Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

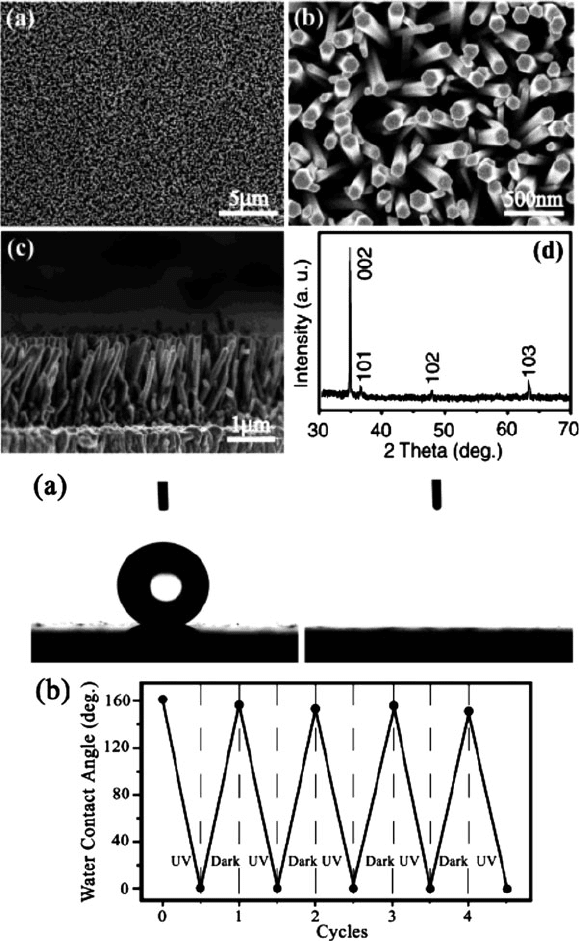
Figure 6.76. SEM images and XRD pattern of ZnO nanorod films, and photographs of water droplet
shapes on the aligned ZnO films before (left) and after (right) UV illumination. Bottom: the reversible
superhydrophobicity/superhydrophilicity character of the films is demonstrated by cycling with UV
irradiation/dark storage. Reproduced with permission from J. Am. Chem. Soc. 2004, 126, 62. Copyright
2004 American Chemical Society.
548 6 Nanomaterials
carbon growth initiates at approximately same time from multiple metal sites.
Subsequent growth is self-directed into a parallel array by interactions amongst
neighboring CNTs.
For some applications, a main drawback of CVD is that MWNTs are often
generated alongside SWNTs. Since the size of the catalyst governs the diameter of
resultant tubes, surface-immobilized nanoclusters of iron oxide@PAMAM dendri-
mer, with well-defined sizes, has been shown to yield only SWNTs with an
extremely narrow diameter distribution.
[201]
Two advanced CVD proce sses,
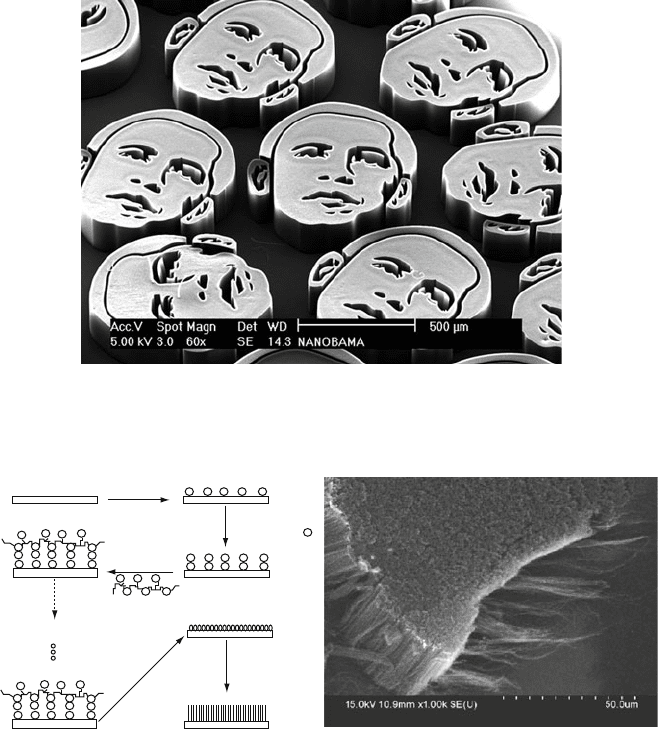
Figure 6.77. SEM image of ‘NanObamas’ comprised of vertically-aligned carbon nanotubes. Image
provided courtesy of Prof. John Hart, Dept. o f Mechanical Engineering, Univ. of Michigan.
