Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

204
Resasco, D. E.; Alvarez, W. E.; Pompeo, F.; Balzano, L.; Herrera, J. E.; Kitiyanan, B.; Borgna, A.
J. Nanopart. Res. 2002, 4, 131.
205
Nikolaev, P.; Bronikowski, M. J.; Bradley, R. K.; Rohmund, F.; Colbert, D. T.; Smith, K. A.;
Smalley, R. E. Chem. Phys. Lett. 1999, 313, 91. It should be noted that Fe(CO)
5
is not the only
system in which the precursor acts as the metal catalyst and carbon source. A number of metallo-
cenes (e.g., ferrocene, cobaltocene, and nickelocene) have also been used; however, they typically
result in MWNT growth rather than SWNTs. This is most likely due to the larger number of carbon
atoms from cyclopentadienyl groups that must self-assemble, relative to smaller carbon precursors
(e.g., CH
4
,C
2
H
2
, etc.) used for SWNT growth.
206
Wagner, R. S.; Ellis, W. C. Appl. Phys. Lett. 1964, 4, 89. For a recent review of the solid–liquid–
solid (SLS) and supercritical fluid–liquid–solid (SFLS) mechanisms for semiconductor nanowire
growth, see: Wang, F.; Dong, A.; Sun, J.; Tang, R.; Yu, H.; Buhro, W. E. Inorg. Chem. 2006, 45,
7511. A recent precedent for the epitaxial growth of ZnO nanowires at the junction of nanowalls: Ng,
H. T.; Li, J.; Smith, M. K.; Nguyen, P.; Cassell, A.; Han, J.; Meyyappan, M. Science 2003, 300,
1249.
207
For a review of CNT growth mechanisms, see: Harris, P. J. F. Carbon 2007, 45, 229. A recent in situ
TEM study of Si nanowire growth has been reported by Hofmann, S.; Sharma, R.; Wirth, C. T.;
Cervantes-Sodi, F.; Ducati, C.; Kasama, T.; Dunin-Borkowski, R. E.; Drucker, J.; Bennett, P.;
Robertson, J. Nature Mater. 2008, 7, 372.
208
The first precedent of solution-liquid-solid (SLS) growth of nanowires: Trentler, T. J.; Hickman, K.
M.; Goel, S. C.; Viano, A. M.; Gibbons, P. C.; Buhro, W. E. Science, 1995, 270, 1791.
209
For instance, see: Paulose, M.; Varghese, O. K.; Grimes, C. A. J. Nanosci. Technol. 2003, 3, 341.
210
For example, see: Adhikari, H.; Marshall, A. F.; Chidsey, E. D.; McIntyre, P. C. Nano Lett. 2006, 6,
318.
211
(a) Huang, S.; Cai, Q.; Chen, J.; Qian, Y.; Zhang, L. J. Am. Chem. Soc. 2009, 131, 2094, and
references therein. (b) Liu, B.; Ren, W.; Gao, L.; Li, S.; Pei, S.; Liu, C.; Jiang, C.; Cheng, H. -M.
J. Am. Chem. Soc. 2009, 131 , 2082.
212
Cantoro, M.; Hofmann, S.; Pisana, S.; Scardaci, V.; Parvez, A.; Ducati, C.; Ferrari, A. C.; Blackburn,
A. M.; Wang, K.-Y.; Robertson, J. Nano Lett. 2006, 6, 1107.
213
Deng, W.-Q.; Xu, X.; Goddard, W. A. Nano Lett. 2004, 4, 2331.
214
It has been shown that reducing the catalyst size causes an increase in the growth rate, whereas
varying the catalyst composition affects the growth rate, activation energy, and onset temperature for
CNT growth: Chiang, W. -H.; Sankaran, R. M. Diam. Rel. Mater. 2009, 18, 946. For the influence of
catalyst morphology on CNT growth termination, see: Kim, S. M. et al. J. Phys. Chem. Lett. 2010, 1,
918, and references therein.
215
The top VLS mechanism was predicted using molecular dynamics calculations. The image was
reproduced with permission from Ding, F.; Bolton, K.; Rosen, A. J. Phys. Chem. B 2004, 108,
17369. The middle VLS mechanism shows both “root growth” (c–d) and “folded growth” (e–g). The
image was reproduced with permission from Lee, D. C.; Mikulec, F. V.; Korgel, B. A. J. Am. Chem.
