Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c027” — 2007/4/9 — 15:53 — page4—#4
27-4 Tissue Engineering
(b)
10 msec
L
T
100 msec
1 sec
Ion channel
current
Cell
action potential
Tissue
anisotropic propagation
Heart ECG
Heart
Cardiac bundles and sheets
5 cm
Anisotropic cell network
100 mm
Intercalated disk
2 mm
Gap junction plaque
50 nm
5 nm
Cx-4 3
(a)
Gap junction
channel
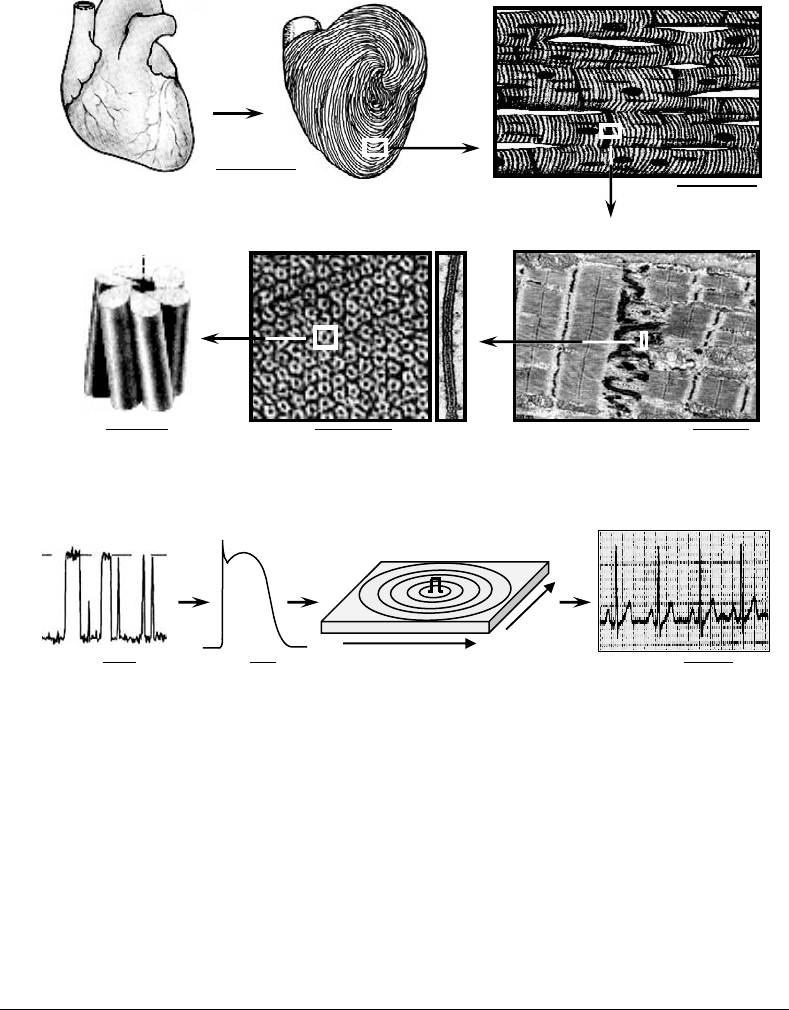
FIGURE 27.1 Levels of anatomical and electrophysiological organization in cardiac muscle. (a) Intercalated disk is
specialized end-to-end connection between cardiac cells. Gap junction plaque is shown in cross-section and en face.
Cx-43 is gap junction protein connexin-43. Note that structural complexity in heart spans many orders of magnitude
from nanometer-size scale in single channels to centimeter-size scale in the heart. (b) Time constants of electro-
physiological function in cardiac muscle range from nanoseconds for a single channel gating to seconds for heart
beats. L and T denote longitudinal and transverse direction, respectively. Pulse sign denotes site of stimulus.
in the heart [59]. In addition, presence of noncontractile scar in heart milieu can cause locally increased
stress gradients, which through mechano-electric feedback may yield in stretch-induced arrhythmias [60].
