Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c027” — 2007/4/9 — 15:53 — page 24 — #24
27-24 Tissue Engineering
[136] Rohr S. and Salzberg B. Characterization of impulse propagation at the microscopic level across
geometrically defined expansions of excitable tissue: multiple site optical recording of transmem-
brane voltage (MSORTV) in patterned growth heart cell cultures. J. Gen. Physiol. 1994; 104:
287–309.
[137] Kucera J., Kleber A., and Rohr S. Slow conduction in cardiac tissue, II. Effects of branching tissue
geometry. Circ. Res. 1998; 83: 795–805.
[138] de Bakker J.M., van Capelle F.J., Janse M.J., Tasseron S., Vermeulen J.T., de Jonge N., and Lahpor J.R.
Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation 1993; 88:
915–926.
[139] Folch A. and Toner M. Microengineering of cellular interactions. Annu. Rev. Biomed. Eng. 2000;
2: 227–256.
[140] Khademhosseini A., Suh K.Y., Yang J.M., Eng G., Yeh J., Levenberg S., and Langer R. Layer-by-layer
deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials 2004;
25: 3583–3592.
[141] Fast V.G. and Ideker R.E. Simultaneous optical mapping of transmembrane potential and
intracellular calcium in myocyte cultures. J. Cardiovasc. Electrophysiol. 2000; 11: 547–556.
[142] Sperelakis N. and Haddad G. Developmental changes in membrane electrical properties of the
heart. In Sperelakis N., Ed. Physiology and the Pathophysiology of the Heart.3rded.Norwell,MA:
Kluwer Academic Publishers, 1995, pp. 669–700.
[143] Athias P., Frelin C., Groz B., Dumas J., Klepping J., and Padieu P. Myocardial electophysiology:
intracellular studies on heart cell cultures from newborn rats. Path. Biol. 1979; 27: 13–19.
[144] Joyner R. Interactions between spontaneously pacing and quiescent but excitable heart cells. Can.
J. Cardiol. 1997; 13: 1085–1092.
[145] Wagner M., Golod D., Wilders R., Verheijck E., Joyner R., Kumar R., Jonsma H., Van Ginneken A.,
and Goolsby W. Modulation of propagation from an ectopic focus by electrical load and by
extracellular potassium. Am. J. Physiol. (Heart Circ. Physiol.) 1997; 272: H1759–H1769.
[146] Antonutto G. and di Prampero P.E. Cardiovascular deconditioning in microgravity: some possible
countermeasures. Eur. J. Appl. Physiol. 2003; 90: 283–291.
[147] Decker M.L., Simpson D.G., Behnke M., Cook M.G., and Decker R.S. Morphological analysis of
contracting and quiescent adult rabbit cardiac myocytes in long-term culture. Anat. Rec. 1990;
227: 285–299.
[148] Clark W.A., Decker M.L., Behnke-Barclay M., Janes D.M., and Decker R.S. Cell contact as an
independent factor modulating cardiac myocyte hypertrophy and survival in long-term primary
culture. J. Mol. Cell. Cardiol. 1998; 30: 139–155.
[149] Simpson D.G., Decker M.L., Clark W.A., and Decker R.S. Contractile activity and cell–cell contact
regulate myofibrillar organization in cultured cardiac myocytes. J. Cell Biol. 1993; 123: 323–336.
[150] Clark W.A., Rudnick S.J., LaPres J.J., Andersen L.C., and LaPointe M.C. Regulation of hypertrophy
and atrophy in cultured adult heart cells. Circ. Res. 1993; 73: 1163–1176.
[151] Ferrick K.J., Maher M., Roth J.A., Kim S.G., and Fisher J.D. Reproducibility of electrophysiological
testing during antiarrhythmic therapy for ventricular arrhythmias unrelated to coronary artery
disease. Pacing Clin. Electrophysiol. 1995; 18: 1395–1400.

mikos: “9026_c028” — 2007/4/9 — 15:53 — page1—#1
28
Tissue Engineering of
Heart Valves
K. Jane Grande-Allen
Rice University
28.1 The Native Heart Valve as a Design Goal............... 28-2
Anatomy and Terminology • Microstructure and Material
Behavior • Valvular Cells
28.2 Approaches to the Tissue Engineered Heart Valve .... 28-6
Biodegradable Polymeric Scaffolds • Decellularized Leaflet
Scaffolds • Cell Seeding • Natural Materials • Building
Block Approach • Cell Origin • Bioreactors for
Conditioning and Proof of Concept • In Vivo Testing in
Animal Models • Clinical Experience
28.3 Conclusions and Future Challenges .................... 28-13
Defining Terms .................................................. 28-14
References ....................................................... 28-15
Heart valves are essential to the normal function of the heart and cardiovascular/cardiopulmonary systems.
