Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

instance, SiO
2
nanoparticles of ca. 50–100 nm are obtained through the controlled
hydrolysis of a silicon alkoxide (e.g., tetraethyl orthosilicate, TEOS) in a mixture of
ethanol, aqueous ammonia (21%) and water. Particle sizes were controlled by the
addition of an entraining silane such as 3-aminopropyltriethoxysilane that was added
to TEOS prior to hydrolysis.
[151]
Iron oxide nanoparticles may be synthesized by
the dissolution of an iron salt in an acidic solution (Eqs. 12–14).
[152]
2 NaOH þ Hf(OHÞ
4
! Na
2
HfO
3
þ 3H
2
Oð11Þ
In addition to oxides, a number of other compositions may be synt hesized.
In general, any stoichiometry is “fair game” through the same reactions that one
does in bulk scale. For example, sulfides through reaction of precursors with H
2
S,
nitrides through NH
3
exposure, etc.
[153]
Reactions with sodium chalcogenides
represent a safer means to introduce chalcogens to the structure; for instance, the
formation of L-cysteine stabilized Mn
2+
-doped ZnS photoluminescent phosphor
nanoparticles (Eq. 15). The reaction of Eq. 16 shows a clever way to generate
SnO
3
2−
SnO
2
2 Na
+
CO
2(g)
Na
2
CO
3
N
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
NN
N
N
N
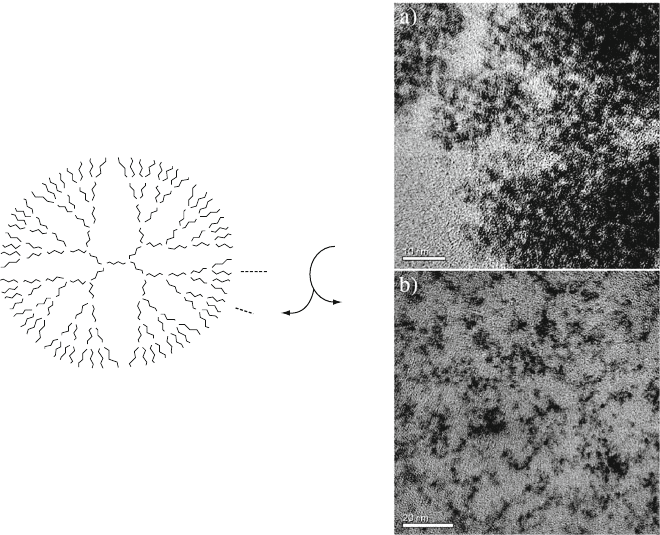
Figure 6.51. Schematic of the growth of tin oxide nanoclusters at room temperature. The TEM images on
the right illustrate dendritic-stabilized nanoclusters using (a) PAMAM and (b) amine-terminated poly
(ethyleneimine) hyperbranched polymer hosts. The latter is a less efficient entraining agent, as observed
by the greater degree of nanocluster agglomeration. Reproduced with permission from Juttukonda, V.;
Paddock, R. L.; Raymond, J. E.; Denomme, D.; Richardson, A. E.; Slusher, L. E.; Fahlman, B. D. J. Am.
Chem. Soc. 2006, 128, 420. Copyright 2006 American Chemical Society.
518 6 Nanomaterials
H
2
S gas in situ, rather than having to handle a cylinder of the dangerous nerve gas.
As long as a suitable entraining agent is used, one is able to control the resultant
size/morphology of the nanostructures. With current advances in compound and
metallic nanoclusters, and the properties of each being fine-tuned via quantum
confinement effects, one can begin to imagine intriguing designs for future micro-
electronic devices.