27.3 Current State of Cardiac Tissue Engineering
Over the last several years different strategies have been developed to design engineered cardiac tissues
that could be used for pharmacological, genetic, and functional studies in vitro and possible implantation
in vivo, as outlined in Table 27.1. These studies have shown that structure and function of cardiac tissue
constructs depend on the animal species used for the cell dissociation [61–63], composition of seeded
cells [14,64], initial cell seeding density [62,63,65,66], scaffold characteristics [40,67–70], composition
of culture medium [69], type of bioreactor [63,65,69], and applied physical forces [61,71]. Most of these
results are based on the evaluation of general histology, and assessment of cellular properties including cell

mikos: “9026_c027” — 2007/4/9 — 15:53 — page5—#5
Cardiac Tissue Engineering 27-5
TABLE 27.1 Cardiac Tissue Engineering In Vitro and In Vivo
In vitro
Cell source Scaffold Bioreactor Assessment References
Neonatal rat
ventricle
Microcarrier
beads
HARV Immunohistology, ultrastructure,
pharmacology
[77,78]
Embryonic chick
ventricle
Planar collagen gel
with
supplements
Static petri dish
attachment to velcro
Immunohistology,
pharmacology, ultrastructure,
gene manipulation, mechanical
contractile force
[79]
Neonatal rat
ventricle
planar collagen gel
with
supplements
Static petri dish
attachment to velcro
Immunohistology,
pharmacology, ultrastructure,
contractile force
[62]
Neonatal rat
ventricle
Collagen gel ring
with
supplements
Static petri dish and
cyclic stretch
Immunohistology,
pharmacology, ultrastructure,
contractile force
[72]
Rat smooth
muscle, skin
fibroblasts, fetal
ventricle, human
atria and
ventricle
Rectangular
gelatin mesh
Static petri dish Histology, cell proliferation [86]
Young human
ventricle
Rectangular
gelatin mesh
Cyclic stretch in dish Histology, proliferation,
mechanical
[71]
Neonatal rat
ventricle
Rectangular
collagen scaffold
(“tissue fleece”)
Static petri dish RT-PCR, pharmacology,
ultrastructure, mechanical
[73,87]
Neonatal rat
ventricle
Fibrin glue and
thick collagen gel
around aorta
Unperfused and
perfused through
aorta
FDG-PET, Immunohistology [88]
Neonatal rat
ventricle
Cross-linked
collagen mesh
HARV Immunohistology, ultrastructure [89]
Fetal and neonatal
rat ventricle
Electrospun
tubular collagen
scaffold
HARV Immunohistology, ultrastructure
mechanical stress–strain curves
[74,90]
Neonatal rat
ventricle
No scaffold Static petri dish Immunohistology, ultrastructure,
electrical connectivity,
mechanical, subcutaneous
implantation
[75,92]
Embryonic chick
and neonatal rat
ventricle
Fibrous PGA disk Static petri dish,
spinner flask, HARV
Viability, metabolic activity,
immunohistology,
ultrastructure
[63]
Neonatal rat
ventricle
Fibrous PGA disk Spinner flask Viability, metabolic activity,
immunohistology,
ultrastructure, tissue scale
electrophysiology
[64]
Neonatal rat
ventricle
Fibrous PGA disk Perfusion cartridge Viability, metabolic activity,
immunohistology,
ultrastructure
[94,95]
Neonatal rat
ventricle C2C12
myoblasts
collagen sponge
disk with
matrigel
Orbitally mixed dish,
Perfusion cartridge
Viability, metabolic activity,
immunohistology,
pharmacology, excitation
threshold, capture rates
[65,96]
Neonatal rat
ventricle
Surface
hydrolyzed,
laminin-coated
PGA disk
Spinner flask,
3D gyrator, HARV
Immunohistology,
immunoblotting, ultrastructure,
viability, metabolic activity,
tissue electrophysiology
[69]
Continued
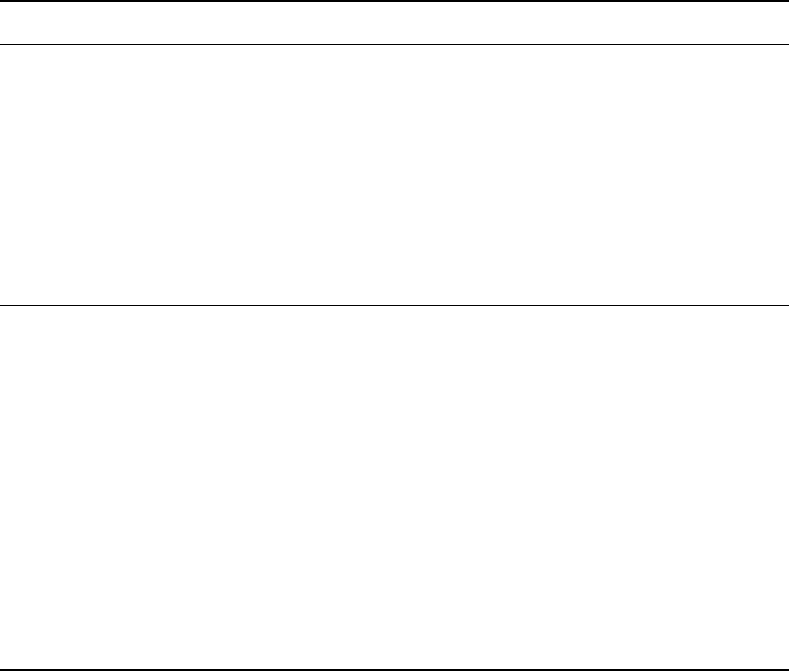
mikos: “9026_c027” — 2007/4/9 — 15:53 — page6—#6
27-6 Tissue Engineering
TABLE 27.1 Continued
In vitro
Cell source Scaffold Bioreactor Assessment References
Neonatal rat
ventricle
Surface hydrolyzed,
laminin-coated
PGA disk
HARV Immunohistology,
immunoblotting,
pharmacology, cell
electrophysiology
[76]
Neonatal rat
ventricle
Fibronectin coated
PLGA disk
HARV Histology, ultrastructure,
optical mapping of
action potentials
[121,122]
Fetal rat ventricle Alginate disk Static petri dish Viability, metabolic
activity, histology
[66]
In vivo implantation in heart
Postoperative
Cell source Scaffold Site of implantation assessment References
Fetal rat ventricle Gelatin mesh cube Infarct in cryoinjured
left rat ventricle
Histology, ultrastructure,
ventricular pressure in
Langendorf preparation
[97]
Rat aortic smooth
muscle
Gelatin mesh, PTFE
patch, PGA, and
PCLA sponge
Defect in right
outflow ventricular
tractinrat
Immunostaining, cell
proliferation,
morphometry
[67,98]
Rat aortic smooth
muscle
PCLA sponge Postinfarct aneurysm
in rat left ventricular
Immunohistology,
echocardiography,
ventricular pressure
[99]
Fetal rat ventricle Alginate disk Rat coronary
occlusion site
Immunohistology,
echocardiography
[100]
Neonatal rat
ventricle
Collagen gel ring with
supplements
Perimeter of healthy
rat ventricle
Immunohistology,
ultrastructure,
pharmacology,
echocardiography
[85]
HARV — high-aspect-ratio-vessel, HFDG-PET — Fluor-Deoxy-Glucose-Positron-Emission-Tomography, PLGA —
poly(lactic-co-glycolic) acid, PTFE — polytetrafluoroethylene, PCLA — ε-caprolactone-co-l-lactide reinforced with knitted
poly-l-lactide fabric.