When functioning properly, the heart valves allow unrestricted, unidirectional blood flow through the
heart for subsequent distribution throughout the body. Consequently, valve disease or dysfunction can
result in significant harm, as the reduction in the forward flow of blood limits the oxygenation of the tissues
and can induce cardiac, cardiovascular, or cardiopulmonary compensation. Valve disease is prevalent in
our society, with valve replacement or repair in approximately 90,000 people in the United States in 2001
[1] (275,000 worldwide [2]). Moreover, valve disease can be either congenital or acquired. For example,
approximately 9 to 14 of every 10,000 children born are affected with the Tetralogy of Fallot [3,4],
a congenital heart disorder characterized by a narrowing of the pulmonary valve among other anomalies.
Acquired valve disease can affect people of all ages and may be due to an infectious agent (rheumatic
heart disease, endocarditis), systemic diseases (lupus, carcinoid syndrome), other cardiac disease, trauma,
pharmacologic agents, aging-related changes, or many other causes, some of which remain unknown [5].
The majority of current treatments for heart valve disease involve elective surgical replacement of
the valve with a mechanical, bioprosthetic, or cryopreserved allograft (homograft) valve. The allograft
is the treatment of choice for children, because bioprosthetic valves will calcify rapidly in children and
mechanical valves cannot growwith the child [6]. Aortic and pulmonary allografts havealso been used very
successfully in adults, with the pulmonary conduit having a 90% freedom from replacement at 20 years
[7], but the vascular remnant of these allografts eventually calcifies. Unfortunately, allografts, much like
other donated organs, are in scarce supply. Moreover, allografts needs to be matched to the recipient tissue
type to prevent immunological rejection [7], which narrows the diminishing pool of donated organs
28-1

mikos: “9026_c028” — 2007/4/9 — 15:53 — page2—#2
28-2 Tissue Engineering
even further. Alternative options such as mechanical or bioprosthetic heart valves can be used in many
situations, and are widely available, but have their own limitations [5]. Mechanical heart valves require
anticoagulation therapy, which some patients cannot tolerate. Bioprosthetic valves do not require any
anticoagulation, but do not contain any living tissues, and they undergo stiffening, calcification, and
structural deterioration in vivo as a result of their glutaraldehyde fixation during manufacturing [8].
Bioprosthetic valves demonstrate a freedom from structural deterioration of 49% at 10 years and only
32% at 15 years, [9] and eventually require another surgical replacement. Overall, there is great need
for a living, unfixed tissue-engineered heart valve (TEHV) or valved conduit in adults who require valve
replacements. A TEHV with the potential for growth would also provide pediatric patients with a superior
alternative for the treatment of valve defects.
28.1 The Native Heart Valve as a Design Goal
28.1.1 Anatomy and Terminology
The aortic valve is one of four valves in the heart, but it is replaced most frequently, and therefore will be
discussed in greater detail. The aortic valve consists of three pieces of connective tissue (the right, left, and
noncoronary leaflets) that are attached to the aorta at one edge, and are free to move at the other edge.
These free edges meet centrally to close the valve and keep the blood from reentering the left ventricle. The
valve is located in the bulbous base of the aorta, which is known as the aortic root (an anatomic recreation
for a TEHV is shown in Figure 28.1). There are several distinct anatomic regions of the valve leaflet itself
(Figure 28.2). The leaflet attachment edge inserts into the aortic root wall at the crown-shaped annulus
[10]. The common region where two leaflets insert into the root wall is their commissure. The leaflet
belly, or body, is the main portion of the leaflet (0.4 mm thick in humans [11]), and bears the majority
of the pressure load when the valve is closed [12]. The coaptation area is the 0.5 to 0.6 mm thick [11]
region of the leaflet that is in contact with the two other leaflets when the valve is closed. The free margin
is the unattached edge of the leaflet, and suspends the leaflet between the tops of the commissures, much
like cables of a suspension bridge [10,13]. Finally, the central portion of the edge of the valve leaflet is the
Nodule of Arantius, a thickened area (0.95 to 1.2 mm [11]) that helps maintain valve closure [14].
The pulmonary valve (the other “semilunar” valve) is located in the pulmonary root between the right
ventricle and pulmonary artery, and is thinner and more delicate than but otherwise almost identical to the
aortic valve. The semilunar valves are quite different structurally from the “atrioventricular” valves. The
mitral valve consists of two differently shaped leaflets attached at their outer border to the junction between
the left atrium and left ventricle. The free edges and ventricular surfaces of the leaflets are connected to
the papillary muscles of the left ventricle by numerous chordae tendineae. Likewise, the tricuspid valve is
located between the right atrium and right ventricle. The tricuspid valve also contains chordae, but has
three differently shaped leaflets as opposed to two. The tricuspid leaflets and chordae are thinner, shorter,
and more delicate than those in the mitral valve.