−
−
−
−
− − − − −
−
− − −
− − − −
+ + + + +
+
−
−−−−
−
−
−−
−
+
+
++
+
−
−
−
−
−
−−−−
−
−
−−
−
+
+++
+
− − −
−
+
−
+
−
+
−
+
−
+
−
+
−
+
−
+
−
+
−
+
−
+
−
+
quartz
repeating
the LBL
process
plasma
PSS
CNT growth
iron colliod
deposition
CH
3
COOH
reducing
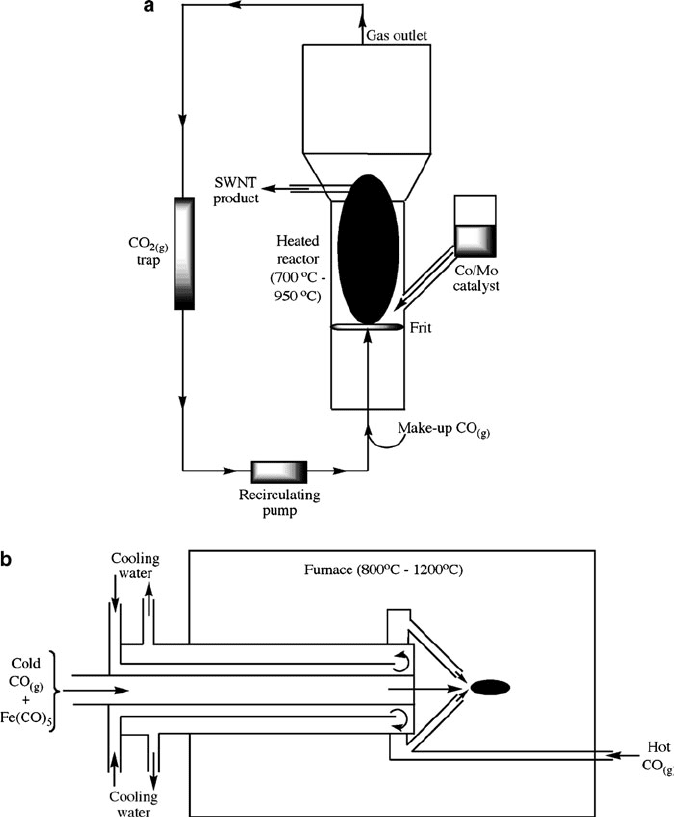
Figure 6.78. Schematic and cross-section SEM image of a layer-by-layer (LbL) approach to deposit iron
nanoclusters onto a surface to yield vertically aligned SWNTs. Reproduced with permission from Liu, J.;
Li, X.; Schrand, A.; Ohashi, T.; Dai, L. Chem. Mater. 2005, 17, 6599. Copyright 2005 American Chemical
Society.
6.3. Nanoscale Building Blocks and Applications 549
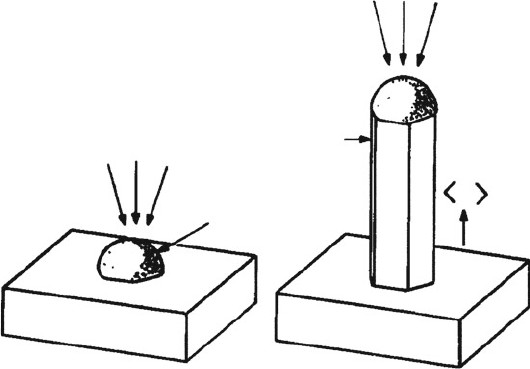
CoMoCAT
®
(fluidized-bed CVD) and HiPCO
®
(high-pressure CO CVD), have
recently been developed for the commercial production of SWNTs (Figure 6.79).
Though the experimental setup of these methods are significantly more complex
than standard hot-walled CVD, these techniques are still considered an extensi on of
Figure 6.79. Illustration of two methods used for the commercial production of SWNTs. Shown are
(a) the CoMoCat fluidized bed method using CO as the precursor and a Co/Mo bimetallic catalyst,
[204]
and
(b) the HiPco “floating catalyst” process using the thermal decomposition of iron pentacarbonyl at
pressures of 1–10 atm.
