Gerya T. Introduction to Numerical Geodynamic Modelling
Подождите немного. Документ загружается.


9.3 Heat generation and consumption 127
in Eq. (9.7b) can be replaced by derivatives and we obtain the Lagrangian heat
conservation equation
ρC
P
DT
Dt
=−
∂q
x
∂x
−
∂q
y
∂y
−
∂q
z
∂z
+ H.
9.3 Heat generation and consumption
There are several types of heat generation/consumption processes that should be
taken into account in the temperature equation
ρC
P
DT
Dt
=−
∂q
i
∂x
i
+ H
r
+ H
s
+ H
a
+ H
L
, (9.8)
where i is a coordinate index, x
i
is a spatial coordinate and H
r
, H
s
, H
a
and H
L
are
the radioactive, shear, adiabatic and latent heat productions (W/m
3
), respectively.
The radioactive heat production (H
r
) is due to the decay of radioactive
elements that are present in rocks. The amount of radioactive heat production
depends strongly on the type of rocks and typical, easy-to-remember values are:
2 × 10
−6
W/m
3
for granites, 2 × 10
−7
W/m
3
for basalts and 2 × 10
−8
W/m
3
for
mantle rocks (Turcotte and Schubert, 2002).
The shear heat production (H
s
) is related to dissipation of the mechanical energy
during irreversible non-elastic (e.g., viscous) deformation and can be calculated
via the deviatoric stresses and strain rates as follows
H
s
= σ
ij
˙ε
ij
(9.9a)
where i and j are coordinate indices (x, y, z) and the repeated ij indices denotes
summation. In the case of 3D viscous deformation of an incompressible fluid Eq.
(9.9a) becomes
H
s
= σ
xx
˙ε
xx
+ σ
yy
˙ε
yy
+ σ
zz
˙ε
zz
+ 2(σ
xy
˙ε
xy
+ σ
xz
˙ε
xz
+ σ
yz
˙ε
yz
). (9.9b)
The adiabatic heat production/consumption (adiabatic heating/cooling) is related
to changes in pressure and can be calculated via pressure changes as follows
H
a
= Tα
DP
Dt
, (9.10)
where
DP
Dt
is the substantive time derivative of pressure. In contrast to shear
and radioactive heating, adiabatic effects can be either positive or negative. It is
known from thermodynamics that the temperature of a substance under conditions
of no thermal exchange increases with increasing pressure and decreases with
decreasing pressure, which thus directly reflects the sign of
DP
Dt
. The effects of
128 The heat conservation equation
adiabatic heating can be very significant in cases of strong changes in pressure, a
fact which has implications for mantle convection.
The latent heat production/consumption (H
L
) is due to the phase transformations
in rocks subjected to changes in pressure and temperature. A very common type of
latent heat is the latent heat of melting, which is negative (heat sink, H
L
< 0) for
melting and positive (heat production, H
L
> 0) for crystallisation.
9.4 Simplified temperature equations
In a complete form, the temperature equation looks quite complicated, but at least
it does not ‘hide’ three equations in one in contrast to the momentum equation.
In the case of constant thermal conductivity k =const, the temperature equation
simplifies to
ρC
P
DT
Dt
= k
∂
2
T
∂x
2
+ k
∂
2
T
∂y
2
+ k
∂
2
T
∂z
2
+ H
r
+ H
s
+ H
a
+ H
L
(9.11a)
or,
ρC
P
DT
Dt
= kT + H
r
+ H
s
+ H
a
+ H
L
. (9.11b)
When the internal heat production is negligible and there is no advection of
material (purely conductive heat transport), the temperature equation takes a form
which is similar to the Poisson equation
∂T
∂t
= κT, (9.12)
where κ =
k
ρC
P
is thermal diffusivity (m
2
/sec).
If temperature does not change with time, heat conservation is described by a
steady-state temperature equation. The steady-state, Eulerian temperature equation
∂T
∂t
= 0, corresponds to the case when temperature remains constant at immobile,
Eulerian observation points, while the temperature at Lagrangian points can change.
In this case, the temperature equation is as follows,
ρC
P
(
¯v ·grad(T )
)
=−
∂q
i
∂x
i
+ H
r
+ H
s
+ H
a
+ H
L
, (9.13)
where i is a coordinate index and x
i
is a spatial coordinate. This form of the
equation is frequently used for computing equilibrium temperature profiles across
9.5 Heat diffusion timescales 129
a deforming medium, for example in the case of steady magma flow in a channel.