28.1.2 Microstructure and Material Behavior
The semilunar valve leaflets consist of three histologically defined layers: the ventricularis forms the lower
surface, the fibrosa forms the upper surface, and the spongiosa layer lies in between [15] (Figure 28.3 and
Figure 28.4). The ventricularis contains a meshwork of elastic fibers along with loosely scattered collagen
fibers [15]. The predominant elastic makeup allows this layer to expand in response to tension in the
closed state of the valve, and then retract when the valve opens in response to ventricular ejection [15].
The fibrosa contains collagen fibers (predominantly type I), which are aligned largely circumferentially,
although radially aligned fibers are found near the root-valve annulus [15]. The collagen fiber bundles
in the fibrosa serve as the main source of strength for the diastolic pressure. The ridged appearance
of the fibrosa is attributed to a corrugation of that tissue layer, in addition to the collagen bundles
[16]. The spongiosa is a gelatinous layer containing loose connective tissue that is rich in proteoglycans
mikos: “9026_c028” — 2007/4/9 — 15:53 — page3—#3
Tissue Engineering of Heart Valves 28-3
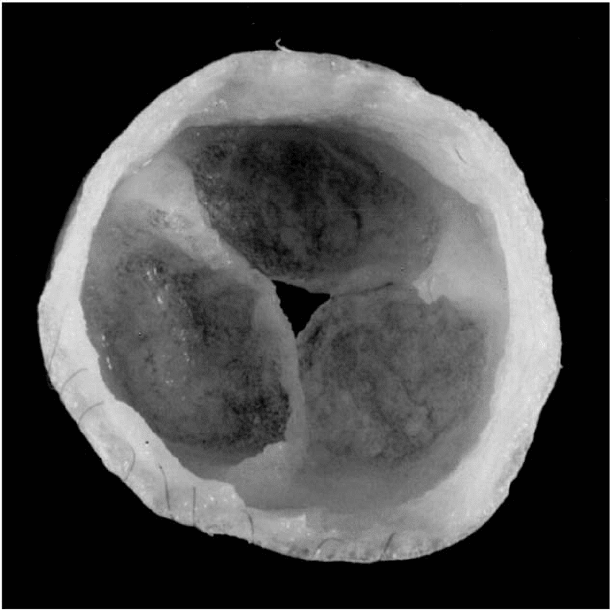
FIGURE 28.1 PGA/P4HB scaffold after cell seeding and 2 weeks of bioreactor conditioning. (Reprinted from
Hoerstrup, S.P., Sodian, R., Daebritz, S., Wang, J., Bacha, E.A., Martin, D.P., Moran, A.M., Guleserian, K.J.,
Sperling, J. S., Kaushal, S., Vacanti, J.P., Schoen, F.J., Mayer, J.E., Circulation, 102, III-46, Copyright 2000, with
permission from Lippincott Williams & Wilkins.)
(PGs), and serves as a mechanism for compressive resistance [17] and shear between the fibrosa and
ventricularis [16].
The mitral and tricuspid valves have a similar laminated structure, except the respective outer layers are
“upside down” from the arrangement shown in Figure 28.3. In these valves, the thick, heavily collagenous
layer is located on the ventricular side, whereas the thin, predominantly elastic layer is found on the atrial
side; these layers are also separated by a spongiosa. These similarities, which may be beneficial in future
tissue engineering of atrioventricular valves, end with the chordae tendineae, which are not found in
semilunar valves. The chordae are strong, thin, cable-like structures that contain a core of highly aligned
collagen inside a thin outer sheath of elastic fibers and endothelial cells.