number, viability, metabolic activity, expression of cardiac-specific proteins, and ultrastructural features.
Few groups also focused on measurements of contractile force at tissue scale [62,72–75], while only
one group has studied in detail microscopic and macroscopic electrical properties of tissue constructs
[64,69,76]. The following paragraphs will give an overview of existing in vitro and in vivo efforts in the
emerging field of cardiac tissue engineering.
27.3.1 Cardiogenesis In Vitro
Akins et al. [77,78] have shown that neonatal rat ventricular myocytes can form multilayered inter-
connected structures when cultivated on fibronectin-coated polystyrene beads or collagen fibers inside
high-aspect-ratio-vessel (HARV) bioreactors. After 6 days in culture, cardiac cells formed small, several
layers thick clusters in the regions between the beads, exhibited presence of sarcomeres and gap junctions,
and rhythmically contracted at rates that were slower in the presence of propranolol. The nonmyocytes
were distributed throughout the tissue clusters, with most of the endothelial cells lining on the interface
between the cluster and culture medium.
Group of Eschenhagen has done some of the most comprehensive work in the field, using mixtures of
embryonic chick [79] or neonatal rat cardiac cells [62,72] and gels made of collagen type I supplemented
with matrigel, chick embryo extract, and horse serum. Their initial work was based on Vandenburgh’s
mikos: “9026_c027” — 2007/4/9 — 15:53 — page7—#7
Cardiac Tissue Engineering 27-7
approach for engineering of skeletal muscle [80], where cell–gel mixture was cast in the thin planar
geometry between two parallel Velcro-coated glass tubes. Firm attachment to Velcro-imposed static stress
on free edges of the gel resulting in thin biconcave tissue construct (8×15×0.18 mm
3
) with loose, aligned
cardiac cell network formed mostly along the construct edges [79]. The alignment and density of this net-
work was improved by use of the chronic cyclic stretch during cultivation [61]. In their current approach
[72], cardiac constructs termed engineered heart tissues (EHTs) are made by embedding neonatal rat
ventricular cells in circularly molded collagen gels, which are subsequently cultivated in static conditions
for 7 days and subjected to chronic cyclic stretch (10%, 2 Hz) for additional 7 days. Resulting submillimeter
thick rings of tissue contain aligned cardiomyocytes organized in loose but uniform tissue-like network
with frequently forming 20 to 50 µm thick cardiac fibers [72]. Myocytes in this network spontaneously
contract at steady rates of ∼2 Hz, and exhibit differentiated cardiac-specific ultrastructure including par-
allel sarcomeres, T-tubules, SR vesicles, formed dyads, and basement membrane [72]. The initial seeding
of unpurified cell mixture (no differential preplating) result in the presence of microphages and abundant
fibroblasts, scattered throughout the EHT, as well as endothelial and smooth muscle cells, packed more
densely in the outer compared to inner region. When electrically and pharmacologically stimulated, EHTs
exhibit cardiac-specific mechanical properties including Frank–Starling behavior, a positive inotropic
response to extracellular calcium and isoprenaline, and negative inotropic effect to carbachol. Although
recorded twitch amplitudes of 1 to 2 mN/mm
2
are an order of magnitude lower than those found in native
cardiac tissues [81], the twitch to resting tension ratio is larger than 1, similar to native muscle. The use of
rat cells, horse serum, chick embryo extract, matrigel, and unpurified cell seeding mixture are all found
to increase the maximum developed force and mechanical integrity of EHTs, while increase in collagen
content seems to decrease twitch tension [14,70]. Up to now, EHTs have been used for studying the effect
of genetic and pharmacological manipulations on cardiac contractile function [62,82–84], and were also
implanted in vivo (see work by Zimmermann et al. [85]).
Group of Li [86] seeded biodegradable gelatin meshes with different cell types including stomach
smooth muscle cells, skin fibroblasts and fetal ventricular myocytes from rat, and adult atrial and
ventricular myocytes from humans. Rat cells and human atrial, but not ventricular, cells proliferated
over 3 to 4 weeks in culture. All cells migrated in a 300 to 500 µm thick outside layer of gelatin scaffold,
which slowly degraded with the highest degradation rate found in the presence of fibroblasts. In separate
in vitro study [71], the same group showed that 2 weeks of cyclic mechanical stretch improved cell prolif-
eration, distribution, and mechanical strength of tissue constructs made using gelatin scaffolds and heart
cells isolated from children who underwent repair of Tetralogy of Fallot.