The steady-state Lagrangian temperature equation
DT
Dt
= 0 corresponds to the
case when the temperature does not change at Lagrangian points but can vary at
Eulerian observation points according to the purely advective heat transport,
∂T
∂t
+ ¯v · grad(T ) = 0. (9.14)
In this case, the temperature equation is as follows
−
∂q
i
∂x
i
+ H
r
+ H
s
+ H
a
+ H
L
= 0. (9.15)
The steady-state Eulerian–Lagrangian temperature equation (
∂T
∂t
= 0and
DT
Dt
=
0) holds for the case when no displacement of the medium occurs, pressure and
temperature are constant and therefore H
s
= 0, H
a
= 0andH
L
= 0. This equation
has the simple form
−
∂q
i
∂x
i
+ H
r
= 0, (9.16)
and is often used for the calculation of steady-state geotherms that characterise
changes of temperature with depth in a layered sequence of rocks with variable
radioactive heat production.
Simplified steady-state temperature equations are often used for obtaining
analytical solutions which are used for testing accuracy of numerical codes
(Chapter 16). Indeed some analytical solutions also exist for more complicated
non steady-state (transient) cases (e.g. Tikhonov and Samarsky, 1972; Shukla,
2005) which will be further discussed in Chapter 16.
9.5 Heat diffusion timescales
One important aspect that can be analysed analytically concerns the timescales of
heat diffusion processes. Heat generated within any region is spread by conduction
(i.e. diffused) on a characteristic timescale (t
diff
) that depends on the width L,of
the region according to
t
diff
=
L
2
κ
. (9.17)
Therefore, the duration of heat dissipation via conduction grows as the square of
the width (L) of the region. For instance, although the shear heat produced within a
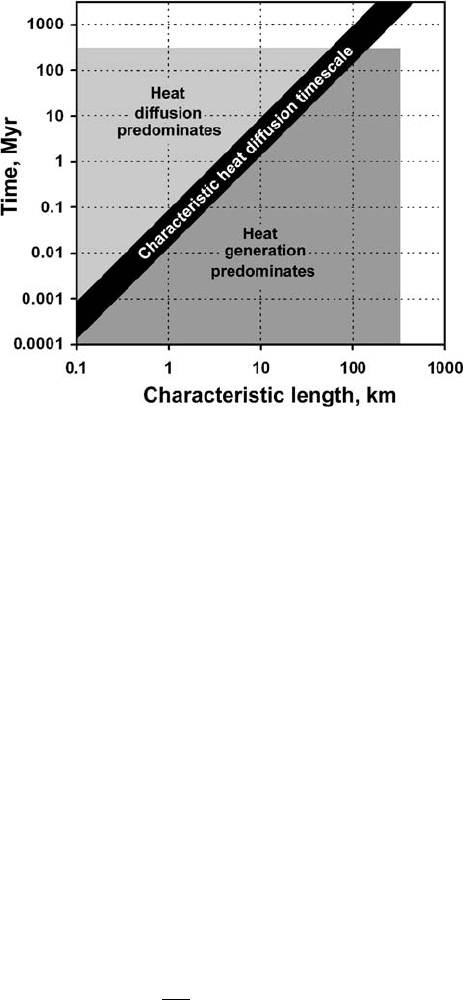
130 The heat conservation equation
Fig. 9.2 Timescales for different thermal regimes calculated according to the equa-
tion t
diff
= L
2
/κ with κ =10
−6
m
2
/s. Shaded areas show length and timescales
characteristic of collisional orogens (Burg and Gerya, 2005).
100 metre wide shear zone dissipates in only ∼1000 yr, the heat generated within a
1 km wide shear zone requires about 100 000 yr for the similar degree of conductive
cooling (Fig. 9.2).
Analytical exercises
Exercise 9.1
Integrate Equation (9.16) in order to calculate the steady-state temperature profile
across the continental crust with radioactive heat production H
r
=1 × 10
−6
W/m
3
,
if the temperature at the surface is 300 K and temperature at the bottom of the crust
is 700 K. Take the thermal conductivity of the crust to be k = 2W/(mK).