Step 1:
[Fe(H
2
OÞ
6
3þ
$ [Fe(OH)(H
2
OÞ
5
2þ
þ H
þ
(pH: 0 2Þð12Þ
Step 2:
2 [Fe(OH)(H
2
OÞ
5
2þ
$½ðH
2
OÞ
4
Fe(OHÞ
2
Fe(H
2
OÞ
4
4þ
þ 2H
2
O (pH: 2 3Þ
ð13Þ
Step 3:
½ðH
2
OÞ
4
Fe(OH)
2
Fe(H
2
OÞ
4
4þ
$ isopolyoxo - cations
$ Fe
2
O
3
xH
2
O (pH : 3 5Þð14Þ
Overall:
ZnSO
4
+Na
2
S + (MnCl
2
Þ
!
L
cysteine
ZnS(:Mn
2þ
)+Na
2
SO
4
+ (Cl
Þð15Þ
H
3
C C
k
S
NH
2
Thiacetamide
þH
2
O ! H
2
S+CH
3
COO
+NH
4
þ
ð16Þ
Mechanisms for the nucleation/growth/agglomeration of metal nanoclusters
In order to maintain control over the composition and morphology of a 0-D
nanostructure, it is essential that we understand the self-assembly mechanism for
these structures. Only within the last decade have we figured out ways to repeat-
ably control the morphology and composition of nanoparticles/nanoclusters. This
explains why the mechanistic details of nanocluster growth have not surfaced until
recently. The first system to be investigated was the synthesis of metallic iridium
nanoclusters, formed through the hydrogenation of a polyoxoanion-supported Ir
complex.
[154]
Since Ir does not have an observable surface plasmon resonance
profile, the rate of nanocluster growth was determined by following the hydrogena-
tion of alkenes over time. This is possible in this system since the initial complex is
not an active toward alkene hydrogenation; catalytic activity arises only from the
reduced metal (Ir
0
).
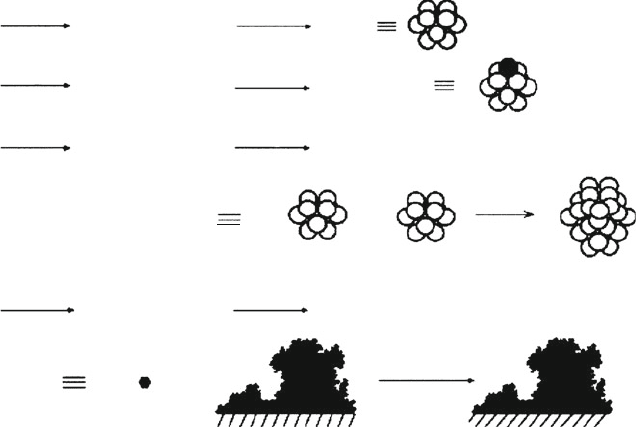
The overall four-step mechanism for nanocluster growth/agglomeration is shown
in Figure 6.52.
[155]
Although this pathway is based on metallic nanocluster studies,
other types of nanoclusters/nanoparticles would likely follow a similar route.
[156]
The first step involves the slow, continuous nucleation of clusters that are much
6.3. Nanoscale Building Blocks and Applications 519
smaller than 1 nm. When an energetically favored critical nucleus size is reached
(ca. 15 atoms for Ir
0
; varies depending on the metal), additional atoms rapidly
aggregate to the surface – a process referred to as autocatalytic surface growth.
The term “autocatalytic” is used since the nanoclusters formed from the nucleation
step are also reactants for subsequent surface growth. Accordingly, the autocatalytic
step will proceed faster as the reaction progresses, effectively shutting down the
nucleation step – essential to achieve monodisperse nanocluster growth. That is, the
size of the growing nanocluster may be controlled by varying the relative values of
k
1
and k
2
, as well as the availabili ty of additional precurso r molecules, both of which
separate the nucleation and growth events in time. In this fashion, metal nanoclusters
have been designated as “living metal polymers,” analogous to organic living
polymers discussed in Chapter 5.