The interaction between the extracellular matrix (ECM) constituents within the valve microstructure
allows distensibility, strength, elastic recovery, viscoelasticity, and an even distribution of deformation over
a wide range of loading [18]. Like many other biological soft tissues, the stress–strain and load elongation
curves of heart valve tissues are characterized by a low pretransition elastic modulus at initial strain (due to
elastic fibers), followed by a transition zone to a higher posttransition elastic modulus at higher strains (due
to the uncrimped collagen) [18]. The unique collagen and elastic fiber arrangements in the different layers,
however, bestow the leaflet with anisotropic behavior (Figure 28.5). The greater circumferential stiffness
(due to collagen) contributes to normal aortic valve function by restricting downward leaflet motion,
while the lower radial stiffness permits the inward motion toward leaflet coaptation. This properly closing
aortic valve will allow blood flow from the left ventricle into the ascending aorta, and prevent reverse
flow. During this functional cycle, the leaflets interact with complex patterns of blood flow [5], and are
mikos: “9026_c028” — 2007/4/9 — 15:53 — page4—#4
28-4 Tissue Engineering
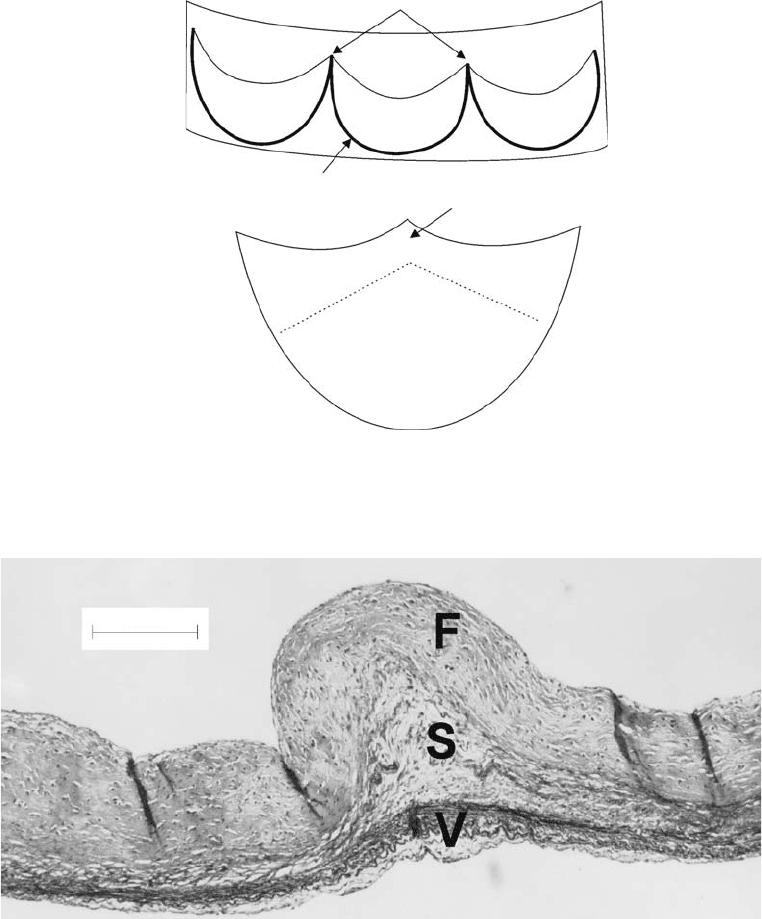
Commissures (2 of 3)
Annulus
Free margin
Coaptation area
Belly
Attachment edge
Nodule of arantius
(a)
(b)
FIGURE 28.2 Semilunar valve leaflet anatomy. (a) Illustration of aortic valve leaflets within an opened aortic root.
(b) Single valve leaflet.
200 mm
FIGURE 28.3 The histological layers of the aortic valve leaflet. Movat’s Pentachrome stain. F, fibrosa; S, spongiosa;
V, ventricularis.
subjected to transvalvular pressures as high as 120 mmHg [19] and shear stresses as high as 7.9 Pa [20].
These magnitudes of load are lower in the pulmonary circulation, where the transvalvular pressure across
the pulmonary valve is only 25 mmHg [19].
28.1.3 Valvular Cells
Heart valves contain both endothelial and interstitial cells [21–23]. The valvular endothelial cells (VECs)
populate the outer surfaces of the valves, whereas “interstitial cells” are all the cells that populate the inside
mikos: “9026_c028” — 2007/4/9 — 15:53 — page5—#5
Tissue Engineering of Heart Valves 28-5
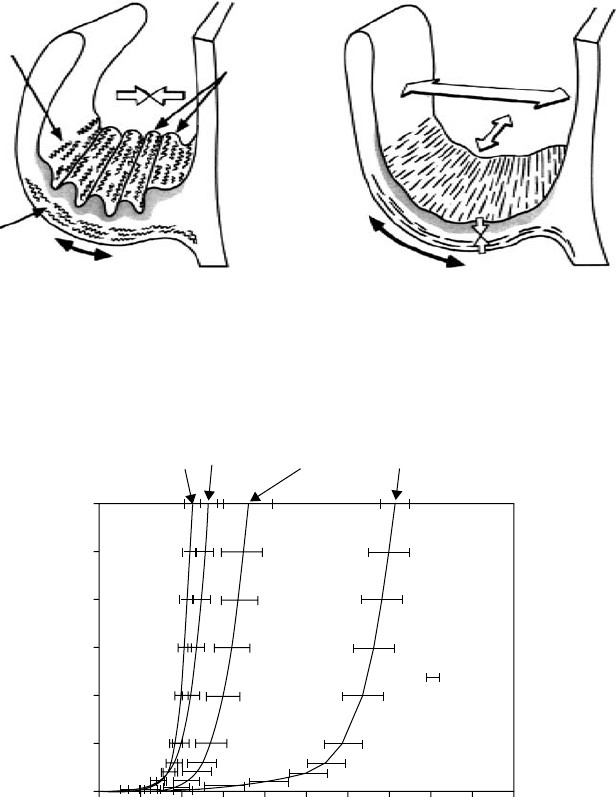
Collagen
crimp
Elastin
Systole
Corrugations
Diastole
FIGURE 28.4 Illustration of the histological layers of the valve during systole and diastole. (Reprinted from
Schoen, F. J., J. Heart Valve Dis., 6, 2, Copyright 1997, with permission from ICR Publishers.)