Exercise 9.2
Compute from Eq. (9.13) the steady-state Eulerian temperature profile across the
magmatic channel described in Exercise 5.1 (Fig. 5.2). Assume the temperature
at the channel walls, as well as the temperature gradient along the channel to be
constant. Use T
0
= 1300 K and
∂T
∂y
= 1 K/m for the analysed horizontal section
across the channel. Take the thermal conductivity to be k = 2 W/(m K) and the
isobaric heat capacity as C
P
= 1000 J/(kg K).
Programming exercises and homework 131
x
j
j+1
(a)
(b)
j
v
x(i, j)
σ
xy(i,j)
v
y(i, j)
η
s(i, j)
η
n(i, j)
i+1
i
i
ε
xy(i,j)
•
ε
xx(i,j)
•
σ
xx(i,j)
′
y
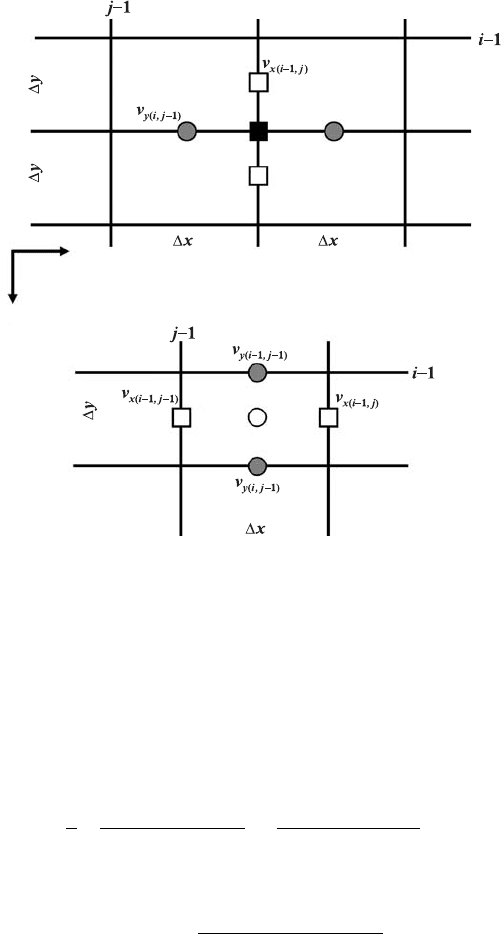
Fig. 9.3 Stencils used for the discretisation of the shear (a) and normal (b) strain
rates and the deviatoric stress components. Indexing of gridlines corresponds to
the basic (density) nodal points. Indexing of different unknowns is made according
to Fig. 7.15.
Programming exercises and homework
Exercise 9.3
Use the variable viscosity model (Exercise 7.2) to compute the strain rate compo-
nents and deviatoric stress components as follows (Fig. 9.3)
˙ε
xy(i,j)
=
1
2
v
x(i,j)
− v
x(i−1,j )
y
+
v
y(i,j)
− v
y(i,j−1)
x
, (9.18)
σ
xy(i,j)
= 2η
s(i,j)
˙ε
xy(i,j)
, (9.19)
˙ε
xx(i,j)
=
v
x(i−1,j )
− v
x(i−1,j −1)
x
, (9.20)
σ
xx(i,j)
= 2η
n(i,j)
˙ε
xx(i,j)
. (9.21)
Compute the σ
xx
˙ε
xx
term for the internal basic nodes (see the solid rectangle
in Fig. 9.3(a)) by averaging its values computed at the four surrounding pressure

132 The heat conservation equation
nodes (see open circle in Fig. 9.3(b)). Compute and visualise (pcolor) the shear
heating distribution (log values) for all internal basic nodes by using the following
equation based on Eq. (9.9)
H
s
= 2σ
xx
˙ε
xx
+ 2σ
xy
˙ε
xy
. (9.22)
Eq. 9.22 is valid for an incompressible medium in 2D (˙ε
xx
= ˙ε
xx
=−˙ε
yy
=
−˙ε
yy
, σ
xx
=−σ
yy
).
An example is in Shear_heating.m.
Exercise 9.4
Compute the adiabatic heating for the same buoyancy driven flow using the fol-
lowing approximate formula derived from Eq. (9.10) under the assumption that
DP
Dt
≈
∂P
∂y
v
y
≈ ρg
y
v
y
,
H
a
= Tαv
y
ρg
y
. (9.23)
Use T = 1300 K and α = 3 × 10
−5
1/K and compute the adiabatic heating for the
internal basic nodes (see solid rectangle in Fig. 9.3(a)). Interpolate the v
y
velocity
component for these nodes from the two nearest staggered v
y
-nodes (see grey
circles in Fig. 9.3(a)). Compare the magnitudes of the shear and adiabatic heating.