[205]
550 6 Nanomaterials
CVD, as the precursor is decomposed on the surface of the catalyst. Most
noteworthy about these and o ther recent CVD methods is that they offer the ability
to fine-tune resultant lengths, diameters, and even chiralities
[202]
by varying the
operating conditions. Think of the possibilities for applications, as it would not be
long until you can place an order such as: “10 g of pure (5,5) SWNTs with an
average diameter of 7 nm and length of 500 nm.” However, this degree of control
will only be possible once the exact growth mechanism is known, rather than current
efforts that invoke “blind” modifications of experimental variables.
[203]
Growth mechanisms of carbon nanotubes
Though CNT growth dates back to the early 1990s, details of the growth mechanism
are still unclear. The most commonly accepte d description is based on the vapor-
liquid-solid (VLS) model, which was first proposed for the growth of semiconductor
whiskers (Figure 6.80).
[206]
This model assumes that the catalyst liquefies , which
then acts as a preferential adsorption site for gaseous precursors. Subsequent growth
of the nanotubes/nanowires occurs by supersaturation of the catalyst droplet, and
precipitation at the liquid–solid interface.
[207]
However, the amount of carbon in a
supersaturated catalyst nanocluster will never be sufficient to account for the length
or number of nanostructures observed in practice. As a result, additional precursor
atoms must be supplied continuously to the catalyst nanocluster in order for sus-
tained growth to occur.
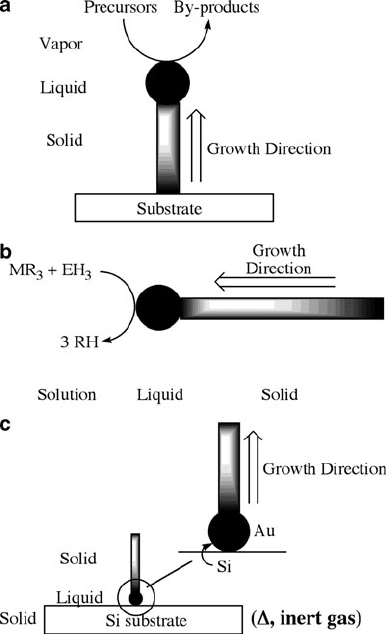
VAPOR
VAPOR
111
SILICON
CRYSTAL
SILICON SUBSTRATE
Au-Si LIQUID
ALLOY
a
b
Figure 6.80. The original schematic used to describe vapor–liquid–solid (VLS) growth of semiconductor
nanowires. Reproduced with permission from Wagner, R. S.; Ellis, W. C. Appl. Phys. Lett. 1964, 4, 89.
Copyright 1964 American Institute of Physics.
6.3. Nanoscale Building Blocks and Applications 551
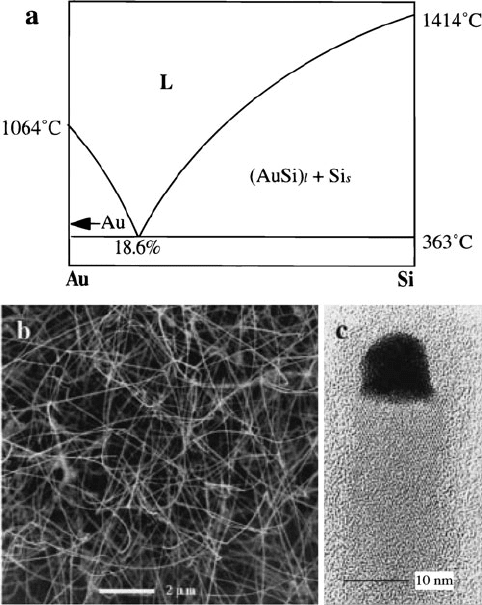
By examining the phase diagram for a binary system (precursor and catalyst
species), one can easily determine the best temperature range for VLS growth –
any value above the eutectic temperature, where the cat alyst remains a liquid
(Figure 6.81). As we saw earlier for gold nanoparticles, as the diameter decreases,
the melting point is significantly decreased relative to the bulk solids. As a result,
“size-corrected eutectics” must be determined for the specific nanopart icle diameter
in order to determine experimental conditions for nanowire growth.