Soc. 2004, 126, 4951. The bottom mechanism, predicted by quantum mechanics/molecular mechan-
ics, is one of the first examples of an atomic-level picture of CNT growth. The image was reproduced
with permission from Deng, W.-Q.; Xu, X.; Goddard, W. A. Nano Lett. 2004, 4, 2331. Other atomic-
level mechanistic calculations: (a) Wang, Q.; Ng, M. -F.; Yang, S. -W.; Chen, Y. ACS Nano 2010, 4,
939; (b) Page, A. J.; Irle, S.; Morokuma, K. J. Phys. Chem. C 2010, 114, 8206.
216
Considering a VLS growth mechanism, the catalyst nanocluster must be molten during nucleation.
However, the growth temperature of carbonaceous nanostructures is much lower than the melting
point of binary C/M systems (M ¼ catalytic metals such as Fe, Ni, Co, etc.), which lends credence to
the existence of “liquid-like” deformable nanoclusters during growth.
217
Graphite-encapsulated metal nanostructures are of increasing importance for magnetic applications
such as high-density magnetic recording media; for example, see: Flahaut, E.; Agnoli, F.; Sloan, J.;
O’Connor, C.; Green, M. L. H. Chem. Mater. 2002, 14, 2553, and references therein. Encapsulation
dominates over CNT growth at low temperatures since the kinetic energy is not sufficient for
578 6 Nanomaterials
graphitic islands to lift off the catalyst surface. Hence, encapsulation may easily be limited, which
enhances CNT growth, by maintaining elevated temperatures. Experimental results also show that
small catalyst nanoclusters (diameters <2 nm) are free of graphite encapsulation since they do not
contain a sufficient number of dissolved C atoms. However, for metal nanostructures >3nmin
diameter, calculations suggest that graphite encapsulation is thermodynamically preferred over
SWNT growth. This is confirmed by the empirical observation that SWNTs form only on catalyst
particles with diameters <2 nm.
218
For example, see: (a) Lin, M.; Tan, J. P. Y.; Boothroyd, C.; Loh, K. P.; Tok, E. S.; Foo, Y. -L. Nano
Lett. 2006, 6, 449. (b) Yoshida, H.; Takeda, S.; Uchiyama, T.; Kohno, H.; Homma, Y. Nano Lett.
2008, 8, 2082.
219
Ding, F.; Harutyunyan, A. R.; Yakobson, B. I. Proc. Nat’l Acad. Sci. 2009, 106, 2506.
220
Using field-emission microscopy (FEM) to observe axial rotation and preferential adsorption of
dimeric C
2
units during growth: Marchand, M.; Journet, C.; Guillot, D.; Benoit, J. -M.; Yakobson, B.
I.; Purcell, S. T. Nano Lett. 2009, 9, 2961.
221
Jin, S.; Bierman, M. J.; Morin, S. A. J. Phys. Chem. Lett. 2010, 1, 1472, and references therein.
222
Hofmann, S.; Sharma, R.; Ducati, C.; Du, G.; Mattevi, C.; Cepek, C.; Cantoro, M.; Pisana, S.;
Parvez, A.; Cervantes-Sodi, F.; Ferrari, A. C.; Dunin-Borkowski, R.; Lizzit, S.; Petaccia, L.;
Goldoni, A.; Robertson, J. Nano Lett. 2007, 602.
223
Lee, Y. H.; Kim, S. G.; Jund, P.; Tomanek, D. Phys. Rev. Lett. 1997, 78, 2393.
224
A recent paper by Hata et al. discusses the variables that govern highly-efficient carbon nanotube
growth – an oxygen-containing “growth enhancer” (e.g., water, alcohols), and a carbon source not
containing oxygen: Futaba, D. N.; Goto, J.; Yasuda, S.; Yamada, T.; Yumura, M.; Hata, K. Adv.
Mater. 2009, 21, 4811.
225
Hata, K.; Futaba, D. N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Science 2004, 306, 1362.
