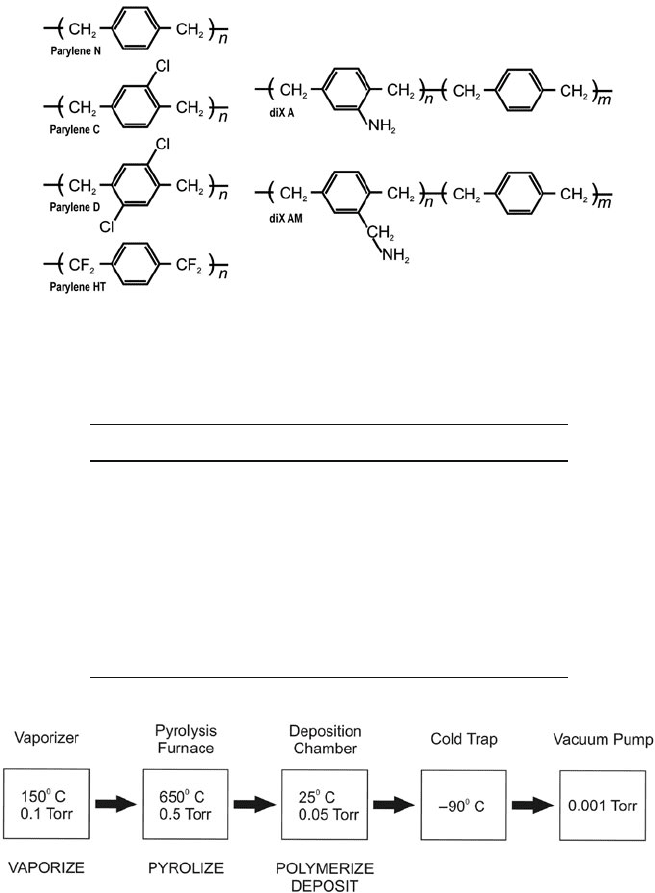
Fig. 4.24 Chemical structure of poly (p-xylylene)s (Original figure from [346], used with
permission of Institute of Physics Publishing Ltd. 2008)
Table 4.3 Comparison of parylene types and their key features
Parylene type Key features
N High crevice penetration, lubricity,
excellent electrical properties
C Low permeability barrier, excellent
combination of electrical and
mechanical properties
D High thermal stability
HT Superior thermal, barrier, and electrical
properties
A and AM Amino group convenient for bonding to
biomolecules
Fig. 4.25 Summary of deposition process (After [350])
4.5.1 Material Properties
Parylene is chemically inert, coats surfaces conformally, and is an excellent poly-
mer barrier [339]. Historically, it has been used as a medical coating, an insulator
4 Additive Processes for Polymeric Materials 225
for implantable neural wire probes [351–354], an interlayer dielectric [355], and
was later introduced to the MEMS community in 1997 as a coating material for
fluidic interconnects [356]. Parylene has gained popularity as a standard MEMS
material for its deposition method (vacuum-based), pinhole-free deposition, low
process temperature, transparency, and compatibility with microfabrication pro-
cesses [342]. Since its introduction to MEMS, Parylene was explored as a structural
material [357] in a wide variety of MEMS applications [123, 204, 205, 329,
358–370]. Parylene in carbonized/pyrolyzed [371–373] and ion-implanted [374]
forms have also found applications in MEMS as a sacrificial and sensing mate-
rial. Further modification of Parylene surfaces has been demonstrated, especially
for biological/biomedical applications [375–377].
4.5.2 Processing Techniques
Parylene is resistant to removal by solvents below its melting temperature. Only
chloronaphthalene or benzoyl benzoate above 150
◦
C are effective [378]. Liftoff
is not possible due to the conformal structure obtained during the deposition
process.
Physical processes are best for removing Parylene. Plasma processes effectively
remove both Parylene N and C; the removal mechanism is discussed in [379, 380].