Sum up the shear and adiabatic heating terms and visualise the results.
An example is in Shear_adiabatic_heating.m.

10
Numerical solution of the heat
conservation equation
Theory: Discretisation of the heat conservation equation with finite
differences. Conservative and non-conservative discretisation schemes.
Explicit and implicit solution schemes of the heat conservation equation.
Advective terms: upwind differences, numerical diffusion. Advection
of temperature with markers. Subgrid diffusion. Thermal boundary con-
ditions: constant temperature, constant heat flux, combined boundary
conditions. Numerical implementation of thermal boundary conditions.
Exercises: Programming various thermal boundary conditions. Solving
the heat conservation equation in the case of constant and variable ther-
mal conductivity with explicit and implicit solution schemes. Advecting
temperature with Eulerian schemes and markers.
10.1 Explicit and implicit formulation of the temperature equation
We now start with the numerical formulation and solution of the temperature
equation. Discretisation of this equation with finite differences can be done in
an explicit and an implicit manner. In order to understand the differences, let us
consider an example of heat diffusion in a non-deforming medium with constant
thermal conductivity (k)
∂T
∂t
=
k
ρC
P
T . (10.1)
In 2D, this discretisation is as follows.
Explicit FD (Fig. 10.1):
T
n
3
− T
o
3
t
=
k
ρC
P
T
o
1
− 2T
o
3
+ T
o
5
x
2
+
T
o
2
− 2T
o
3
+ T
o
4
y
2
. (10.2)
133

134 Numerical solution of the heat conservation equation
X
y
T
1
ο
T
3
ο
T
5
ο
T
2
ο
T
3
n
T
4
ο
Fig. 10.1 Stencil of the 2D grid used for an explicit discretisation of the temperature
equation with constant thermal conductivity.
This form is called explicit because the new temperature (T
n
) at the next time instant
t
n
can be explicitly calculated from the known temperatures (T
o
) at the current time
instant t
o
(t
n
= t
o
+ t,wheret is time step):
T
n
3
=
kt
ρC
P
T
o
1
− 2T
o
3
+ T
o
5
x
2
+
T
o
2
− 2T
o
3
+ T
o
4
y
2
+ T
o
3
. (10.3)
The explicit formulation does not require composing and solving of a global system
of equations and is therefore very convenient to program. However, this formulation
has a strong limitation on the time step that can be used in the calculations. The
time step must satisfy,
t <
x
2
3κ
, (10.4)
where κ =
k
ρC
P
is thermal diffusivity and x is the minimum grid spacing. This
limitation means that the number of time steps increases as the square of the
decrease in the grid spacing, which can be quite inconvenient for high-resolution
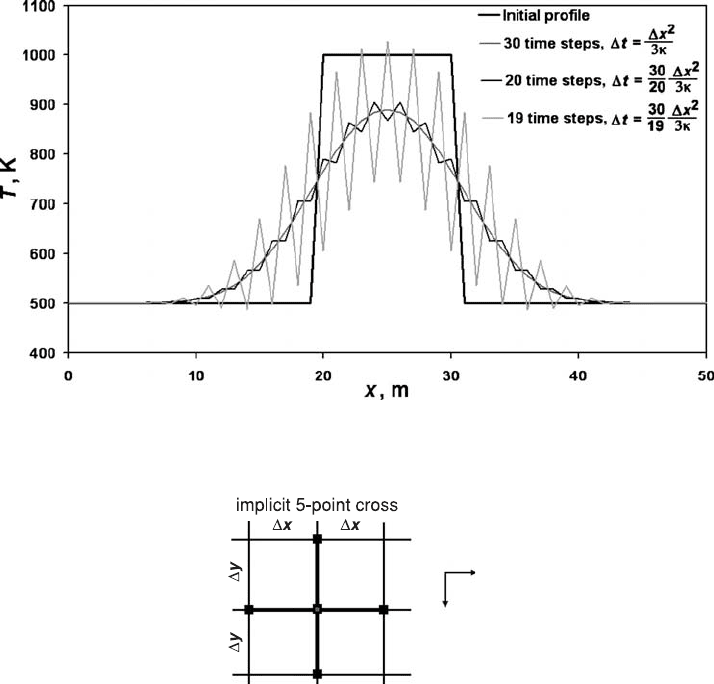
thermal models. If larger time steps are indeed employed, numerical oscillations
occur that increase with the number of time steps (Fig. 10.2, see also program
example Explicit_implicit_1D.m).