226
Rummeli, M. H.; Borowiak-Palen, E.; Gemming, T.; Pichler, T.; Knupfer, M.; Kalbac, M.; Dunsch,
L.; Jost, O.; Silva, S. R. P.; Pompe, W.; Buchner, B. Nano Lett. 2005, 5, 1209.
227
(a) For a recent review of inorganic-based nanotubes, see: Goldberger, J.; Fan, R.; Yang, P. Acc.
Chem. Res. 2006, 39, 239, and references therein. (b) Mukherjee, S. Synthesis, Characterization and
Growth Mechanism of Single-Walled Metal Oxide Nanotubes, Ph.D. Dissertation (Chem. Eng.),
Georgia Institute of Technology, August 2007. May be accessed online at: http://etd.gatech.edu/
theses/available/etd-06302007-202542/unrestricted/mukherjee_sanjoy_200708_phd.pdf
228
Lu, J. G.; Chang, P. C.; Fan, Z. Y. Mater. Sci. Eng. Rep. 2006, 52, 49.
229
Hulteen, J. C.; Martin, C. R. J. Mater. Chem. 1997, 7, 1075.
230
For example, see: (a) Wang, J. M.; Gao, L. J. Mater. Chem. 2003, 13, 2551. (b) Liu, B.; Zeng, H. C.
J. Am. Chem. Soc. 2003, 125, 4430.
231
Mukherjee, S.; Bartlow, V. M.; Nair, S. Chem. Mater. 2005, 17, 4900.
232
(a) Kamat, P. V. J. Phys. Chem. C 2008, 112, 18737, and references therein. (b) Bang, J. H.; Kamat,
P. V. ACS Nano 2009, 3, 1467. (c) Farrow, B.; Kamat, P. V. J. Am. Chem. Soc. 2009, 131, 11124.
233
(a) Baker, D. R.; Kamat, P. V. J. Phys. Chem. C 2009, 113, 17967. (b) Yu, P.; Zhu, K.; Norman, A.
G.; Ferrere, S.; Frank, A. J.; Nozik, A. J. J. Phys. Chem. B 2006, 110, 25451. (c) Wen, X.; Junchao,
T.; Sun, Y.; Sun, Y.; Dai, N. Proc. SPIE 2009, 7381, 73810Z.
234
(a) Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.;
Grigorieva, I. V.; Firsov, A. A. Science 2004, 306, 666.(b) Novoselov, K. S.; Jiang, D.; Schedin,
F.; Booth, T. J.; Khotkevich, V. V.; Morozov, S. V.; Geim, A. K. Proc. Natl. Acad. Sci. U.S.A. 2005,
102, 10451.
235
Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Katsnelson, M. I.; Grigorieva, I. V.;
Dubonos, S. V.; Firsov, A. A Nature 2005, 438, 197.
236
Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov,
A. N.; Conrad, E. H.; First, P. N.; de Heer, W. A. Science 2006, 312, 1191.
237
Graphite intercalation compounds and applications, Endo, M., Ed.; Oxford University Press:
Oxford, U.K., 2003.
References 579
238
Bulk graphite oxide is best prepared from purified natural graphite using the Hummers method:
Hummers, W. S. & Offeman, R. E. J. Am. Chem. Soc. 1958, 80, 1339. For the mechanical exfoliation
of bulk graphene oxide into sheets within an aqueous medium, see: Stankovich, S.; Dikin, D. A.;
Dommett, G. H. B.; Kohlhaas, K. M.; Zimney, E. J.; Stach, E. A.; Piner, R. D.; Nguyen, S. T.; Ruoff,
R. S. Nature 2006, 442, 282.
239
(a) He, H., Klinowski, J., Forster, M. & Lerf, A. Chem. Phys. Lett. 1998, 287, 53. (b) Lerf, A., He, H.,
Forster, M. & Klinowski, J. Structure of graphite oxide revisited. J.Phys.Chem.B102,4477–4482
(1998).
240
For example, see: (a) Tung, V. C.; Allen, M. J.; Yang, Y.; Kaner, R. B. Nat. Nanotechnol. 2009, 4,
25. (b) Allen, M. J.; Fowler, J. D.; Tung, V. C.; Yang, Y.; Weiller, B. H.; Kaner, R. B. Appl. Phys.