Plasma etching [335, 346, 358, 377], reactive ion etching [346, 355, 381], reac-
tive ion beam etching [382], high-density plasma etching [383], and Bosch-like
switched chemistry etching [346, 384, 385] have all been demonstrated. Oxide and
metal masks may redeposit during the etch process and result in rough surfaces or
the formation of micrograss [346, 355, 381]. Spin-on glass, nitride, and sputtered
a-Si are also mediocre masking materials [346, 383]. Photoresist masks are pre-
ferred, however, the etch rate is comparable to that of Parylene and thus exhibits
low selectivity [346]. SU-8 masks exhibit higher selectivity although its removal
after etching was not required in the single study to date [386]. Anisotropic sidewall
profiles are possible by using high-density plasma and switched chemistry etching
[346, 383–385].
Other methods to remove Parylene include ultraviolet laser ablation [353, 354]
and manual removal [352]. Release agents (such as 2% Micro
R
lab cleaning solu-
tion, International Product Corp.) applied to surfaces allows Parylene to be peeled
away after deposition. Peeling of Parylene without damage to the film was also
demonstrated on Si surfaces having a native oxide layer; immersion in water can
facilitate the release [204]. Photoresist sacrificial layers are practical for the release
of smaller Parylene structures [361]. As removal can pose fabrication challenges
in some applications, alternative patterning methods were investigated. Selective
deposition of Parylene is achieved by controlling substrate temperature [387].
This technique exploits the phenomenon that deposition thickness is a function
of substrate temperature; heating the substrate (70
◦
C) selectively limits Parylene
deposition to cooler regions [388, 389].
226 E. Meng et al.
Parylene’s thermoplastic nature also enables thermal imprint patterning (Ni
molds at 250
◦
C) [390]. Micromolding combined with thermocompression bonding
has been used to form channels [370, 391]. Other modes of Parylene-to-Parylene
and Parylene-to-substrate bonding were also developed [392, 393].
4.5.3 Lessons Learned
Adhesion to most substrates (such as silicon) requires pretreatment with adhesion
promotion A-174 (gamma-methacryloxypropyltrimethoxysilane) prior to deposi-
tion. However, A-174 is incompatible with common positive photoresists causing
dissolution of photoresist patterns during the adhesion promotion process. It is
suggested that a short oxygen plasma treatment can promote Parylene-to-Parylene
adhesion by roughening the surface [358]. To further improve Parylene-to-Parylene
adhesion, especially in wet biomedical applications, thermal annealing was per-
formed (2 days at 200
◦
C in vacuum) [204]. Robust Parylene-to-substrate adhesion
was promoted by surface roughening with BrF
3
or XeF
2
gas phase etching [358,
394, 395] of Si or by anchoring Parylene to the substrate by deposition into etched
trenches [366, 396–398].
4.5.4 Case Study
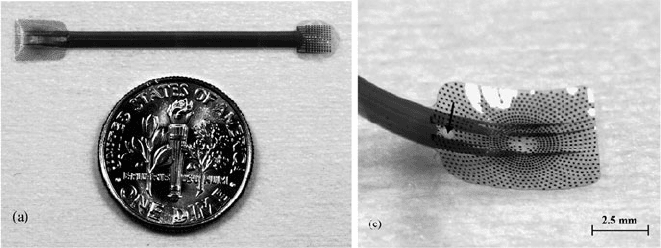
Implantable microelectrode arrays and telemetry coils were fabricated using single-
and dual-layer Parylene/metal processes to realize an intraocular retinal prosthe-
sis (Fig. 4.26)[204, 205, 399]. Parylene was selected over other polymers for its
flexibility, process compatibility, and biocompatibility. Fabricated electrode arrays
and coils, however, cannot tolerate accelerated lifetime testing due to water infil-
tration between the Parylene-to-Parylene interface. Thermal annealing was found to
Fig. 4.26 Parylene multielectrode array with biomimetic arrangement of electrodes. The close-
up shows an array that has been annealed and heat formed to match the curvature of the retina
(Reprinted from [204] with permission from Elsevier, copyright 2006)
4 Additive Processes for Polymeric Materials 227
improve Parylene-to-Parylene adhesion (200
◦
C, 2 days, vacuum with nitrogen back-
fill) and provide an extrapolated mean time to failure exceeding 20 years following
accelerated lifetime testing [399]. As the interface between the Parylene array and
the tissue is curved, thermoforming of the thin-film devices was performed using
aluminum molds to impart an anatomically matched curvature to the array portion.