Kofidis et al. [73,87], used 20 × 15 × 2mm
3
commercially available collagen-based scaffolds (“tissue
fleece”) that were inoculated with neonatal rat cardiac cells and cultured in petri dishes. The randomly
distributed cells formed sparse synchronously contractile networks, and exhibited cardiac specific mech-
anical responses to stretch, extracellular calcium, and epinephrine. In an attempt to increase the thickness
of the engineered cardiac tissue, the same group recently embedded a rat aorta in the 8.5 mm thick mixture
of collagen gel and cardiac cells, and used pulsatile flow through the aorta for 2 weeks as a vehicle for
nutrition and oxygen delivery [88]. The aorta remained patent throughout the culture and viability was
increased compared to unperfused controls.
van Luyn et al. [89] have also used neonatal rat cells and commercially available cross-linked collagen I
bovine matrices, and cultured them in HARV bioreactors for up to 3 weeks. Spatially scattered cells
exhibited immature sarcomeres, gap junctions, and stained for troponin-T.
In recent studies, Evans et al. [90] and Yost et al. [74] cultured embryonic and neonatal rat cardiac
cells on fibronectin coated aligned tubular scaffolds (15 mm long, 4 mm inner, 5 mm outer diameter)
made from extruded collagen I fibers [91]. After 3 to 6 weeks in HARV bioreactors, cardiac cells aligned,
contracted spontaneously, formed few interconnected cell layers (with total thickness of ∼20 µm) on
the inside and outside lumen of the tube, and exhibited registered sarcomeres and randomly distributed
gap junctions. Tubular collagen scaffolds exhibited viscoelastic properties qualitatively resembling those
of native papillary muscle [74] only when seeded with cardiac cells, as inferred from the shape of the
stress–strain hysteresis loops.
mikos: “9026_c027” — 2007/4/9 — 15:53 — page8—#8
27-8 Tissue Engineering
Very elegant studies by Shimizu et al. [75,92,93] have demonstrated that cardiac cells can form 3D
multilayer tissue-like structures without the use of any type of scaffold. Isotropic monolayers of puri-
fied cardiac cells were cultured to confluence on the surfaces made of temperature responsive polymer
poly(N-isopropylacrylamide). This polymer is slightly hydrophobic and cell adhesiveat 37
◦
C and becomes
hydrophilic and cell repellent when cooled below 32
◦
C. After 4 days, up to four cardiac sheets were detached
(together with secreted extracellular matrix) from polymer surface by cooling to 20
◦
C and overlaid using
pipette or polyethylene mesh. Overlaid sheets exhibited uniform gap junction distribution, connected
electrically, and formed compact multilayered spontaneously contractile cardiac constructs with area
of1cm
2
and thickness of up to 50 µm. After subcutaneous implantation, cardiac constructs survived
up to 12 weeks, spontaneously contracted, appeared vascularized and at 3 weeks exhibited twitch tension
of 1.2 mN [75].
Group of Freed and Vunjak-Novakovic has utilized various approaches to engineering of cardiac tissue
based on the use of biodegradable polymer scaffolds and different tissue culture bioreactors. Initial studies
of Bursac et al. [64], and Carrier et al. [63] have demonstrated that cardiac cells formed tissue-like con-
structs when seeded on 5 mm diameter×2 mm thick fibrous poly(glycolic) acid (PGA) disks inside spinner
flask bioreactors. Cells in the outer 50 to 70 µm thick region were randomly oriented and connected in
the relatively dense multilayer network. These cells expressed cardiac specific proteins (α-sarcomeric actin
and troponin-T), end ultrastructural features characteristic of cardiac myocytes (parallel sarcomeres, all
types of specialized junctions, dense mitochondria, glycogen granules). The cells in the interior of these
tissue constructs were sparsely distributed and often necrotic. Spontaneous macroscopic contractions
were observed at days 2 to 4 of culture and generally ceased thereafter, with occasional activity at rates of
less then 1 Hz on culture day 7. Action potential propagation and electrical excitability were studied using
linear array of eight metal microelectrodes with 500 µm spatial resolution (Figure 27.2a). Constructs were
electrically excitable and exhibited isotropic, macroscopically continuous electrical propagation with velo-
cities as high as 60% of those found in native ventricles [64]. Use of purified cell mixture (after differential
preplating) for seeding resulted in superior electrophysiological properties including higher velocity of
propagation, increased maximum rates of tissue capture and lower excitation threshold compared with
use of unpurified (no preplating) cell mixture. In addition, use of neonatal vs. chick cardiac cells, dynamic
seeding and cultivation in bioreactors vs. static petri dishes, and increase in the number of seeded cells up
to 8×10
6
cells per scaffold have all improved cell packing density, metabolic activity, and electrophysiolo-
gical properties of constructs [63,64]. In further studies, Carrier et al. [94] looked in the use of perfusion
through tissue construct as means to improve the cellularity and tissue architecture, and studied effect of
oxygen deprivation on engineered cardiac muscle [95]. In the most recent studies from the same group,
Radisic et al. [65,96] used cell–matrigel mixture to densely inoculate cardiac cells inside collagen sponge