Lett. 2008, 93, 193119. (c) Fowler, J. D.; Allen, M. J.; Tung, V. C.; Yang, Y.; Kaner, R. B.; Weiller,
B. H. ACS Nano 2009, 3, 301.
241
(a) Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M. S.; Kong, J. Nano
Lett. 2009, 9, 30. (b) Kim, K. S.; Zhao, Y.; Jang, H.; Lee, S. Y.; Kim, J. M.; Kim, K. S.; Ahn, J. -H.;
Kim, P.; Choi, J. -Y.; Hong, B. H. Nature 2009, 457, 706.
242
For a recent review of graphene properties and applications, see: Allen, M. J.; Tung, V. C.; Kaner, R.
B. Chem. Rev. 2009, 21, 3056.
243
The quantum Hall effect is the integer quantization of the Hall conductance for a 2-D system
subjected to low temperatures and strong magnetic fields. However, for graphene, an anomalous
fractional quantization is observed in which electrons exhibit much smaller effective masses. This
corresponds to extremely facile transport through graphene, which is analogous to the mobility of
relativistic particles such as photons. For example, see: (a) http://icmt.illinois.edu/ICMT-talks/
20080114-Abanin-Talk.pdf. (b) Toke, C.; Lammert, P. E.; Crespi, V. H.; Jain, J. K. Phys. Rev. B
2006, 74, 235417. (c) Novoselov, K. S.; Jiang, Z.; Zhang, Y.; Morozov, S. V.; Stormer, H. L.;
Zeitler, U.; Maan, J. C.; Boebinger, G. S.; Kim, P.; Geim, A. K. Science 2007, 315, 1379. (d)
Ozyilmaz, B.; Jarillo-Herrero, P.; Efetov, D.; Abanin, D. A.; Levitov, L. S.; Kim, P. Phys. ReV. Lett.
2007, 99, 186804.
244
For instance, see: Bolotin, K. I.; Sikes, K. J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.;
Stormer, H. L. Solid State Commun. 2008, 146, 351.
245
Li, D.; Kaner, R. B. Science 2008, 320, 1170.
246
Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. J. Am. Chem. Soc. 2008, 130, 5856.
247
Stankovich, S.; Piner, R. D.; Chen, X. Q.; Wu, N. Q.; Nguyen, S. T.; Ruoff, R. S. J. Mater. Chem.
2006, 16, 155.
248
Bon, S. B.; Valentini, L.; Verdejo, R.; Garcia Fierro, J. L.; Peponi, L.; Lopez-Manchado, M. A.;
Kenny, J. M. Chem. Mater. 2009, 21, 3433.
249
Note: the term ‘zero-bandgap semiconductor’ should be distinguished from a metal, even though
both of these materials exhibit zero bandgaps. Within a graphene sheet, the Dirac point represents
the sole electronic state responsible for its electrical conductivity; in contrast, electrons in many
other states within the vicinity of the Fermi level may participate in electrical conductivity.
250
http://www-mtl.mit.edu/researchgroups/palacios/graphene/palacios.html; Ambipolarity is also
shared by organic semiconductors: a) Chua, L. L.; Zaumseil, J.; Chang, J. F.; Ou, E. C. W.; Ho, P.
K. H.; Sirringhaus, H.; Friend, R. H. Nature 2005, 434, 194. (b) Meijer, E. J.; De Leeuw, D. M.;
Setayesh, S.; van Veenendaal, E.; Huisman, B. H.; Blom, P. W. M.; Hummelen, J. C.; Scherf, U.;
Klapwijk, T. M. Nat. Mater. 2003, 2, 678.
251
Zhang, Y.; Tang, T. -T.; Girit, C.; Hao, Z.; Martin, M. C.; Zettl, A.; Crommie, M. F.; Shen, Y. R.;
Wang, F. Nature 2009, 459, 820.
252
Zhang, Y.; Tan, Y. W.; Stormer, H. L.; Kim, P. Nature 2005, 438, 201.
253
(a) Son, Y. -W.; Cohen, M. L.; Louie, S. G. Phys. Rev. Lett. 2006, 97, 216803. (b) Han, M. Y.;
Ozyilmaz, B.; Zhang, Y.; Kim, P. Phys. Rev. Lett. 2007, 98, 206805.