In electronic neuroprostheses, it is desirable to fabricate electrodes using materials
with high charger delivery capacity. Ir is thus preferred over Pt. However, the high
melting temperature of Ir results in cracking of films deposited in Parylene C. In
this case, Parylene HT, which has greater thermal stability, was used to successfully
fabricate Parylene HT–Ir electrode arrays [204].
4.6 Conductive Polymers
Polymers are usually considered as solely having plastic properties. They are excel-
lent insulators and have been extensively used as coatings to protect electrical wires
from short-circuits. However, Alan J. Heeger, Alan G. MacDiarmid, and Hideki
Shirakawa changed this view with their discovery of a new class of conducting
polymers in 1974 for which they were awarded the Nobel Prize in Chemistry in
2000. Although this class of polymer is in its infancy, their potential applications
have significant implications.
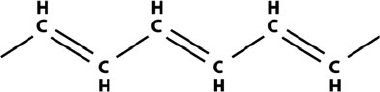
Polyacetylene was the first conducting polymer. The key feature of the polymer
is that it consists of alternating single and double bonds, called conjugated double
bonds along the backbone of the polymer chain [231] (Fig. 4.27). The π-electron in
a double bond has less energy and can be delocalized.
Fig. 4.27 Polyacetylene
structure
However, it is not enough to have conjugated double bonds. To become electri-
cally conducting, the polymer has to be disturbed: either by removing electrons from
(oxidation), or inserting them into (reduction), the material. The process is known
as doping. In the doped state, the polymer can achieve conductivity even compa-
rable to metals, which is an increase of about 13 orders of magnitude compared to
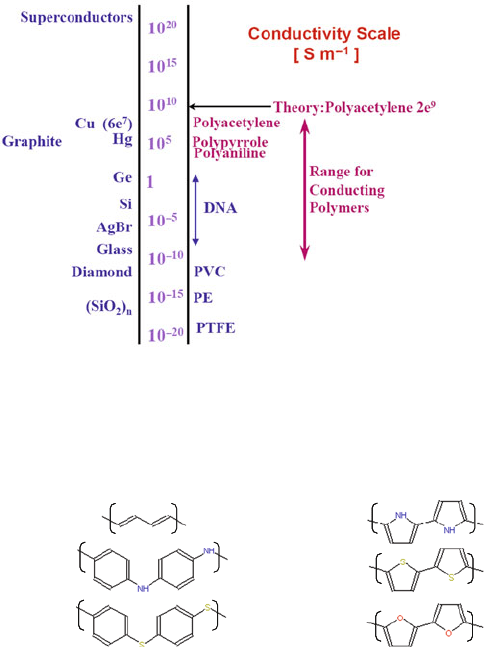
common nonconducting polymers [400] (Fig. 4.28).
Although featuring the highest conductivity among other conducting polymers,
polyacetylene is not stable, which limits its use. In the next three decades following
the development of polyacetylene, a wide range of derivatives were developed, such
as polypyrrole, polythiophene, and polyaniline [402] (Fig. 4.29).