Fibrosa
fresh
cirumferential
Ventricularis
fresh
cirumferential
Fibrosa
fresh
radial
Vertricularis
fresh
radial
300
200
100
0
0 20406080100
Strain (%)
Stress (kPa)
SEM
FIGURE 28.5 Radial and circumferential stress–strain curves of the ventricularis and fibrosa of aortic valve leaflets.
(Reprinted from Vesely, I. and Noseworthy, R., Micromechanics of the fibrosa and the ventricularis in aortic valve
leaflets, J. Biomech., 25, 107, Copyright 1992, with permission from Elsevier.)
of the leaflets. Although it is presumed that the VECs provide the tissue with a nonthrombogenic sur-
face, their functions otherwise are only beginning to be explored [23,24]. The valvular interstitial cells
(VICs), which are only slightly better understood, are responsible for the synthesis of extracellular matrix
components, including collagen, elastin, proteoglycans, and hyaluronan (for reviews see References 25
and 26). A key characteristic of VICs is that this group of cells exhibits a mixed phenotype of both fibro-
blastic and smooth muscle cell characteristics and are yet uniquely different from both these cell types
[21,22,27,28]. It remains unclear whether this dual phenotype is caused by a single population of cells
that express both features simultaneously [22,27], a single population of cells that can switch between
these two phenotypes [21], or a population of several types of cells [29,30]. The different phenotypes of
VICs are typically distinguished by their morphological appearance and immunohistochemical staining
[21,27,28]. The fibroblastic phenotype is marked by elongated cells that contain numerous organelles for

mikos: “9026_c028” — 2007/4/9 — 15:53 — page6—#6
28-6 Tissue Engineering
matrix synthesis, and stain for prolyl-4-hydroxlyase. The smooth muscle cell phenotype is denoted by
cobblestone cells that stain for smooth muscle α-actin and stress fibers. VICs display this dual phenotype
consistently throughout passaging [22,27]. Although in native valves these different cell phenotypes are
slightly segregated [29,30], cells harvested from different regions of the valve had a consistent dual phen-
otypic appearance and growth characteristics [22]. It has also been difficult to separate these phenotypes
in culture [28].
28.2 Approaches to the Tissue Engineered Heart Valve
The basic approach to constructing a TEHV, as with many other engineered tissues, is first to seed an
appropriate cell type onto or within a suitable scaffold, and then to have a period of incubation during
which the cells remodel or otherwise become integrated with the scaffold, and form a neotissue. This defin-
ition is intentionally ambiguous because a wide range of cells, scaffolds, and incubation environments
have been used. All of the proposed TEHVs, however, have been designed to have as many of the ideal
features of a heart valve substitute as possible [31,32] (i) maintain normal structural and biological func-
tion over the patient’s lifetime, (ii) not elicit any inflammatory, foreign body, or immunologic responses,
(iii) have antithrombotic surfaces and the potential for growth and self-repair, (iv) manufactured for each
individual, (v) easy to implant with little technical variability, and (vi) available in an unlimited supply
[31,32]. The majority of research of TEHVs has focused upon the structural, antithrombotic, immuno-
logic, and availability aspects of this lofty goal. Although the studies described here do not represent an
exhaustive discussion of TEHVs, several reviews provide more thorough detail [2,26,33].
28.2.1 Biodegradable Polymeric Scaffolds
Almost half of the proposed designs for TEHVs involve seeding cells on or within a polymeric biode-
gradable scaffold. The purpose in using a biodegradable scaffold is to anchor the seeded cells within an
environment that is originally strong enough to withstand the in vivo mechanical forces, yet will sub-
sequently degrade slowly, thereby transferring the function of load-bearing to the nascent ECM produced
by the cells. Ideally, scaffold degradation rate and cellular synthesis rate should be balanced so that the
scaffold has been completely degraded when the seeded cells have generated an amount of ECM compar-
able to native heart valves. The scaffold should also have initial material properties that are comparable to
native valves.