254
http://www.lbl.gov/enews/back-issues/2007/Feb/SABLSelectZigzag.pdf
580 6 Nanomaterials
255
(a) Bhardwaj, T.; Antic, A.; Pavan, B.; Barone, V.; Fahlman, B. D. J. Am. Chem. Soc. 2010, 132,
12556. (b) Uthaisar, C.; Barone, V. Nano Lett. 2010, 10, 2838.
256
Uthaisar, C.; Barone, V.; Peralta, J. E. J. Appl. Phys. 2009, 106, 113715.
257
Kosynkin, D. V.; Higginbotham, A. L.; Sinitskii, A.; Lomeda, J. R.; Dimiev, A.; Price, B. K.; Tour,
J. M. Nature 2009, 458, 872.
258
Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H. Nature 2009, 458, 877.
259
(a) Han, M. Y.; Oezyilmaz, B.; Zhang, Y.; Kim, P. Phys. Rev. Lett. 2007, 98, 206805. (b) Chen, Z.;
Lin, Y. -M.; Rooks, M. J.; Avouris, P. Physica E 2007, 40, 228.
260
Campos-Delgado, J. et al. Nano Lett. 2008, 8, 2773.
261
Yang, X.; Dou, X.; Rouhanipour, A.; Zhi, L.; Joachim Rader, H.; Mullen, K. J. Am. Chem. Soc.
2008, 130, 4216.
262
Zaumseil, J.; Meitl, M. A.; Hsu, J. W. P.; Acharya, B. R.; Baldwin, K. W.; Loo, Y.-L.; Roger, J. A.
Nano Lett. 2003, 3, 1223.
263
Ginger, D. S.; Zhang, H.; Mirkin, C. A. Angew. Chem. Int. Ed. 2004, 43, 30. This review contains all
of the references for the various experimental conditions.
264
Yang, Y. T.; Callegari, C.; Feng, X. L.; Ekinci, K. L.; Roukes, M. L. Nano Lett. 2006, 6, 583.
265
Collin, J.-P.; Dietrich-Buchecker, C.; Gavina, P.; Jimenez-Molero, M.; Sauvage, J.-P. Acc. Chem.
Res. 2001, 34, 477.
266
Geeves, M. A. Nature 2002, 415, 129.
267
Liu, Y.; Flood, A. H.; Bonvallet, P. A.; Vignon, S. A.; Northrop, B. H.; Tseng, H.-R.; Jeppesen, J. O.;
Huang, T. J.; Brough, B.; Baller, M.; Magonov, S.; Solares, S. D.; Goddard, W. A.; Ho, C.-M.;
Stoddart, J. F. J. Am. Chem. Soc. 2005, 127, 9745.
Topics for Further Discus sion
1. From your knowledge of semiconductor and metallic 0-D nanostructures, think about how you would
design a coating that would sense the surrounding wall colors of walls and adapt its color to match
(i.e., color-adapting furniture!).
2. Mercedes-Benz and other manufacturers feature scratch-resistant clear coats as standard on new
vehicles. What are these coatings comprised of, and how does this prevent surface scratching?
3. Carbon nanotubes have been touted as being useful to store large amounts of hydrogen gas for fuel
cell applications. From the literature, how is this contained – within the interior or adsorbed along the
sidewall?
4. What are some examples of “self-cleaning” coatings? How do these work?
5. What factors govern the tilt angle (between the substrate and alkyl chain) of a SAM on a gold or silver
surface?
6. The growth mechanism of carbonaceous nanostructural materials is generally thought to be via VLS.
Cite some recent examples of carbon nanotube/nanofiber growth at temperatures far below the
melting point of the nanoparticulate catalyst species.
7. Interestingly, 1-D nanostructures such as nanocrystals or nanowires may also be formed on the reactor
sidewalls by laser ablation. Explain the growth mechanism of these structures, as opposed to more
commonly-formed 0-D nanostructures within these systems.
8. In the Finke four-step mechanism for nanocluster growth, explain why higher temperatures are most
conducive for the growth of monodisperse nanoclusters.
9. Think of a new device that you could fabricate by DPN that would be comprised of both nanoclusters
and nanotubes/nanowires. What are some potential applications for this device?
