Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

When molecular precursors are decomposed in the gas phase, they first exhibit
homogenous nucleation which leads to supersaturation. Condensation of sub-
nanosized particulates will ensue, followed by growth via Ostwald ripening. Particle
growth is quenched by shutting off the flow of precursor, or cooling the system by
either cool-gas dilution or free-jet expansion through a narrow nozzle. The overall
size and morphology of the nanoparticles may be controlled by favoring either
nucleation or growth via sintering. That is, if sintering/annealing is faster than
collisions, large spheres will be formed; in contrast, aggregates of smaller nanopar-
ticles will be produced if nucleation processes are faster than sintering.
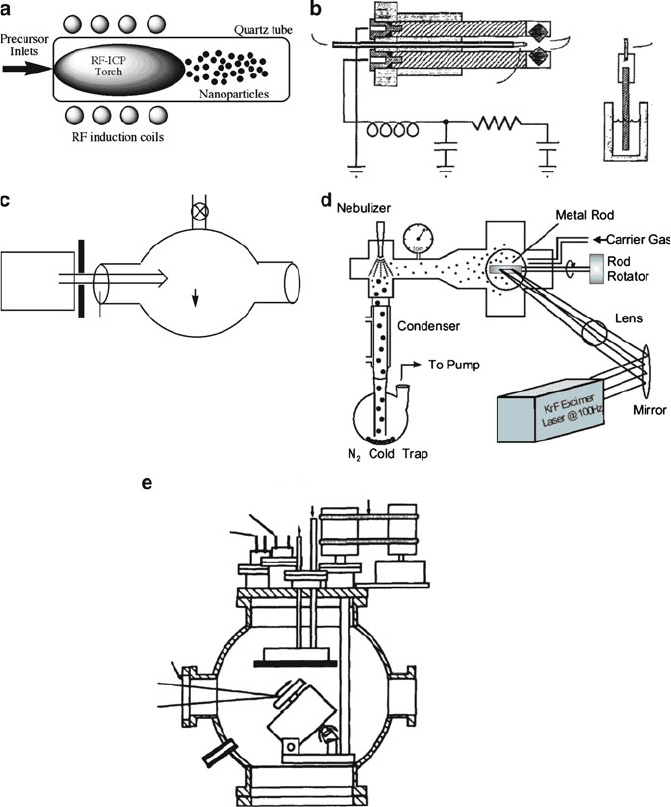
Figure 6.36 shows a variety of gas-phase techniques that have been used to
synthesize 0-D nanoparticles. Radio frequency plasma sources have long been
used for quantitative analysis by atomizing component species in liquid or solid
samples – a technique referred to as inductively-coupled plasma atomic emission
spectroscopy (ICP-AES). The extreme energy of an ICP may also be exploited to
vaporize precursor sources to afford the growth of nanoparticles (Figure 6.36a).
[112]
In this system, the nanoparticle size/morphology would be mostly controlled by the
concentration of precursor in the plasma, and the rate of cooling – a function of its
distance from the plasma source.
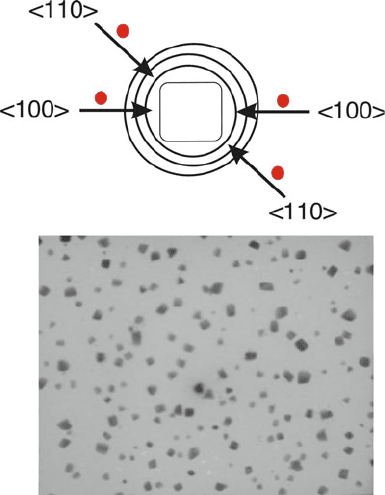
Though gas-phase techniques are not generally useful for shape-control of
nanoparticles, plasma techniques have been used to generate cubic nanocrystals.
[120]
Using a precursor gas mixture of SiH
4
/H
2
, cubic nanocrystals are thought to be
formed due to preferential etching of spherical Si nanoparticles by H atoms formed
in the plasma, which would more readily attack (100) facets than (110) or (111) –
Figure 6.37.
As one would expect, simple flame pyrolysis has been used to generate 0-D
nanoparticles. In fact, millions of metric tons of carbon black and metal oxide
nanoparticles are synthesized by this method each year. The complex flame chemis-
try is difficult to control, which often generates a broad distribution of nanoparticle
sizes, compositions, and morphologies. A degree of growth control may be afforded
by placing electrodes at the exit side of the flame reactor. By varying the applied
field strength, one may control the sizes and degree of agglomeration of the formed
nanoparticles.
[121]
A spark formed between two charged electrodes may also be used
to form nanoparticles (Figure 6.36b). Such spark-facilitated methods are used for
solid sources with a high melting point such as Si or C, which are not easily
evaporated in a furnace.
Laser sources are also useful for either pyrolysis of precursor vapors
(Figure 6.36c), or vaporization of solid precursor targets (Figure 6.36d ). Using a
CO
2
laser (100 W), one is able to grow nanoparticles from precursor powders,
crystals, and sintered blocks of Fe
3
O
4
, CaTiO
3
,Mg
2
SiO
4
, and metal carbides.