It is widely believed that Au nanoparticle growth by the Tu rkevich method occurs
via the LaMer model,
[157]
in which the concentration of metal atoms in solution
constantly increases (by decomposition of a precursor compound) until supersatura-
tion occurs. Atomic nucleation then occurs, followed by growth via the adsorption of
additional gold atoms. This may be obser ved experimentally by measuring a sharp
drop in the concentration of metal atoms in solution. Below a certain level of
supersaturation, no further nucleation events will take place, which is required to
effectively control the monodispersity of the resultant nanoparticles. Further growth
A
A+B
B+C 1.5C
2B
2B C
B n Pt''
+
+
Pt
0
n
Pt
0
n-1
Pt
0
n+m
Pt
0
n
+ Pt''
Pt
0
n
+ Pt
0
m
Pt
0
n
+ Pt
0
bulk
Pt
0
bulk
k
1
H
2
k
1
H
2
k
2
k
3
k
4
H
2
k
2
k
3
k
4
H
2
Figure 6.52. Schematic of the four-step mechanism for transition metal (e.g., Pt) nanocluster formation.
Shown are (i) nucleation to a desired cluster size; (ii) autocatalytic growth onto the cluster surface;
(iii) diffusive agglomerative growth of two nanoclusters; and (iv) autocatalytic agglomeration into bulk
metal particulates. Reproduced with permission from Besson, C.; Finney, E. E.; Finke, R. G. J. Am. Chem.
Soc. 2005, 127, 8179. Copyright 2005 American Chemical Society.
520 6 Nanomaterials
will be accompanied by coarsening or Ostwald ripening, whereby larger partic ulates
grow at the expense of smaller ones. Though this may yield a high degree of
monodispersity, it may also yield bulk metal particles via a second autocatalytic
surface-growth process (step 4, Figure 6.52). A general prediction of the overall
mechanism is that lower concentrations and higher temperat ures are most conducive
to yield nanoclusters rather than bulk metal (i.e., k
1
>> k
3
, k
4
). Indeed, this
prediction is backed up by experimental data.
The sizes of resultant Au nanoparticles may be fine-tuned by varying the experi-
mental conditions
[158]
; in fact, a recent study has even elucidate d a non-LaMer
growth mode via nanowire intermediates (Figure 6.53).
[159]
It should be noted that
in the Turkevich process, the citrate ion serves as a reducing agent by undergoing
oxidative decarboxylation to yield an acetone dicarboxylic acid intermediate. This
species is then proposed to chelate gold [AuCl
x
]
n
ions as they undergo stepwise
reduction from Au
3+
! Au
+
! Au
0
.
[160]
Hence, it appears as though the reduction
of metal ions in solution occurs via the aggregation/nucleation of unreduced ions,
rather than the nucleation of zero-valent atoms that then coalesce into larger metallic
nanostructures.
[161]
Interestingly, it has been shown that the growth of nanoclusters proceeds through
the formation of “magic number” (or closed shell) clusters that exhibit unusual
electronic stability. It should be noted that once the diameter of the cluster is <ca.
10 nm, a significant portion of the total atoms lie at its surface, which will result in
different physical/optical properties than exhibited by the bulk metal.