The first such scaffold used to generate a TEHV was a woven mesh of 90% poly(glycolic acid)
(PGA)/10% poly(lactic acid) (PLA) sandwiched between nonwoven PGA mesh, which was seeded with
ovine vascular myofibroblasts and endothelial cells and used to replace the right leaflet of the pulmonary
valve in a lamb model [34]. Although the resulting pulmonary valve was functional in the short term
(3 weeks), this scaffold was found to be too stiff and thick for long-term use [35,36]. Conversely, seeding
cells in PGA mesh alone produced a neotissue that was too delicate to handle [37], although the high
porosity of this scaffold (95%) encouraged high seeding efficiencies and subsequent ECM production [35].
To avoid the mechanical limitations of the previous scaffolds, Sodian et al. [38] developed a new TEHV
scaffold using poly(hydroxyalkanoate) (PHA), a thermoplastic, easily moldable polymer. The polymer was
cast into a valved-conduit-shaped mold, made porous through a salt leaching process, and seeded with
autologous ovine vascular myofibroblasts and endothelial cells. Although this valved conduit functioned
normally in a sheep model for up to 17 weeks, the PHA scaffold material did not degrade fully by that time,
and the developing neotissue did not contain any histologically detectable elastin or have an endothelial
cell coating [38]. Moreover, PHA has a high echocardiographic density, which prevented the TEHV
performance from being evaluated by Doppler echocardiography.
Because the PHA did not degrade rapidly enough, valved conduits were next assembled from nonwoven
PGA mesh coated with a thin layer of poly-4-hydroxybutyrate (P4HB), a biodegradable thermoplastic
moldable polymer that provided the nonwoven PGA with additional strength [39]. After seeding with
mikos: “9026_c028” — 2007/4/9 — 15:53 — page7—#7
Tissue Engineering of Heart Valves 28-7
(a)
(a)
(a) (b)
(b)
(b)
(c)
(c)
(c)
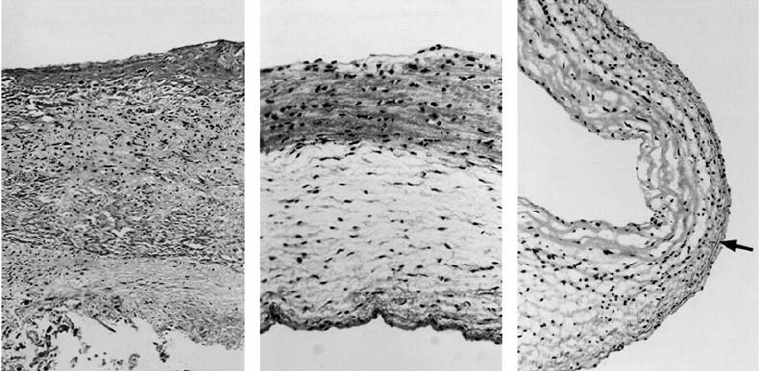
FIGURE 28.6 After6weeksin vivo (a), the TEHV from Figure 28.5 demonstrates preliminary organization (50×).
After 16 weeks (b) and 20 weeks (c), the TEHV leaflet demonstrates organized, dense collagen on the outflow
surface, elastic fibers on the inflow surface (arrow), and spongy organization within (both 100×). (Reprinted from
Hoerstrup, S. P., Sodian, R., Daebritz, S., Wang, J., Bacha, E.A., Martin, D.P., Moran, A.M., Guleserian, K.J.,
Sperling, J. S., Kaushal, S., Vacanti, J.P., Schoen, F.J., Mayer, J.E., Circulation, 102, III-48, Copyright 2000, with
permission from Lippincott Williams & Wilkins.)
autologous ovine vascular cells and 2 weeks of dynamic conditioning in a bioreactor (Figure 28.1), these
TEHVs were implanted in the pulmonary position of sheep for 20 weeks. These TEHVs functioned well,
with only mild to moderate pulmonary regurgitation at 16 and 20 weeks. Upon explant, the TEHV leaflets
were found to have the normal three-layered structure of native heart valves (Figure 28.6), although their
biochemically measured concentrations of collagen, elastin, and GAGs, as well as their ultimate tensile
strength, were significantly higher than normal native pulmonary leaflets. Overall, the PGA/P4HB has
been considered a very successful scaffold for TEHV development and is still being investigated [40,41].
Other biodegradable scaffolds that have been explored include biodegradable polyurethane, which was
found to have not degraded completely at 6 weeks [42]. Finally, new classes of biodegradable scaffolds
are being designed, such as a combination of poly(vinyl alcohol) (PVA) with brush groups of modified
PLA [43]. This novel scaffold should combine the advantages of PVA, a high water content hydrogel with
high elasticity and the ability to incorporate biologically active molecules on its hydroxyl groups, with the
features of PLA, which is biodegradable, can be crosslinked, and is hydrophobically attractive to cells.