228 E. Meng et al.
Fig. 4.28 Conductivities of conducting polymers relative to other common polymers and inor-
ganic materials. PE is polyethylene, PVC is polyvinylchloride and PTFE is polytetrafluoroethylene,
also known as Teflon
©
. The upper limits in conductivity for the conducting polymers polyaniline,
polypyrrole, and polyacetylene are shown (Reprinted from [401] with permission of, the MIT
Technology Licensing Office)
PolythiophenePolyaniline
Polyfuran
Polyphenylene
sulfide
PolypyrrolePolyacetylene
n
n
n
n
n
n
Fig. 4.29 Types of conducting polymers (Reprinted from [402] with permission from ASME)
4.6.1 Material Properties
Some of the properties of conducting polymers can be controlled by changing the
redox state. By an electrochemical process, electrons can be added to or removed
from the polymer via an electrode, thereby changing the oxidation state. More
important, t his change is reversible through chemical or electrochemical means. For
example, the conductivity can be switched by 13 orders of magnitude, an effect
used in organic transistors. Optical absorption, permeability, hydrophobicity, stored
charge, and volume all change in a controllable manner. This enables devices such
as chemical sensors, filters, capacitors, and batteries [403].
Here, we focus on the actuator applications of conducting polymer, where the
volume change relies on the ion and solvent migrations [404–407]. By applying
a positive potential, electrons are removed from the polymer via an electrode and
the polymer is oxidized. To compensate, oppositely charged ions are incorporated
4 Additive Processes for Polymeric Materials 229
into the polymer from the supporting electrolyte and thus the volume expands. The
polymer can be reversibly reduced to neutral state by applying a more negative
potential and allowing the polymer to shrink to the original volume.
Although electrostatic and piezoelectric materials have major technological
importance for the direct conversion of electrical energy to mechanical energy in
actuators, conducting polymers, as an alternative, could provide a similar function
in a manner analogous to natural muscle. Key features include:
1. Large strain (2∼20%)
2. High stress (100 times greater than muscle)
3. Low actuation voltage (∼1 V or less)
4. Can be positioned continuously between minimum and maximum values
5. Can be kept in a fixed position without consuming additional power; consumes
current only when switching states
6. Can operate in liquid electrolytes, including body fluids
However, conducting polymer-based actuators have a low actuation rate and
low conversion efficiency of electrical energy to mechanical energy. These can be
improved through miniaturization (fabrication of conducting polymer-based devices
on a smaller scale). Miniaturization could lead to improvements in conductivity and
electroactivity [408]. Higher conductivity is important, but reducing resistive loss is
the key requirement. One attractive feature of miniaturization is the possibility of
having highly ordered and less defective material to contribute to high conductivity.
In addition, the efficiency of the electroactive process through a redox cycle can
be improved because the surface area to volume ratio is higher, providing a large
interface between the electrode and electrolyte. This can increase the rate-limiting
ion diffusion process, and reduce the double-layer capacitance and the RC time
constant.
4.6.2 Actuation Mechanism and Theories
During the last few decades, researchers have proposed many models to describe
the actuation mechanism of conducting polymer actuators. The complex nature
of the actuation mechanism involves a large number of electrical, chemical, and
mechanical variables, some of which are interrelated. Madden, Madden, and Hunter
described the actuation of a free-standing polypyrrole film as a simple elastic
mechanical model superimposed with the electrochemically generated strain that
is assumed to be directly proportional to the charge density [409]. Della Santa, De
Rossi, and Mazzoldi investigated the modeling and characterization of a muscle-
like conducting polymer axial/linear actuator operating in an electrolytic cell [410].
Their model utilized simple lumped parameters identified using force and length
change data. Based on the beam-bending model, Pei and Inganaes developed a
mathematical model for a bilayer strip made of a gold-coated polyethylene layer
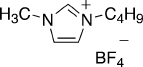
230 E. Meng et al.
and a polypyrrole layer [411]. It was concluded that the actuation consisted of
a fast cation insertion for volume expansion and a subsequent slow salt draining
process for phase relaxation. Alici et al. developed a mathematical model of a strip-
type polypyrrole/PVDF/polypyrrole trilayer actuator operated in air, using a similar
beam-bending method [412]. The static characteristics of the actuator were ana-
lyzed and verified by experiments, such as the relationship among the input voltage,
output deflection, and force.