[162]
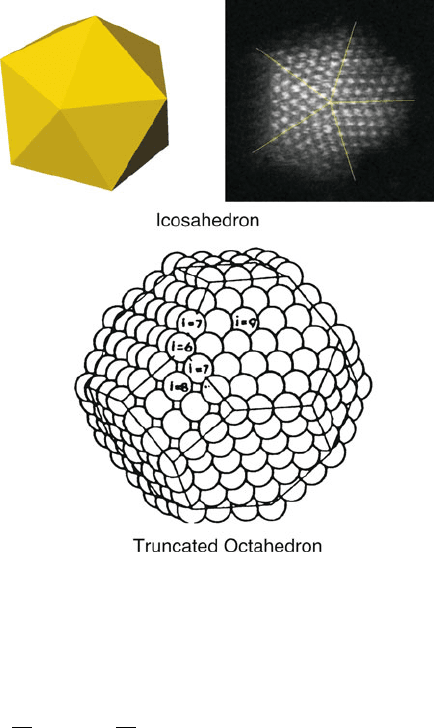
A fcc or
hcp transition metal will form stable clusters with icosohedral or truncated octahe-
dral (TO; analogous to C
60
) symmetry (Figure 6.54 ). The number of metal atoms
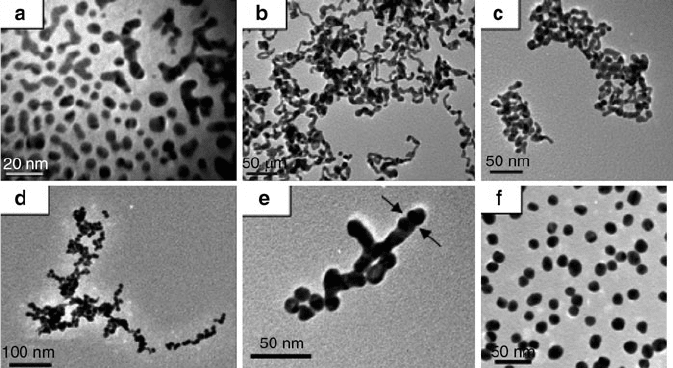
Figure 6.53. TEM micrographs of various stages of Au nanoparticle synthesis via the citrate reduction of
AuCl
4
, showing nanowire intermediate formation. The colors of the solutions are: (a) colorless, (b) dark
blue, (c) dark purple, (d) - (e) purple, and (f) ruby-red. Reproduced with permission from J. Phys. Chem.
C 2007, 111, 6281. Copyright 2007 American Chemical Society.
6.3. Nanoscale Building Blocks and Applications 521
within an icosohedral cluster is given by the Mackay sequence of Eq. 17; i.e., 13, 55,
147, 309, 561, 923, 1,415, 2,057, 2,869, etc.:
N
T
¼
10
3
n
3
þ5n
2
þ
11
3
nþ1;ð17Þ
where n ¼ packing shell (n ¼ 1, 2, 3, 4,. ..). The number of surface atoms in the nth
shell is given by 10n
2
+2.
[163]
For instance, the first stable shell consists of a central
atom surrounded by 12 surface atoms, corresponding to 92% of the total atoms
housed on the surface.
In contrast, the number of total and surface atoms within a TO array are given by
Eqs. 18 and 19, respectively. For instance, the first stable cluster of 38 atoms consists
of 32 surface atoms surrounding a core of 6 atoms (84% of the total atoms located on
the surface). Subsequent shells yield stable clusters with 201, 586, 1289, 2406, 4033,
6266, etc. atoms. Further, the number of TO edge atoms is represented by Eq. 20,
corresponding to the most reactive atoms that exhibit the lowest number of nearest
neighbors. Hence, the first shell of a TO structure would contain 24 edge atoms –
63% of the total number of atoms in the cluster. A regular TO cluster may be
Figure 6.54. Illustrations of icosahedral and truncated octahedral (TO) clusters. The numbers listed on
the TO faces indicate atomic coordination.
522 6 Nanomaterials
designated by its (n, m) indices with n – m ¼ 0(n, m > 1), where n is the number of
atoms on an edge adjoining (111) facets and m that between adjoining (111) and
(100) faces.
[164]
However, gold nanoclusters with N
T
> 20 have been shown to
exhibit the irregular TO
+
structure, with 0 < n – m 4, m > 1. Stable clusters
within the TO
+
set contain 79, 140, 225, 314, 459, ... metal atoms.