The implicit finite-difference discretisation is given by (Fig. 10.3):
T
n
3
− T
o
3
t
=
k
ρC
P
T
n
1
− 2T
n
3
+ T
n
5
x
2
+
T
n
2
− 2T
n
3
+ T
n
4
y
2
. (10.5)
This form is called implicit because the new temperature (T
n
) for the next time
instant t
n
cannot be explicitly calculated from the temperatures (T
o
) known from
the current time instant. In order to obtain new temperatures, the global system of
equations written for all points of the model has to be solved:
T
n
3
t
−
k
ρC
P
T
n
1
− 2T
n
3
+ T
n
5
x
2
+
T
n
2
− 2T
n
3
+ T
n
4
y
2
=
T
o
3
t
. (10.6)
10.2 Conservative finite differences 135
Fig. 10.2 Oscillations of explicit numerical solution of Equation (10.1) due to the
use of a too large time step. An example is in Explicit_implicit_1D.m.
X
y
T
1
n
T
3
n
T
5
n
T
2
n
T
3
ο
T
4
n
Fig. 10.3 Stencil of the 2D grid used for implicit discretisation of the temperature
equation with constant thermal conductivity.
It is important to mention that the implicit formulation places no limitation on the
size of the time step (in the absence of internal heat sources and advective terms).
Indeed, very large implicit time steps do not necessarily guarantee an accurate
solution (since the time derivative in Eq. (10.5) is only first-order accurate in time.
10.2 Conservative finite differences
Conservative finite-difference discretisation should be used in cases when the
heat conservation equation contains a variable thermal conductivity. Such finite
differences ensure conservation of heat fluxes between nodal points, so allowing a
correct numerical solution. In a general sense, this is analogous to the formulation of
conservative finite differences for the Stokes equation with variable viscosity, which
136 Numerical solution of the heat conservation equation
x
1A2B3 C 4
k
1
T
1
k
A
q
xA
q
xB
q
xC
T
A
∆x
1
∆x
2
∆x
3
k
2
T
2
k
B
T
B
k
3
T
3
k
C
T
C
k
4
T
4
Fig. 10.4 1D staggered grid used for the discretisation of the temperature equation
with variable thermal conductivity. 1, 2, 3, 4 are basic nodes (squares) of the grid
where the temperature equations are formulated. A, B, C are additional nodes
(circles) of the grid where the heat fluxes are defined.
was described in Chapter 7. Below, examples of non-conservative and conservative
finite differences are compared for the 1D heat conservation equation (Fig. 10.4)
ρC
P
DT
Dt
=−
∂q
x
∂x
,
where q
x
=−k
∂T
∂x
.
An erroneous non-conservative FD formulation of the heat flux terms, in either
the explicit or the implicit formulation, for the two basic nodes 2 and 3 can for
example be obtained (we can ‘arrive’ at this equation by assuming erroneously
that all we need to do is to use Eq. 10.5 and put different thermal conductivity for
different nodes):
node 2:
∂q
∂x
2
=−k
2
(
T
3
− T
2
)
/x
2
−
(
T
2
− T
1
)
/x
1
(x
1
+ x
2
)/2
(10.7a)
node 3:
∂q
∂x
3
=−k
3
(
T
4
− T
3
)
/x
3
−
(
T
3
− T
2
)
/x
2
(x
2
+ x
3
)/2
, (10.7b)
which implicitly means that the formulations of the horizontal heat flux q
xB
for
temperature equations at nodes 2 and 3 are different due to the different thermal
conductivities k
2
and k
3
:
node 2: q
xB
=−k
2
T
3
− T
2
x
2
node 3: q
xB
=−k
3
T
3
− T
2
x
2
.
This implies that heat flux is not conserved and artificially ‘jumps’ between basic
nodes in response to the difference in the thermal conductivity at these nodes.
On the other hand, a proper conservative FD formulation of the heat flux term
in either the explicit or implicit temperature equations for the two basic nodes 2