The VLS mechanism is generally sufficient to model the growth of nanowires; in
addition, nanowire growth may occur in solution via solution-liquid-solid (SLS)
[208]
or via solid-liquid-solid
[209]
via simple annealing of substrate-immobilized nano-
clusters (Figure 6.82). However, the VLS route is som ewhat controversial in its
description of CNT growth, since there are many recent reports of CNTs being
Figure 6.81. Silicon nanowire growth from a gold nanocluster catalyst. Shown is (a) the phase diagram
for the Au/Si system, showing the eutectic temperature/composition; (b) SEM image; and (c) high-
resolution TEM image of the nanowires grown at a temperature of 450
C. The dark tip of the nanowire
is from the gold nanocluster. Reproduced with permission from Hu, J.; Odom, T. W.; Lieber, C. M. Acc.
Chem. Res. 1999, 32, 435. Copyright 1999 American Chemical Society.
552 6 Nanomaterials
grown at temperatures below the size-corrected eutectic,
[210]
or from non-melting
nanoclusters such as SiO
2
,Al
2
O
3
, etc.
[211]
Though small nanoparticles may exhibit
“fluid-like” behavior by altering their surface geometry, empirical data has shown
that the catalyst particle may remain crystalline, rather than an amorphous liquid
phase, duri ng growth. This suggests that nucleation may be dominated by the
catalyst surface – not unlike most heterogeneous catalysis processes (e.g.,
Ziegler–Natta polymerization).
[212]
As with any complicated system, it is much easier to study growth mechanisms
using computational techniques. For CNT growth, it has been shown that small
graphitic islands form on the supersaturated catalyst surface. When the island cover s
half of the catalyst particle, it lifts off and forms the SWNT – with the same diameter
as the catalyst. Based on empirical data and theoretical predictions,
[213]
the catalytic
ability of the metal/alloys for SWNT growth follows the order: Ni/Mo > Ni/Cr >
Figure 6.82. Schematic illustrations of (a) VLS, (b) SLS: solution-liquid-solid, and (c) SLS: solid-liquid-
solid 1-D nanostructural growth.
6.3. Nanoscale Building Blocks and Applications 553
Ni/Co > Ni/Pt > Ni/Rh > Ni/Fe > Ni > Fe/Mo > Fe/Cr > Fe/Co > Fe/Pt > Fe/Rh
> Fe > Ni/Mo > Fe/Mo > Co/Mo > Co > Pt > Cu. Depending on whethe r the
mechanism proceeds via VLS or surface-addition mechanism, the above order may
be related to the ease of carbide formation and carbon diffusion through the
nanocluster interior, or the morphology/composition of the catalyst surface and
rate of carbon diffusion on the catalyst surface, respectively.
[214]
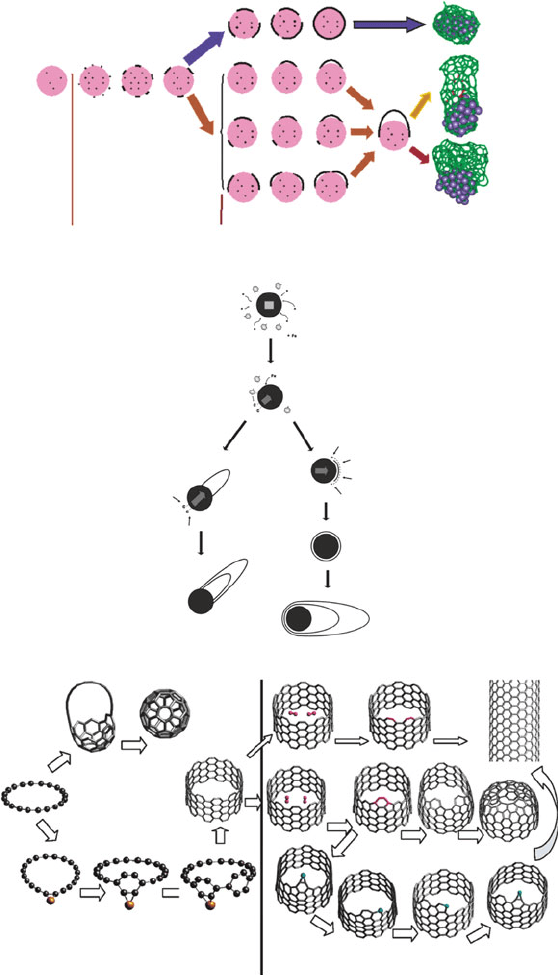
Figure 6.83
shows some recent mechanistic proposals, based on experimental and theoretical
data. The VLS-based proposals (Figure 6.83, top and middle) illustrate the following
basic steps:
(i) At initial growth stages, carbon dissolves in the (semi)-molten “liquid-like”
catalytic nanocluster .