N
T
¼ 16ðn þ1Þ
3
33ðnþ1Þ
2
þ24ðnþ1Þ6ð18Þ
N
S
¼ 30ðn þ1Þ
2
60ðnþ1Þþ32ð19Þ
N
E
¼ 36ðn þ1Þ48ð20Þ
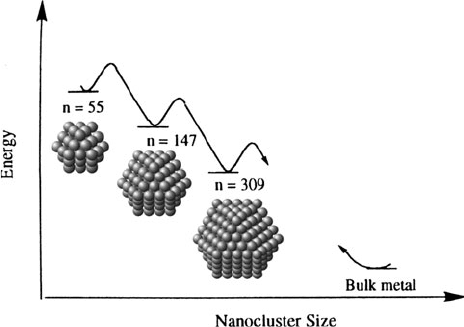
It should be noted that intermediate magic-number nanoclusters represent only local
minima in the potential energy surface, relative to the globa l minimum of a bulk
metal with the lowest possible surface area (Figure 6.55).
[165]
The high yield
of magic number nanoclusters is a consequence of kinetically controlled surface
growth. That is, once these favored intermediate structures are formed, they are less
reactive toward autocatalytic surface growth relative to non-magic number clusters.
Thus far, we have considered mechanisms to explain the relat ive sizes of 0-D
nanostructures, without consideration of their shapes. The observed shapes of
nanocrystalline facets may be controlled by either thermodynamics or kinetics.
For those reactions under thermodynamic control, growth will occur in such a way
to minimize the total interfacial free energy, g – i.e., the energy associated with the
nucleation/growth of a surface of a unit area. For a fcc structure, g {110} > g
{100} > g {111}; hence, a single crystal should prefer an octahedral structure to
maximize the numb er of (111) facets. However, as we have seen, a truncated
Figure 6.55. Comparative energy levels for “magic number” noble metal nanoclusters relative to bulk
metal. Also shown are the perfect fcc arrays of the nanoclusters, in which more than 75% of the atoms are
located on the surface. Adapted with permission from Finke, R. G. in Metal Nanoparticles: Synthesis,
Characterization, and Applications Feldheim, D. L.; Foss, C. A. eds., Dekker: New York, 2002.
Copyright 2002 Taylor & Francis.
6.3. Nanoscale Building Blocks and Applications 523
octahedron (TO) structure is often observed since a cube will have a lower g than an
octahedron of the same volume.
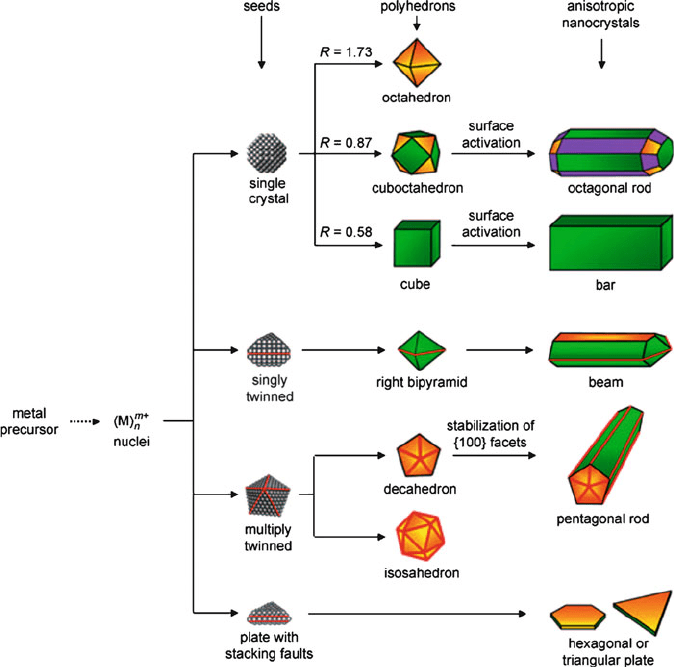
In contrast, to achieve kinetic control over the resultant nanostructure, one must
generally slow down the precursor decomposition/reduction while taking advantage
of Ostwald ripening. Once the atomic nucl eation and growth becomes slow, the
nanostructures will exhibit random hexagonal close-packing in addition to a variety
of stacking faults, leading to thermodynam ically-disfavored shapes (Figure 6.56).