10. How would one utilize inert gas evaporation to pattern micron-sized arrays of 0-D nanoparticles?
11. How would you synthesize free-standing nanorings using both the top-down and bottom-up
approaches?
References 581
12. Calculate the total number of atoms AND number of surface atoms contained within a Fe nanoparticle
with a diameter of 10 nm. Calculate the number of C atoms in a (10,0) SWNT with a length of 25 mm
and diameter of 1.5 nm.
13. How would you synthesize metal oxide nanotubes, using a sacrificial template? Cite any related
precedents from the literature.
14. Describe how the band diagram of silicon nanowires (SiNWs) differs from bulk Si (see Chapter 4).
Does this imply the use of SiNWs for applications that differ from bulk Si? Elaborate.
15. What are some examples of NEMS devices currently under development?
16. What are the major developmental efforts underway to power nanodevices?
17. How could you deposit a square grid of TiO
2
nanowires with a controllable spacing between adjacent
nanowires?
18. Describe how LbL may be used to deposit coatings onto complex, nonplanar substrates. Cite
examples for this strategy from the literature.
19. Cite examples of materials designs where both “top-down” and “bottom-up” approaches were used.
20. Explain the phenomena of “optical tweezers” and “nano pens”, and describe how these techniques
have been used to assemble nanostructures on various surfaces.
21. There are recent reports that N-doped CNTs are less toxic than SWNTs or MWNTs (e.g., Nano Lett.
2006, 6, 1609). Provide some likely rationales for the varying toxicological effects for these doped
nanostructures.
22. Describe a technique (with illustrations of your experimental setup, if necessary) to synthesize the
following heterostructures. FOR EACH, describe how you control the diameter of the core and
thickness of the shell. (a) a 0-D quantum dot comprised of a CdSe core and GaP shell; (b) a 1-D
nanowire comprised of a CdSe core and GaP shell.
23. Explain how light has been used to assemble 0-D nanostructures into 2-D arrays on planar substrates.
24. Describe a “top-down” and “bottom-up” approach to synthesizing 0-D nanostructures.
25. Describe the Vapor-Liquid-Solid, Solution-Liquid-Solid, and Solid-Liquid-Solid synthetic routes for
1-D nanostructural growth. Make sure you discuss the experimental setup and required precursor(s)
for each technique, as well as the morphological control (i.e., control over thickness, length, chirality,
etc.) one would have for each technique.
Further Reading
1. http://cohesion.rice.edu/centersandinst/icon/virtualjournal.cfm (The Virtual Journal of Nano-
technology Environment, Health and Safety)
2. http://www.nanohub.org, http://www.azonane.com (free registration)
3. http://www.nanowerk.com (updated news stories related to nanotechnology).
4. Springer Handbook of Nanotechnology, Bhushan, B. ed., 2nd ed., Springer: New York, 2007.
5. Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications, Imperial Col-
lege Press: London, U.K., 2004.
6. Ozin, G. A.; Arsenault, A. C.; Cademartiri, L. Nanochemistry: A Chemical Approach to Nanomater-
ials, 2nd ed., Royal Society of Chemistry Publishing: Cambridge, U.K., 2009.
7. Kostarelos, K. Nanomedicine, Pharmaceutical Press: New York, 2010.
8. Dresselhaus, M. S.; Dresselhaus, G.; Eklund, P. C. Science of Fullerenes and Carbon Nanotubes,
Academic: New York, 1996.
9. Dendrimers and Dendritic Polymers, Frechet, J. M. J.; Tomalia, D. A. eds., Wiley: New York, 2001.
10. Advanced Materials 2004, 16, 15. Special issue dedicated to George Whitesides.
11. MRS Bulletin 2006, 31, 5 – May 2006. Special issue on materials for magnetic data storage.
12. MRS Bulletin 2005, 30, 12 – December, 2005. Special issue on fabrication of sub-45-nm structures
for the next generation of devices.
13. Madou, M. J. Fundamentals of Microfabrication, 2nd ed., CRC: Boca Raton, 2002.
14. Alternative Lithography – Unleashing the Potentials of Nanotechnology, Sotomayor-Torres, C. M.
ed., Kluwer/Plenum: New York, 2003.