[216]
Calculations indicate that there is a dynamic process
of carbon precipitation onto the catalyst surface and re-dissolution, until a
highly supersaturated catalyst is obtained.
(ii) Carbon precipitates on the surface of the highly supersaturated catalyst
nanoclusters, forming carbon strings/polygons. This causes a decrease in the
dissolved carbon concentration.
(iii) The carbon nuclei form graphitic islands on the surface of the catalyst, which
aggregate into larger graphitic clusters.
(iv) At low temperatures, the graphitic islands are not able to lift off the catalyst
surface, resulting in graphite-encapsulated metal nanoclusters.
[217]
(v) At relatively high temperatures (ca. 500–1,200
C), when the diameter of the
island becomes ca. 1/2 that of the catalyst, the graphitic nucleus lifts off the
catalyst surface to form the SWNT endcap. Subsequent graphitization and
growth propagation of the SWNT may occur through two routes (Figure 6.83 –
middle):
(v.1) “Root (base) Growth” (c–d): carbon atoms migrate through the catalyst
nanocluster and precipitate from the liquid-like catalyst to the open end
of the growing CNT. This route has recently been confirmed for
MWNT growth via in situ TEM measurements, from Fe
3
C catalyst
nanoclusters (Figure 6.84 – top) .
[218]
Recent theoretical
[219]
and empir-
ical
[220]
studies have addressed the question of how growth occurs
at the atomic level, described by a ‘screw-dislocation-like’ (SDL)
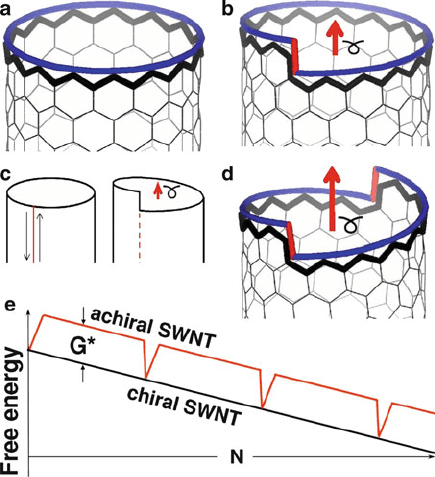
mechanism (Figure 6.85).
[221]
(v.2) “Tip (folded) Gr owth” (e–g): carbon atoms precipitate directly onto the
catalyst surface, and are added to the graphitic endcap. This route has
not been demonstrated for CNTs, but has been shown to occur for
carbon nanofiber (CNF) growth using in situ TEM measurements
(Figure 6.84 – bottom).
[222]
In contrast, a surface-governed route is characterized by negligible carbon dissolu-
tion in the catalyst bulk. Since the growth is not propos ed to occur through
supersaturation/precipitation, any species that are chemisorbed onto the catalyst
surface will have a dramatic influence on SWNT growth. Figure 6.83 (bottom)
shows a proposed route for a surface-based SWNT growth mechanism, which
progresses through the continual addition of C
2
units onto the leading edge of the
554 6 Nanomaterials
Low Temperature
Intermediate
Temperature
High
Temperature
Supersaturated
Highly
Supersaturated
Unsaturated
(a)
(b)
(c)
(d)
(f)
(g)
(e)
C
60
Initial tube
Nucleation stage Growth stage
(A)
(B)
(C)
(D)
(E)
F
e
Figure 6.83. Comparison of a VLS-based mechanism (top and middle) and a surface-mediated route
[215]
(see text for details).