Further, if twinned regions are present rather than single-crystal nuclei, one is able
Figure 6.56. Reaction pathways that give rise to varying shapes of fcc metal nanocrystals. First, a precursor
is reduced or decomposed to form the nuclei (small clusters). Once the nuclei have grown past a certain size,
they become seeds with a single-crystal, singly twinned, or multiply twinned structure. If stacking faults
are introduced, then plate-like seeds will be formed. The green, orange, and purple colors represent the {100},
{111}, and {110} facets, respectively. Twin planes are delineated in the drawing with red lines. The parameter
R is defined as the ratio between the growth rates along the <100> and <111> directions. Reproduced
with permission from Xiong, Y.; Xia, Y. Adv. Mater. 2007, 19, 3385. Copyright 2007 Wiley-VCH.
524 6 Nanomaterials
to guide the morphology into 1-D or 2-D arrays via selective oxidation of specific
crystal facets, or by the prefere ntial adsorption of entraining agents to
specific regions of the growing nanocrystal.
[166]
Self-assembly of 0-D nanostructures into arrays
Now that you understand how 0-D nanostructures are synthesized and stabilized, it
is worthwhile noting how these structures are aligned into more complex arrays.
As you recall, the “bottom-up” approach to materials design features the purposeful
placement of individual nanoarchitectures, in order to build specific functional
devices one unit at a time. Though one would have the greatest control over the
device properties through the growth/arrangement of individual atoms, current syn-
thetic methodology most easily yields nanoclusters/nanoparticles that consist of small
groups of atoms (N
atoms
~ 50+). Advanced lithographic techniques such as dip-pen
nanolithography (DPN), micro/nano-contact printing, focused-ion beam (FIB), and
deposition of nanoparticles within lithographically generated surface features have all
been used to pattern 0-D nanostructures into regular 2-D and 3-D arrays.
[167]
As we have seen, a stabilizing agent is used to prevent agglomeration of growing
0-D nanostructures. In fact, this component also provides an effective “handle”
to bind the nanostructure to a particular surface. Once appropriate reactive groups
(e.g., —OH, NH
2
) are placed on a surface through monolayer formation, the stabi-
lizing group surrounding the nanostructure spontaneously becomes chemisorbed
(Figure 6.57). If the nanostructures are encapsulated with stabilizing agents of
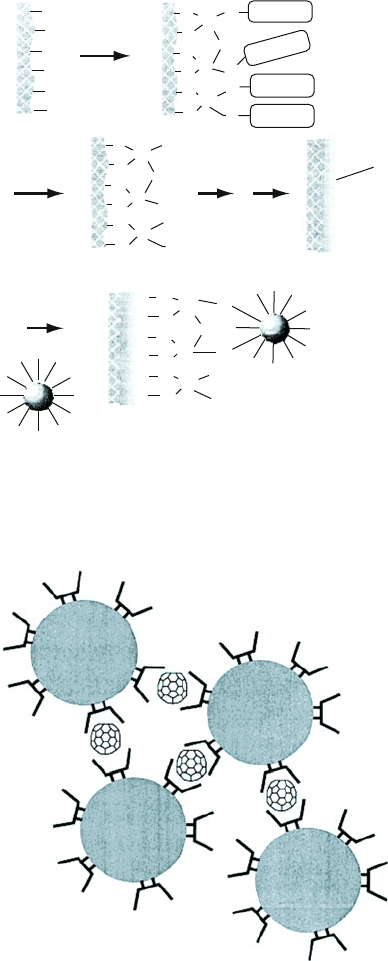
well-defined sizes, then the spacing between adjacent nanostructures will also be
highly ordered and predictable (Figure 6.58). Though this most often results in a
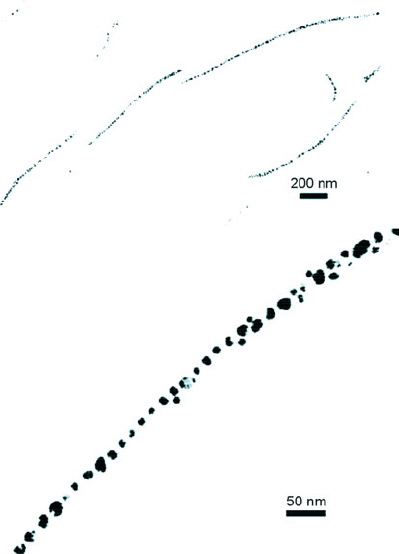
2-D matrix of nanostructures, it is also possible to create alignment into 1-D chains.