582 6 Nanomaterials
15. Wilson, M.; Kannangara, K.; Smith, G.; Simmons, M. Nanotechnology: Basic Science and Emerging
Technologies, CRC: Boca Raton, 2002.
16. Carbon Nanotubes, O’Connell, M. J. ed., CRC: Boca Raton, 2006.
17. Carbon Nanotubes: Synthesis, Structure, Properties and Applications, Smalley, R. E.; Dresselhaus,
M. S.; Dresselhaus, G.; Avouris, P. eds., Springer: New York, 2001.
18. Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications,
Jorio, A.; Dresselhaus, G.; Dresselhaus, M. S. eds, Springer: New York, 2008.
19. D. Tomanek. http://www.pa.msu.edu/cmp/csc/nanotube.html. The Nanotube Site (Internet refer-
ence).
20. Lyshevski, S. E.; Lyshevski, S. E. MEMS and NEMS: Systems, Devices, and Structures, CRC: Boca
Raton, 2002.
21. Nanoparticles: From Theory to Application, Schmid, G. ed., Wiley: New York, 2004.
22. Rotello, V. Nanoparticles: Building Blocks for Nanotechnology (Nanostructure Science and
Technology), Springer: New York, 2004.
23. The Chemistry of Nanomaterials: Synthesis, Properties, and Applications, Rao, C. N. R.; Muller, A.;
Cheetham, A. K. eds., Wiley-VCH: Berlin, 2004.
24. Wolf, E L. Nanophysics and Nanotechnology, Wiley-VCH: Berlin, 2004.
References 583

CHAPTER 7
MATERIALS CHARACTERIZATION
Thus f ar, we have focused on the relatio nship between the structure of a material
and its properties/applications. However, we have not yet focused on how one is
able to det ermine the structure and composition of materials. That is, when a
material is fabricated in the lab, ho w are we able to assess whether our method
was suc cessful? Depending on the nature of the material being investi gated, a suite
of techniques may be utilized to assess its structure and properties. Whereas some
techniques are qualitative, such as providing an image of a surface, others yield
quantitative information such as the relative co ncentrations of atoms that comprise
the ma terial. Recent technologic al advances have allowed materials sc ientists to
accomplish something that was once thought to be impossible: to obtain actual two-
dimensional/three-dimensional images of atomic posit ions in a solid, in real time. It
should be noted that the sensitivity of quantitative techniques also continues t o be
improved, with techniques now being able to eas ily measure parts per trillio n (ppt)
concentrations of impurities in a bulk sample.
This chapter will focus on the most effective and widely used techniques available
to characterize solid-state compounds. The primary objective of this chapter is to
provide a practical description of the methods used to characterize a broad range of
materials. Rather than focusing on the theoretical aspects of each technique, which
may be found in many other textbooks (see “Further Reading” section), our treat-
ment will focus on method suitabilities, sample preparation, and anticipated results.
In this manner, you will be well informed regarding the best method to use for a
particular material. Since techniques such as solution-phase nuclear magnetic reso-
nance (NMR) and infrar ed spectroscopy (IR) are used throughout undergraduate
courses, the background of these methods will not be provided in this textbook.
Further, it is beyond the scope of this chapter to provide detailed background in
optics, electronics, and physical chemistry concepts that underly most of the tech-
niques described herein. For this information, the reader is referred to the “Further
Reading” section at the end of this chapter.
585
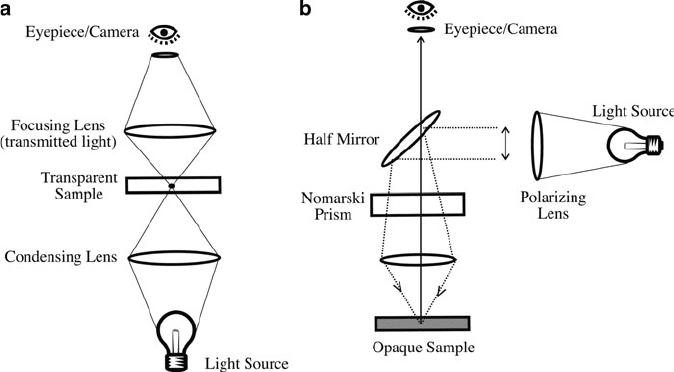
7.1. OPTICAL MICROSC OPY
Of the many techniques available for the analysis of solid mater ials, perhaps the
simplest is optical microscopy. Two modes of optical microscopy are typically
employed, based on the measurement of transmitted or reflected light, from a
transparent or opaque sample, respectively (Figure 7.1). Often, a microscope is
fitted with both modes, allowing one to analyze both types of samples. Most of
the solid-state materials discussed thus far are nontransparent in their as-grown/
as-deposited states. Further, it is usually difficult to prepare thin cross sections for
transmission microscopy. Hence, materials scientists typically employ the reflection
mode, also known as episcopic light differential interference contrast (DIC) micros-
copy.