6.3. Nanoscale Building Blocks and Applications 555
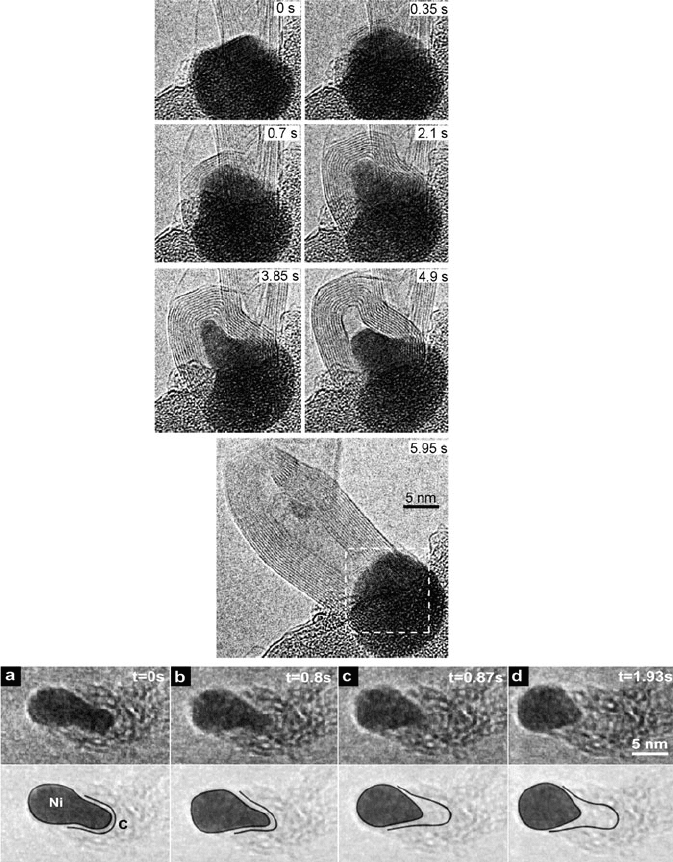
Figure 6.84. Nucleation and growth of a MWNT from a nanoparticulate catalyst (NPC) on a substrate.
(a) Graphene layers are formed on a NPC and then a MWNT is suddenly expelled from the deformed
NPC. The recording time is shown in images. Copyright: Yoshida, H.; Takeda, S.; Uchiyama, T.; Kohno,
H.; Homma, Y. Nano Lett. 2008, 8, 2082. (a–d) ETEM image sequence showing a growing CNF in 3:1
NH
3
:C
2
H
2
at 1.3 mbar and 480
C. The video was recorded at 30 frames/s, and the time of the respective
stills is indicated. Drawings (lower row) indicate schematically the Ni catalyst deformation and C-Ni
interface. Hofmann, S.; Sharma, R.; Ducati, C.; Du, G.; Mattevi, C.; Cepek, C.; Cantoro, M.; Pisana, S.;
Parvez, A.; Cervantes-Sodi, F.; Ferrari, A. C.; Dunin-Borkowski, R.; Lizzit, S.; Petaccia, L.; Goldoni, A.;
Robertson, J. Nano Lett. 2007, 7, 602.
556 6 Nanomaterials
growing nanotube. As indicated in this scheme, the absence of an active catalyst will
result in the formati on of fullerenes rather than SWNTs. In this route, nanotube
growth is proposed to initiate from surface metal atoms adding to the edge of a
polyyne ring, which is subsequently able to add additional carbon atoms.
Whereas path C of Figure 6.83 (bottom) shows the successful addition of C
2
units
at the growth edge, path D shows the formation of a defect, which leads to tube
closure. It should be noted that this route is 1.3 eV more favorable than route C when
there is no metallic catalyst present, thus explaining the preference for closed-cage
fullerenes. Most likely, this is a consequence of pentagonal defe cts that are formed
when carbon atoms add to the open end of the growing SWNT. Hence, calculations
have shown that catalyst-assisted defect repair is a crucial step in the overall
mechanism (Figure 6.83 – bottom, route E). This is proposed to occur through a
“scooter mechanism,”
[223]
whereby the catalyst atoms diffuse along the open-end
surface of the SWNT, facilitating the formation of hexagons that prevent premature
nanotube closure.
Figure 6.85. Illustration of an axial screw dislocation in a CNT. An achiral zigzag (n, 0) tube (a) can be
viewed as a perfect crystal, and transformed into a chiral one by cutting, shifting by a Burgers vector
(red arrows in b–d), and resealing a tube-cylinder (b). The free-energy profile shown in (e) illustrates
the difference in growth of a chiral or achiral CNT with an increasing number of added carbon
atoms. Reproduced with permission from Ding, F.; Harutyunyan, A. R.; Yakobson, B. I. PNAS 2009,
106, 2506.
6.3. Nanoscale Building Blocks and Applications 557