28.2.2 Decellularized Leaflet Scaffolds
Although polymeric biodegradable scaffolds have a long history in TEHV designs, another early approach
that is still in active development today is the use of decellularized semilunar valve leaflets as a scaffold, with
the rationale that they would provide the requisite strength [44], and already contain ECM in the correct
microstructural arrangement [45]. Unlike polymeric designs, there is no need to fabricate or mold these
scaffolds. Moreover, removing the cells would presumably eliminate the most antigenic elements, thereby
avoiding any immunological response in the recipient. This reasoning has enabled the development
of decellularized scaffolds from not only human heart valves (from donated allograft organs [45–47] or
cadavers [48]), but also porcine heart valves. The predominant matrix element in these scaffolds, collagen,
is highly conserved between species and is thus considered minimally antigenic [49,50], although there
are concerns about the potential for transmission of xenogenic diseases [51].
A major area of research in the development of this scaffold is determining the best method to remove
the cells from the original valve leaflet. Several different methods, involving ionic and nonionic detergents,
mikos: “9026_c028” — 2007/4/9 — 15:53 — page8—#8
28-8 Tissue Engineering
solutions that are hypotonic or hypertonic, and enzyme treatments, have been attempted, abandoned,
debated, and revisited, with only few direct comparisons [52]. In early studies, a treatment involving
hypotonic saline to lyse the cells followed by two washes with the nonionic detergent Triton X-100 and
one enzymatic soak in DNAse and RNAse was effective in the removal of all cells and cell debris [44,53].
This method preserved the majority of the thermal, physical, and material properties with the exception
of a slight swelling and a slight increase in the stress relaxation of the tissue. This successful treatment was
in contrast to their previous experimentation with the ionic detergent sodium dodecyl sulfate (SDS), in
which the leaflet matrix swelled up to three times and had significant thermal denaturation [53]. On a
single wash basis, however, SDS appears to remove more cells than Triton X-100 [54]. A combination of
0.5% trypsin and 0.2% EDTA was also successful in removing cells from human and porcine valves [48,55],
as was a solution of 1% deoxycholic acid [56]. A solution of 0.1% N -cetylpyridinium chloride was shown
to remove cells effectively and to preserve the tissue’s microstructure and mechanics but this treatment
induced calcification when the decellularized leaflet was tested in a rat subcutaneous dermal model [57].
Booth et al. [52] found that solutions of 0.03 to 0.1% SDS and 0.5% Na deoxycholate in hypotonic solutions
worked best (with SDS causing a slight increase in tissue extensibility [58]), which they attribute to better
protease inhibition than in the previous studies that implicated SDS in fiber damage. Although a few
studies reported using protease inhibitors [48,53,55], such as phenylmethyl sulfonyl fluoride (PMSF) and
EDTA, to block the endogenous lysosomal proteases released during cell lysis and to prevent degradation
of the matrix scaffold, certain protease inhibitors (including PMSF) are short-lived in aqueous solutions
and a more stable compound such as aprotinin may be preferable [52]. Many studies did not report any
use of protease inhibitors, which could result in partial degradation of the collagen and elastic matrix
components of the scaffold. The partial degradation of elastin in these scaffolds is considered particularly
risky given that decellularized aortic wall, found to contain an abundance of partially degraded elastin,
was prone to calcification in a rat subcutaneous dermal model [59].
Once prepared, the decellularized leaflet scaffold is almost always reseeded with autologous cells derived
from the same host animal to be used in the TEHV study. Several of these scaffolds have been reseeded
with endothelial cells only [56,60]. The main intent of this seeding is to form an antithrombotic coating
around the bare collagen [60], and is an especially important consideration in planning for human TEHV
use, because humans may have a more difficult time endothelizing structures than do the sheep models
used in most of these studies [61]. Many other decellularized scaffolds, however, have also received a
preliminary reseeding with vascular myofibroblasts in attempts to accelerate the eventual remodeling of
the matrix [51,55,62–64]. Despite the preliminary seeding of the decellularized scaffolds, dispersing the
cells within the existing matrix has proven difficult, with some tendency for the cells to remain on the
surface or to merely line the largest pores [65]. In addition, Steinhoff et al. [62] found that the seeded cells
tended to make new matrix on top of as opposed to within the existing scaffold matrix.
The Synergraft™ valve (Cryolife, Inc., Kennesaw, Georgia) consists of a decellularized porcine pul-
monary root or composite aortic root (constructed from three noncoronary root-valve segments);
decellularized human allografts are also available [46,66]. In contrast to the other approaches, the Syner-
graft valved conduits were not reseeded with any cells before implantation in sheep models, but became
entirely repopulated with host cells and were completely functional for one year [45]. Although these scaf-
folds were developed with many techniques similar to those used for other TEHVs, they are not universally
considered tissue-engineered structures because they are not reseeded with cells before implantation.