For instance, Au nanostructures that are stabilized by a long-chain thiolated poly
(ethylene oxide) polymer form a linear array via interactions with sulfate/acid groups
of a polysaccharide compound (chondroitin sulfate c, IX) (Figure 6.59). In addition to
chemisorptive methods, light actuation (e.g., optoelectronic tweezers, OET) and elec-
trostatic forces generated from biased p-n junction-patterned substrates have also been
an effective means to align metallic nanoparticles.
[168]
6.3. Nanoscale Building Blocks and Applications 525
TiO
2
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
HO
HO
HO
HO
Ti(On-Bu)
4
OO
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
Ti
Ti
Ti
Ti
Ti
Ti
Ti
Ti
Ti
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
HO
HO
HO
HO
HO
HO
OH
Hydrolysis
Gold
Nanoparticles
n -Bu
n -Bu
n -Bu
n
-Bu
Figure 6.57. Illustration of the chemisorption of a nanostructure onto a hydroxylated TiO
2
surface. The
hydroxyl groups on the periphery of the nanostructure stabilizing agent provide the handle for surface
adsorption. Reproduced with permission from Liu, S.; Zhu, T.; Hu, R.; Liu, Z. Phys. Chem. Chem. Phys.
2002, 4, 6059. Copyright 2002 PCCP Owners Societies.
Figure 6.58. Schematic of the controlled spacing between individually stabilized gold nanoparticles by
the formation of fullerene inclusion complexes. Reproduced with permission from Yonezawa, T.;
Matsune, H.; Kunitake, T. Chem. Mater. 1999, 11, 33. Copyright 1999 American Chemical Society.
526 6 Nanomaterials
An important precedent for the self-assembly of 0-D nanostructures into a 3-D
superlattice array was reported by O’Brien and coworkers.
[169]
In this work, oleic
acid stabilized g-Fe
2
O
3
nanoclusters were synthesized by the decomposition of Fe
(CO)
5
and subsequent oxidation using trimethylamine N-oxide within a high boiling
solvent (trioctylamine, b.p. 365
C). Depending on the relative size ratios and
concentrations of Fe
2
O
3
:PbSe QDs, AB
13
or AB
2
superlattice arrays were generated
(Figure 6.60) – isostructural to intermetallic compounds such as NaZn
13
and AlB
2
,
respectively. The superlattices were made by simply placing a substrate in a
suspension containing the two types of nanoparticles. Upon evaporation of the
solvent in a low-pressure chamber, the nanoparticles self-assembled into the ordered
arrays. Binary superlattices of this type that feature both magnetic and semiconduct-
ing QDs may have appl ications for data storage applications, whereas arrays of 2+
semiconductor QDs may be exploited for next-generation solar or thermoelectric
devices.
Figure 6.59. TEM images of a 1-D array of Au nanoparticles formed through the interaction of PEO and
polysaccharide chains. Reproduced with permission from Takagi, K.; Ishiwatari, T. Chem. Lett. 2002,
990. Copyright 2002 Chemical Society of Japan.
6.3. Nanoscale Building Blocks and Applications 527