[1]
This technique is useful for imaging of a variety of reflective samples
including minerals, metals, semiconductors, glasses, polymers, and composites.
The semiconductor industry relies heavily on reflective DIC imagi ng for quality
assessment of computer chip components.
Surface artifacts such as depressions and particulates create optical path differ-
ences in the reflected beam. Formation of the final image is the result of interference
between two distinct wave fronts that reach the image plane slightly out of phase with
each other. Unlike the situation with transmitted light and semitransparent phase
specimens, the image created in reflected light DIC can often be interpreted as a true
three-dimensional representation of the surface geometry, provided a clear distinc-
tion can be realized between raised and lowered regions in the specimen. Oftentimes,
the reflected wave fronts emanating from the sample are separated by only fractions
of a micrometer, which is much less than the resolution of the objective.
Figure 7.1. Schematic of the components of (a) transmitted light optical microscope and (b) reflected
light optical microscope.
586 7 Materials Characterization
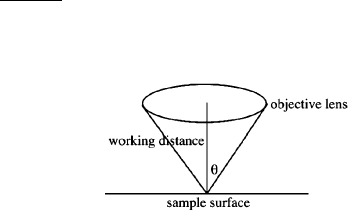
The ability to discern fine details within a magnified image is referred to as the
resolution of a microscope. Since light is used as the illumination source in optical
microscopy, the resolution is expressed in the same units as the wavelength of light
(nm). The theoretical resolution, R, of any optical system may be calculated using
Abbe’s equation (Eq. 1):
R ¼
0:61l
ðsin yÞ
;ð1Þ
where:
The denominator of Eq. 1 represents the numerical aperture (NA) of the objective
lens, related to its light gathering ability. Other primary factors that influe nce
the resolution of a lens is the wavelength of light used, the index of refraction ()
of the environment surrounding the lens (e.g., 1.00 for air), and the angle of
illumination (y).
Examining the above equation, one can see that a resolution limit outside of the
nanoregime will be reached using visible light (350–700 nm) as the illumination
source. That is, using high numerical apertures (e.g ., 1.3–1.4 for oil-immersion
lenses where ¼ 1.5), the theoretical resolution using polychromatic visible light
(ca. 500 nm) is on the order of 220 nm. Hence, any sample features that are less than
220 nm apart from on e another will appear blurry. It should be noted that the
calculated resolution represents the best possible cutoff for clear discernment of
small features. In practice, the observed resolution is often worse than the theoretical
value, depending on the degree of optical aberrations that are inherent in the lens.
It may be seen by Eq. 1 that higher spatial resolutions (i.e., smaller R valu es) are
possible through use of shorter wavelengths. To illustrate this concept, subsequent
sections of this chapter will examine the high resolutions inherent in microscopes
that use an electron beam rather than visible light. However, we first must ask
ourselves whether it is possible to improve the resolution limits of optical micros-
copy. If this is possible, the cost of such a modification would be far less than the
price of electron microscope s (currently $300 K–$1.5 M).
In the late 1920s, Edward H. Synge published a series of articles that conceptua-
lized the idea of an “ultrahigh resolution” optical microscope.
[2]
His original idea
proposed using a screen with an aperture of dimensions much smaller than wave-
length of the illuminating source. Upon irradi ating the screen with a high-intensity
light source, the light is confined to the dimensions of the hole. If this hole is placed
in close proximity (nanometer regime) to the sample surface, the light emerging from
the aperture could be used to image a specimen before it had time to spread out.
7.1. Optical Microscopy 587