scaffolds, and employed similar perfusion bioreactor for construct cultivation. The viability, metabolic
activity, and cellular density through ∼1 mm thick region were higher than in constructs cultured in
orbital shakers and those from studies by Carrier et al. In a different study, Papadaki et al. [69] have
shown significant improvements in structure and function of cardiac constructs when PGA scaffolds were
hydrophilized and coated with laminin, percentage of serum in culture medium reduced after 2 days
of cultivation, constructs seeded with concentrated cell suspension in rotating gyrators, and cultivation
performed in HARV bioreactors. Compared to previous studies, tissue constructs exhibited better cell
viability yielding thicker (120 to 160 µm) cardiac-like outer region, and higher cellular expression of dif-
ferentiation marker proteins including creatine kinase-MM (involved in metabolism), sarcomeric myosin
heavy chain (involved in contractile function), and gap junction protein Connexin-43. Tissue scale elec-
trical properties approached those found in native muscle with conduction velocity at basic stimulation
rate of 1 Hz and maximum capture rate reaching 90 and 70% of those found in donor neonatal ventricles,
respectively (Figure 27.2a). Macroscopic electrical propagation was effectively isotropic due to random
cell orientation and uniform gap junction distribution. Further electrophysiological and pharmacological
studies at microscopic scale by Bursac et al. [76] demonstrated that action potentials in cardiac cells from
7-day constructs were comparable to those in 2-day old donor ventricles with respect to depolarization
upstroke, amplitude, and resting potential. The major difference was prolonged action potential duration
mikos: “9026_c027” — 2007/4/9 — 15:53 — page9—#9
Cardiac Tissue Engineering 27-9
(b)
Tissue
construct
Neonatal
ventricle
Cardiac action potential
20 mV
100 ms
1mm
Tissue sample
S
V
m
x
y
z
O
2
Tyrode’s
T =30°C
Cell scale recording
S
R
Tissue scale recording
z
–
1mm
Tissue sample
S
+
x
y
pH = 7.32
T =37°C
1 2
3 4 5 6
(a)
Electrical impulse propagation
3
2
5
4
6
1
030 300
Tissue construct
Neonatal ventricle
Time (msec)
S
S
R
R
R
R
S
R
FIGURE 27.2 Tissue and cell scale electrophysiological recordings in 7-day old cardiac tissue constructs and 2-day
old neonatal ventricles. (a) Custom-built linear array of two stimulating and eight recording microelectrodes (only six
are shown) was used for assessment of macroscopic impulse propagation. S and R in two tracings denote stimulus
artifact and responses, respectively. Note relatively smooth biphasic shapes of recorded extracellular waveforms in
constructs and ventricles. The response amplitude in constructs is an order of magnitude lower than in ventricles due
to smaller number of cardiac cell layers present. Propagation velocities in constructs and ventricles are comparable
(i.e., times for propagation from electrode 1 to 6 are similar). (b) Glass capillary microelectrodes are used for action
potential recordings from single cells in the tissue sample. Note fast upstroke and similar resting potential, but longer
action potential in constructs than in ventricles. S and R denote stimulus artifact and response, respectively.
(Figure 27.2b) and absence of early repolarization notch due to downregulation of transient outward
potassium current. In addition, cardiac cells cultured in 3D tissue constructs maintained more differen-
tiated phenotype (higher expression of marker proteins) and more in-vivo like action potential features
compared to those cultured in 2D monolayers under similar cultivation conditions.
27.3.2 In Vivo Implantation for Cardiac Repair
By April 2004, in vivo implantation of engineered cardiac tissue in infracted heart was attempted by only
three groups.
Li et al. [97] cultured fetal rat myocytes on biodegradable gelatin meshes (15 ×15 ×5mm
3
)for7days
and implanted cardiac constructs over the scar area in cryoinjured syngeneic rat hearts. Cells populated
sparse interstices of gelatin meshes (see in vitro work by Li’s group) and continued to proliferate and
spontaneously contract in vitro for at least 26 days. Epicardially implanted grafts survived for 5 weeks,
exhibited increased cellularity, slight degradation, and moderate degree of vascularization. Left ventricu-
lar developed pressure, showed no improvement over the control animals. In other studies [67,98], the
same group evaluated use of various scaffold materials seeded with aortic smooth muscle cells (used to

mikos: “9026_c027” — 2007/4/9 — 15:53 — page 10 — #10
27-10 Tissue Engineering
presumably increase elasticity of the patch) for repair of defect in the right ventricular outflow tract in
syngeneic rats. Eight weeks postimplantation constructs made of ε-caprolactone-co-l-lactide reinforced
with knitted poly-l-lactide fabric (PCLA) outperformed those made of gelatin, PGA, and polytetrafluoro-
ethylene (PTFE) with respect to cellularity, elastin content, and preserved thickness. In the next study
[99], grafts made of PCLA and smooth muscle cells were used to repair left ventricular aneurysm in the
rat hearts after transmural infraction. Cell-seeded grafts reduced abnormal chamber distensibility and
improved ventricular function compared with implanted cell-free grafts, as assessed by echocardiography
and constant pressure measurements in Langendorff preparation.