4.6.3 Applications
4.6.3.1 Actuators
Conducting polymer actuators are commonly fabricated in two forms: linear and
bilayer bending actuators. For linear actuators, Hara et al. built a polypyrrole–zigzag
metal wire composite linear actuator and showed an enhanced electrochemical
strain up to 21.4% [413]. Spinks et al. developed a helix tube polypyrrole lin-
ear actuator for a Braille display screen [414]. The bilayer actuator is a simple
mechanical amplification structure that can convert a small linear strain into a
large bending angle. One of the earliest demonstrations was carried out by Smela
et al. with a polypyrrole/gold bilayer actuator to produce an electrically con-
trolled micro finger [415]. This actuator, however, could only be used i n a liquid
electrolyte.
A further improvement was made by Kaneto et al. to enable the actuator to oper-
ate in air [416]. They used an insulating paper soaked with the electrolyte or a solid
polymer electrolyte sandwiched between the two conducting polymer layers. This
type of actuator has some limitations: either the ionic conductivity in solid polymer
electrolyte is very low at room temperature, or the solvent in the solvent-swollen
gel, typically water, will evaporate in the long run.
A promising approach to dry actuators has become feasible with the synthesis of
new molecules called ionic liquids. These materials are salts that are liquid at room
temperature; they typically consist of nitrogen-containing organic cations, such as
1-butyl-3-methyl imidazolium, and inorganic anions (Fig. 4.30)[417]. Furthermore,
some ionic liquid relatives are plastic solids at room temperature, yet still maintain
a reasonable conductivity, whereas others can be transformed into soft elastomeric
solids at room temperature by the addition of small amounts (∼5%) of a suitable
polymer. These materials have only recently been applied to conducting polymer
actuators, but the results are impressive: they show significantly enhanced lifetimes
Fig. 4.30 Structure of the ionic liquid BF
4
1-butyl-3-methyl imidazolium (Reprinted from [417]
with permission from Wiley-VCH)

4 Additive Processes for Polymeric Materials 231
compared to other electrolytes, as well as fast switching speeds [418]. The com-
bination of gels with ionic liquids is therefore the next logical step in the effort to
develop dry actuators.
4.6.3.2 Conducting Polymer as a Strain Gauge Material
Conducting polymers have also been reported to have a strain-sensitive effect,
where the resistance changes as the gauge is stretched. The resistance change is
given by:
R
R
s
= Gε,
where R
s
is the unstrained resistance of the gauge and is the strain. The factor
G is known as the gauge factor of the sensor. A higher gauge factor improves the
sensitivity of the strain gauge. Pure polypyrrole has been used to make strain gauges,
however, the best conducting polymer strain gauges have been made by coating a
flexible fabric with a l ayer of polypyrrole; the reported gauge factor was around 13
[419].
4.6.4 Processing Techniques
4.6.4.1 Deposition
Depositing thin, uniform polymer films by spin-coating is a widely used tech-
nique in microfabrication. This technique can be applied to photoresist, such as
Shipley 1818. For metal layers, such as Cr, Au, Ti, an E-beam evaporation or ther-
mal evaporation can be used. E-beam evaporation has a small effect on samples
thus making it easier to pattern by etching or a lift-off process; thermal evapora-
tion will raise the sample temperature thus it is not suitable for a lift-off process
due to overheating of the photoresist and difficulty stripping the photoresist after
evaporation.
There are many ways to deposit conducting polymers. Polyaniline can be made
into powder and dissolved or dispersed in an acid solution [420]. Polythiophene can
be dissolved in certain types of organic solvent by special treatment such as alkyl
substitution on carbon rings [421]. Then they can be spin-cast into films onto many
types of substrates. If the polymer is insoluble, such as polypyrrole, the electro-
chemical deposition technique is a common alternative. This process is conducted
in a typical three-electrode electrochemical cell setup, which consists of a working
electrode (where the conducting polymer deposits), a counterelectrode, and a ref-
erence electrode (providing precise potential control of the synthesis). The electric
power can be applied by means of either potentiostatic (constant potential) [405,
422] or galvanostatic (constant current) mode [423].