28.2.3 Cell Seeding
The methods used to seed the cells on and within the polymeric biodegradable scaffolds and the decellu-
larized valve leaflets tend to be very straightforward: a concentrated solution containing the cells is dripped
onto the scaffold surface and the cells disperse by gravity [67,68]. This seeding dispersion was encouraged
by gentle agitation in some studies [30], but there was no report of any improved seeding efficiency due
to this method. Many TEHVs have been developed by seeding first with myofibroblasts (several million
cells), incubating 10 to 14 days, and then seeding with endothelial cells [34,37,38,62,69–72]. In preparation
mikos: “9026_c028” — 2007/4/9 — 15:53 — page9—#9
Tissue Engineering of Heart Valves 28-9
for this staged seeding process, a mixed population of vascular cells can separated into endothelial and
nonendothelial cells using fluorescence-activated cell sorting (FACS)-based binding to acetylated low-
density lipoprotein (positive binding in endothelial cells [73]). Staged seeding is not necessarily required
[74]; mixed vascular cells seeded onto PGA/PLA–PGA sandwich scaffolds tended to segregate during
incubation and in vivo implantation, forming neotissue with endothelial cells (staining for Factor XIII) on
the outside and myofibroblasts (negative for Factor XIII) within. Other factors shown to improve seeding
efficiency include 24 or 36 h seeding intervals (as opposed to 2 or 12 h intervals [68]), using polymeric
scaffolds with high porosity such in PGA [67,68], mixing soluble collagen into the cell seeding solution
[70], and coating the scaffold with Matrigel before seeding [75].
28.2.4 Natural Materials
Although the majority of TEHVs to date have been constructed from either biodegradable polymeric or
decellularized leaflet scaffolds, there are a number of alternative scaffolds that have been developed using
natural polymers such as collagen. The rationale behind using these natural materials is that the synthetic
biodegradable polymers may have cytotoxic degradation products such as lactic acid, which can lower
the pH of the culture medium [76]. A very early approach, reported by Carpentier et al. [77], was to
inject solubilized collagen into a leaflet shaped mold. The resulting valve was implanted in sheep in the
tricuspid or mitral position and functioned for up to 10 months without incompetence. Upon explant,
fibroblasts were found within the collagen leaflets, but the leaflets were determined to have thickened
slightly in vivo. More recently, valvular interstitial cells seeded within collagen sponge scaffolds were
found to demonstrate phenotypic characteristics very similar to cells within intact valve leaflets [78].
Rothenburger et al. [71,75] grew human and porcine vascular and valvular cells within a freeze-dried
porous type I collagen matrix in vitro and found that the cells produced the large hydrating proteoglycan
(PG) versican, the small collagen-binding PG decorin, fibronectin, thrombospondin, and a medium-
sized heparan sulfate PG (possibly perlecan or syndecan). The production of these PGs and glycoproteins
indicate that the cells were interacting with and organizing the nascent ECM. Another natural material,
fibrin, is being explored as a natural scaffold because fibrin gels can be made autologously from a patient’s
own blood and thereby prevent an immunologic reaction [67,68,76]. Fibrin gel components can also be
coupled with exogenous biologically functional groups, such as growth factors, for improved neotissue
formation. In addition, the use of injection molding to cast a cell-seeded fibrin gel ensures that the cells
will be evenly distributed throughout the TEHV. This initial even distribution of cells is an advantage
over the seeding of fibrous or sponge structures, where seeding dispersion is due to gravity and barely
50% of cells attach [67]. The disadvantage with fibrin gels is their initial weakness, which needs to be
improved before in vivo studies can be performed. Yet another natural polymer that has been proposed
is chitosan, a polysaccharide derivative that has been used for other tissue engineering applications [23].
Although three-dimensional chitosan scaffolds have yet to be tested in a TEHV, monolayer cultures of
VECs adhered better to chitosan surfaces — and chitosan/collagen IV combinations in particular — than
to PHA surfaces.
28.2.5 Building Block Approach
Almost without exception, the approach to TEHV development has been to use scaffolds that are destined
for degradation or remodeling by the cells that will populate these constructs. The exception to this
paradigm is the approach by Vesely et al. [79], in which the different ECM structural components are
derived and then assembled into an approximation of the native aortic valve microstructure in vitro.The
collagen bundles that provide the leaflet strength are replicated by neonatal rat aortic smooth muscle cells
(NRASMCs) seeded within a type I collagen gel and anchored to promote uniaxial or branched contrac-
tion. The lubricating glycosaminoglycan component will be represented by cross-linked hyaluronan (also
seeded with NRASMCs), and the network and sheets of elastic fibers are synthesized by the NRASMCs atop
the crosslinked hyaluronan and around the collagen bundles. The final valve structure will be assembled