Leor et al. [100] cultured fetal rat myocytes on porous biodegradable alginate disks (6 mm diameter,
1 mm thick) inside 96-well plates for 4 days, and implanted cardiac tissue constructs over infracted region
in rat hearts 7 days after permanent occlusion of left main coronary artery. Nine weeks postimplant-
ation, cardiac constructs survived, while alginate scaffold substantially degraded. Cardiac grafts were
neovascularized, contained infiltrated macrophages and lymphocytes due to use of allogenic cells and no
immunosuppression, and exhibited small number of sparsely distributed cardiac cells presumably due
to low initial seeding density. Echocardiography revealed attenuated left ventricular dilatation and main-
tained contractile function, although it was not clear if implanted cell-free scaffolds would have produced
similar results. Further in vitro study from the same group [66] focused on methods to increase cell density
in alginate scaffolds by applying moderate centrifugal forces during seeding.
Zimmermann et al. [85] implanted 12-day old ring-shaped EHTs (see in vitro work from Eschehagen’s
group) around the circumference of healthy syngeneic rat hearts. Two weeks after implantation, EHTs
were vascularized, innervated, expressed differentiatedcardiac phenotype, and did not alter left ventricular
function compared to preoperative state, as assessed by echocardiography. Spontaneous contractions were
preserved in vivo, but no intercellular coupling of EHTs and host tissue could be demonstrated. Despite the
syngeneic approach, EHTs were completely degraded in the absence of immunosuppression, presumably
due to presence of allogenic components in reconstitution mixture (e.g., matrigel, horse serum, chick
embryo extract).
In all of the described in vivo attempts no electrophysiological studies of engineered patch were done
pre- or postimplantation.
27.4 Design Considerations
Ultimate success of cardiac tissue repair with an implanted cardiac patch depends on thorough under-
standing of the key parameters of tissue design in vitro, and careful definition of the desired tissue
engineering outcomes.
27.4.1 Cell Source and Immunology
One of the crucial aspects for successful engineering of cardiac tissue is a choice of implanted cells.
Experiences from cellular cardiomyoplasty show that for the improvement of heart systolic function
implanted cells need to be (or be capable of becoming) contractile [26]. Although fetal and neonatal
cardiac cells are clearly shown to functionally incorporate in the myocardium [17–19], they are not cells of
choice due to limited proliferation potential, immunological, and ethical issues. For this reason, their use
will probably stay limited to in vitro model systems and proof-of-concept in vivo studies. Possible “ideal”
cell source may be human embryonic or adult stem cells. Although human embryonic stem cells are shown
to differentiate into cardiac myocytes [101,102], their immunogenic and tumorogenic nature, and low
efficiency and specificity of differentiation (<1% of cells differentiate into mixture of atrial, ventricular,
and nodal cells), as well as ethical issues, mayfinally preclude their clinical use. Some hope lies in the nuclear
transfer technology (“therapeutic cloning”) [103], and genetic knock-out of major histocompatibility
complexes [104], which may offer strategies for preventing immune rejection. Autologous adult stem cells
from skeletal muscle, peripheral blood, or bone marrow appear as better choice for cell transplantation
mikos: “9026_c027” — 2007/4/9 — 15:53 — page 11 — #11
Cardiac Tissue Engineering 27-11
than embryonic stem cells. For example, autologous skeletal myoblasts are easy to proliferate in vitro
and implant in vivo and cause no immune response, which currently makes them one of the cell types
used in clinical trials [33]. Unfortunately, they do not express gap junction proteins and are still not
shown to functionally couple with host cardiac tissue when implanted [105,106], although this may be
resolved with stable transgene expression of connexin molecules [107]. Mesenchymal or hematopoietic
stem cells derived from bone marrow may represent an ideal cell source [29,108]. However, the efficiency
of their transdifferentiation into cardiac myocytes remains controversial in light of recent findings that
question techniques used to quantify the number of transdifferentated cells in heart [109,110]. Still
their beneficial effect may come from induced neovascularization in implantation sites. Current research
efforts are focused on increase in percentage of embryonic or adult stem cells that commit to cardiac
phenotype by use of different growth factors or media compositions, introduction of early cardiac genes
in undifferentiated stem cells in vitro, or coculture of stem cells with differentiated cardiac myocytes.
Another promising alternative is in vitro genetic reprogramming of differentiated somatic cells (for review
see Reference 111).
27.4.2 Cellular Composition
The heart is composed of different types of cells. Engineering of functional tissue patch requires selection
of the appropriate composition of seeded cell mixture. For example, if an implant were to be localized
in the ventricle, cardiac myocytes that comprise a main fraction of seeded cells should be of ventricular
origin or with ventricular characteristics. The percentage of nonmyocytes in the seeding mixture is a
parameter that can be varied. Eschenhagen’s group found that it was necessary to use higher percentage
of nonmyocytes (no preplating after cell isolation) to improve mechanical integrity and twitch tension of
EHTs [14]. In contrast, scaffold-free constructs by Shimizu et al. [75] developed similar twitch tensions
despite the fact that they were made of purified cardiac cell mixture. This suggests that the percentage of
“needed” nonmyocytes may actually depend on the presence and type of scaffold. It is possible that in
EHTs, higher number of fibroblasts contributed initial contraction of collagen gel [112], which in turn
increased the proximity and intercellular connectivity between myocytes, yielding increased contractile
force. In addition, Bursac et al. [64] have shown that use of unpurified cardiac cell mixture decreased
propagation velocity and compromised electrical properties in cardiac constructs. Therefore, for a given
type of scaffold, the percentage of seeded nonmyocytes should be selected to optimize for both mechanical
and electrical function of cardiac constructs.