232 E. Meng et al.
4.6.4.2 Patterning
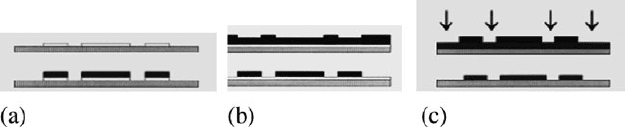
There are several ways to pattern conducting polymers. The main methods are listed
as follows (Fig. 4.31)[424].
1. Patterning the electrode onto which the conducting polymer is deposited.
2. Using photoresist as a mold to template deposition.
3. Depositing the conducting polymer in the whole layer then etching it.
4. Printing the polymer (this only works with soluble conducting polymers).
Fig. 4.31 Patterning methods: (a) patterned electrodes; (b) template deposition; and (c) etching
(Reprinted from [424] with permission from IOP)
Patterning electrodes is the simplest way because conducting polymer will
directly deposit on a substrate with no need of further patterning. The main draw-
back is the nonuniform film thickness; higher electric fields and reactant availability
at the edges of electrodes produce thicker deposits. Also the polymer has finite lat-
eral growth, which may bridge adjacent electrodes or make it harder to release the
structure after deposition.
The second method is template deposition, using photoresist as a mold. The con-
ducting polymer will only deposit in the opening windows, and photoresist can be
subsequently removed with acetone or ethanol. This method can eliminate the lateral
growth in patterned electrodes to benefit the structure release.
Conducting polymers can also be patterned by removal of unwanted material
by reactive ion etching (RIE). First, the polymer is deposited on the entire surface
and then the photoresist is patterned to form an etch mask. Finally, RIE is applied
(usually oxygen plasma). Because oxygen will etch both the photoresist and con-
ducting polymer, the etching process should be carefully monitored. Christophersen
et al. reported that the oxygen plasma etch selectivity (ratio of etch rates) between
polypyrrole and the masking 2 μm thick photoresist was 2.5:1 [425]. However, our
experiments (40 sccm O
2
flow rate, 100 mtorr pressure, 300 W power) demonstrated
an S1818 etch rate of 0.18 μm/min, whereas that of polypyrrole was 0.20 μm/min.
Thus, this method limits the conducting polymer thickness to be less than the
photoresist thickness.
4.6.4.3 Release
Microactuators require a method to partially release them from the substrate. As
shown in Fig. 4.32 [424], a sacrificial layer is commonly used. But for this method,

4 Additive Processes for Polymeric Materials 233
a)
b)
Fig. 4.32 Release methods: (a) sacrificial layer and (b) differential adhesion (Reprinted from
[424] with permission from IOP)
the overhang should not be very large to avoid anchor failure during actuation. Also,
the undercut release of large areas requires long etch times.
The second method uses differential adhesion. It is well known that gold adheres
weakly to bare silicon, silicon dioxide, and glass. Therefore a thin layer of metal
such as chromium is normally deposited first as an adhesion-promoting layer. By
patterning the adhesion layer only at the anchor parts, the structure on the bare
silicon can be released with the stress generated by the activated conducting polymer
layer.
4.6.4.4 Process Considerations
Although there is great flexibility in fabrication techniques, conducting polymers are
nevertheless s ensitive to some chemicals. Therefore care must be taken to devise
an appropriate process. Table 4.4 summarizes some chemical compatibilities and
processes [424].
Table 4.4 Processing hazards
Damaging Use care, test first Harmless
Cr etchant Acids Au etchant
High temperatures Hot plate at 100
◦
C
Developer (KOH),
bases
Solvents Photoresist
Resist
stripper/remover
UV light in mask
aligner
Reprinted from [424] with permission from IOP
4.6.5 Case Study
In this case study, we design two structures with detailed processes based on the
aforementioned fabrication techniques (Fig. 4.33). The conducting polymer we
use is dodecylbenzene sulfonate (DBS
–
)-doped polypyrrole. The synthesis is con-
ducted in 0.1 M pyrrole monomer (Sigma Aldrich) and 0.1 M NaDBS (Sigma
Aldrich) aqueous solution, by applying a constant potential of 0.5 V versus Ag/AgCl
reference electrode.