27.4.3 Tissue Architecture
The anisotropic architecture and dense cell packing of native cardiac tissue impose important design
rules in engineering of functional cardiac patch. For instance, velocity of electrical propagation and
mechanical stiffness in healthy adult human ventricles are on average 2 to 3 times larger in longitudinal
(fiber) than in transverse (cross-fiber) direction [113,114]. This can vary widely with location in the
heart, age of individual, and heart disease. Therefore, only a cardiac patch with dense 3D network of
elongated and aligned cardiac myocytes that mimic architecture of native tissue can generate desirable
spatio-temporal distribution of electrical and mechanical activity, and result in efficient and safe therapy.
Aligned growth of cardiac cells can be induced by static or dynamic stretch [61,115,116], presence of free
tissue boundaries [117], or by cell guidance with oriented surface topography [49,118,119] and anisotropic
distribution of chemical cues for cell attachment [49,120]. Eschenhagen’s group applied cyclic stretch and
ring geometry creating a sparse network of oriented cardiac cells in the form of a thin (submillimeter
diameter) cardiac cable. Repair of larger injured area in the heart will, however, require engineering of an
anisotropic slab of 3D cardiac-like tissue with controllable shape, size, and geometry.
Bursac et al. [49] have recently shown that anisotropic monolayers of cardiac cells (that mimic longit-
udinal sections of native cardiac tissue) can be designed with highly controllable architecture using surface
microabrasion or micropatterning of extracellular matrix proteins (e.g., fibronectin) (Figure 27.3b). These
mikos: “9026_c027” — 2007/4/9 — 15:53 — page 12 — #12
27-12 Tissue Engineering
(b)
Isochrones
2 mm
50
m
10 m
Sarcomeric a-actin and DAPI
Fibronectin
Excitation
light source
sample
Photodetectors
Recorded signals
Fiber optic
bundle
2s
Data acquisition
Experimental chamber
(cross-section)
(a)
(c)
Isochrones
2 mm
Scanning electron micrograph
50 m
200 m
Light micrograph
17 mm
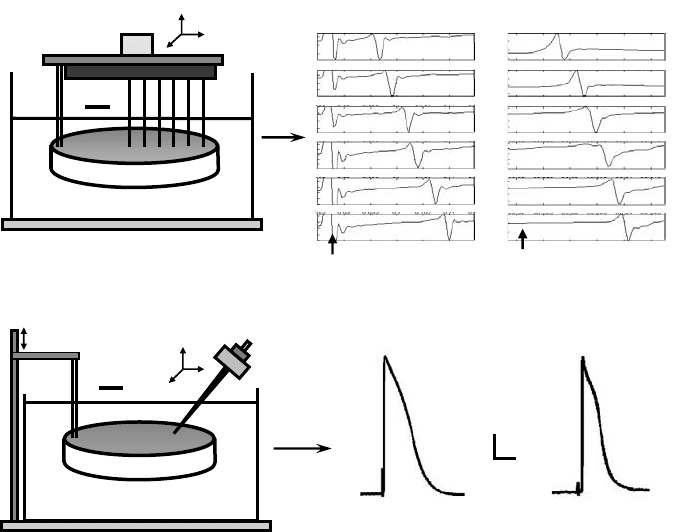
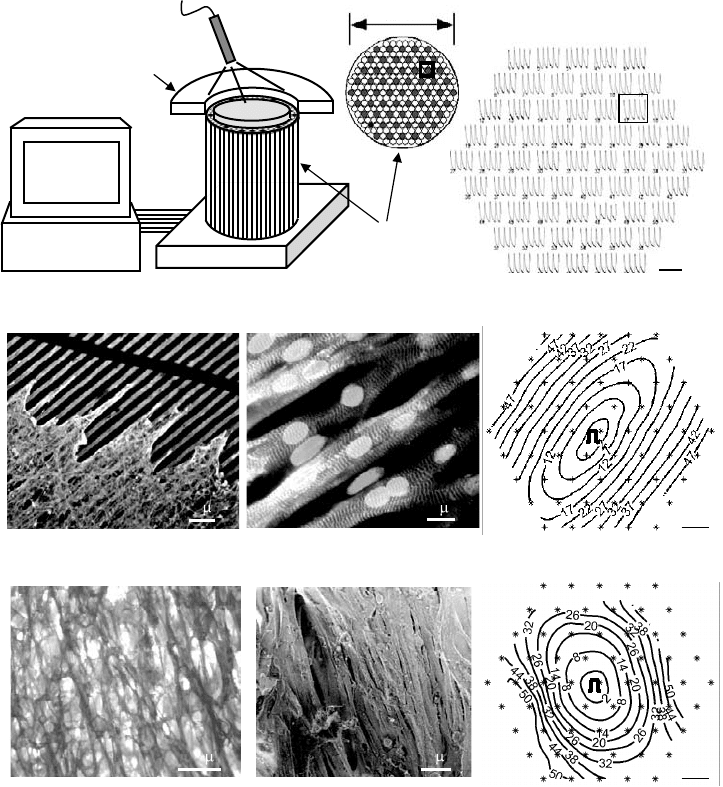
FIGURE 27.3 Architecture and impulse propagation in 2D and 3D anisotropic cultures of neonatal rat ventricular
cells. (a) Optical mapping setup. Tissue samples stained with voltage sensitive dye were positioned over 61 hexagonally
arranged fibers and transilluminated with excitation light. Two seconds of optically recorded action potentials from
a cardiac cell monolayer are shown on the right. Gray circles denote active fibers. Square denotes a recording site in
the bundle and corresponding voltage trace (for details see Reference 49). (b) Anisotropic monolayer of cardiac cells.
Cells were cultured on micropatterned lines of fibronectin. On the left, culture is deliberately scratched to expose
patterned lines and cell-deposited fibronectin. Aligned cells exhibit prominent sarcomeres and elongated nuclei
(middle). Elliptical isochrones demonstrate anisotropic propagation (right). ∗ denotes recording sites. Pulse symbol
denotes site of stimulus. Thedegree of anisotropy can be systematically varied by controlling the amount of intercellular
clefts and cell co-alignment [49]. (c) Anisotropic tissue construct. Oriented fibrous architecture in PLGA scaffolds
(left) was accomplished by leaching sucrose from a polymer-coated template made of aligned sucrose fibers [121].
Cardiac cells were aligned in numerous regions along the direction of PLGA fibers (middle). Macroscopic propagation
was anisotropic (albeit moderately), as assessed by optical mapping of transmembrane voltage (right) [122].
mikos: “9026_c027” — 2007/4/9 — 15:53 — page 13 — #13
Cardiac Tissue Engineering 27-13
methodologies enabled systematic control over the degree of anisotropy (longitudinal-to-transverse velo-
city anisotropy ratios from 1 to 5.6), fiber direction, and amount of longitudinal intercellular clefts in the
2D cardiac monolayers. Optically recorded action potentials with voltage sensitive dye RH237 (at 61 sites
over2cm
2
area) were used to map propagation of electrical activity and degree of functional anisotropy
(Figure 27.3a). It would be ideal if techniques for 3D cardiac tissue culture could be developedwith a similar
level of architectural control as those used for 2D cell culture. In most recent studies Bursac et al. [121,122]
extruded and baked sucrose to form 3D aligned fibrous templates in an attempt to induce anisotropic
architecture in poly(lactic-co-glycolic) acid (PLGA) scaffold disks (Figure 27.3c). After 2 weeks of culture
in HARV bioreactors, 12 mm diameter, 0.7 mm thick cardiac constructs exhibited regions with aligned
cells, and moderate degrees of functional anisotropy (i.e., longitudinal-to-transverse velocity ratio of
up to 2), as assessed by optical mapping of electrical propagation (Figure 27.3c). Using similar techniques
to align elastomeric polymers [123] instead of PLGA in conjunction with chronic mechanical stimula-
tion may yield 3D cardiac patches with physiological degrees of structural and functional anisotropy. An
alternative approach is the use of electrospun scaffolds [74,91,124] providing that obtained degrees of
porosity can support deeper cell penetration during seeding than achievable by current methodologies.
27.4.4 Tissue Thickness
Another important parameter in the design of a functional cardiac patch is thickness of the tissue con-
struct. Compared to other cells in the body, cardiac myocytes require high oxygen and nutrient supply
due to continuous contractile activity. High metabolic demand in myocardium is sustained by dense
vascularization with average arterial intercapillary distances that range from 15 to 50 µm depending on
the size of the heart and basal heart rate [125,126]. Absence of vasculature in tissue constructs is a limiting
factor for engineering thicker (>200 to 300 µm) cardiac patches with physiological cell packing densities.
Nonetheless, high cell density is necessary for establishment of proper intercellular communication, which
in turn enables efficient generation of mechanical force and fast, electrically safe impulse propagation.
Mixing and perfusion of culture medium, and/or cyclic mechanical stimulation are some of the methods
that can alleviate diffusional limits of oxygen and nutrient supply, but only to a certain extent. Neverthe-
less, thin but dense engineered cardiac tissue is still a good in vitro approximation of a viable portion of
explanted ventricular slice after several hours of superfusion [127], and thus could be used for different
tissue-scale functional studies in vitro. These studies would be more versatile and technically easier than
studies in monolayer cultures (e.g., they would enable variety of force measurements, and yield substan-
tially higher signal-to-noise ratio during extracellular and optical recordings of electrical propagation).
Moreover, implantation of even 10 to 20 well-coupled anisotropic cardiomyocyte layers over the infracted
area may still have a significant therapeutic value regarding the facts that after epicardial infarction only
several disarrayed cell layers may survive above the scar, and that increased thickness of survived layers is
directly correlated with decreased incidence of arrhythmias [128]. This is one of the reasons why approach
by Shimizu et al. [75] with scaffold-free cardiac multilayers deserves close attention. On the other hand,
engineering of thicker cardiac slices will depend upon the development of externally perfusable, patent
microcapillary-like networks inside the tissue constructs. Recent work by Vacanti’s group [129] represents
an interesting approach to this problem.
27.4.5 Electrical Function and Safety
One of the most important criteria for successful design of cardiac patch is the issue of electrical and
mechanical safety. It is important to understand that haphazardly adding donor cells or transplanting
a poorly designed tissue patch into an already compromised heart may only increase the likelihood for
aneurisms, tissue rupture, or arrhythmias. In general, the injection of donor cells or implantation of a tissue
patch introduces structural and functional heterogeneity in the cardiac milieu that depends on (1) electro-
mechanical characteristics of donor cells, (2) the density, coupling, and geometrical arrangement of
donor cells within the implant, (3) the degree of interaction between the donor and host cells, and