[122]
The utility of a laser source is the introduction of localized heating, which facilitates
nanoparticle growth in controlled regions such as reactor flask walls or within a
quenching cold trap. Laser ablation (Figure 6.36e) may be distinguished from laser
vaporization by the type of fragments that are produced. Whereas ablation will result
in both sub-nano and micron-sized fragments in the gas phase, vaporization results
498 6 Nanomaterials
Aperature
KBr
Window
Glass irradiation cell
Electric
feedthrough
Thermocouple
Fused
silica
window
Laser
pulse
Target
Liquid
Nitrogen
Belt
Stepping
motor
To load lock
Substrate
He
gas
To turbomolecular
p
um
p
via cold tra
p
CO
2
laser
Fe(CO)
5(g)
Fe
nano
Air inlet
nozzle
Si electrodes
Collection
substrate
thermophorestic
collector
LN
2
R
L
C
V
Figure 6.36. Illustrations of apparati used in the gas-phase synthesis of 0-D nanoparticles. Shown are:
(a) plasma system; (b) spark-facilitated growth
[113]
; (c) laser vaporization/pyrolysis
[114]
; (d) laser
evaporation
[115]
; (e) laser ablation
[116]
; (f) inert-gas evaporation
[117]
; (g) electrospray system
[118]
;
(h) spray pyrolysis.
[119]
6.3. Nanoscale Building Blocks and Applications 499
1000 mm
Ar
Quenching
Gas
3
4
2
6
1
Filter,
Vacuum
Pump
Cooling
Water
5
180 mm
Evaporation
Zone
Ar
Carrier
Gas
Gas Return Cap:
Syringe Pump
Filter
Filter
Flow meter
CO
2
Dryer
75ⴗ
Compressed Air
The Capillary Tested
SMPS
EtOH
evaporation
Organic species
removed
30 nm
30 nm
30 nm
a
b
c
100 ⬚C
400 ⬚C
600 ⬚C
800 ⬚C
1000-1500 ⬚C
Hydroxy groups
removed
Grain growth and
phase change
Grain growth,
crystal habit formation
Increasing temperature
Air
81 μm I.D.
224 μm O.D.
Air
nA
-H.V.
Droplet:
EtOH
Ti(OR)4
H2O
AcOH
Solid particle
formed via
chemical rxn
Hydrolyzed
titanium oxide
and impurities
(amorphous)
Rutile
single crystal
with possible twins
and stackin
g
faults
Polycrystalline
titanium dioxide of
anatase and rutile
Nanocrystalline
titanium dioxide
of anatase
CO
2
Radioactive
Sources, Po
210
(10 mCi)
Flow meter
Quenching Zone
(Diluter)
100 mm 315 mm
h
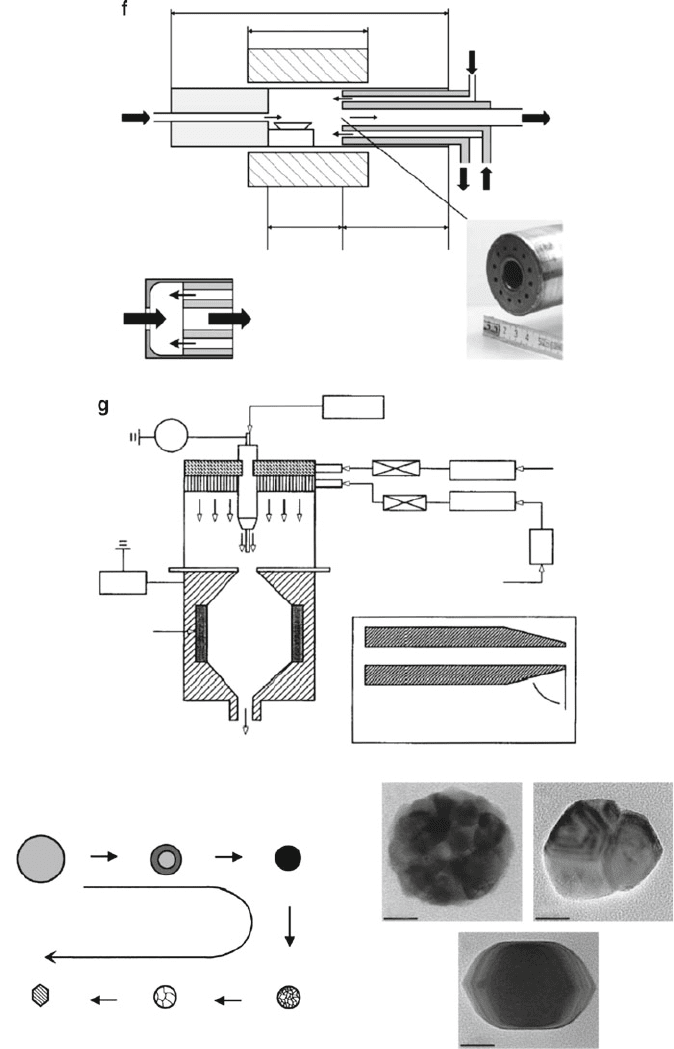
Figure 6.36. Continued
500 6 Nanomaterials
in purely gas-phase molecular precursors. Hence, laser ablation may be considered
a type of ‘top-down’ approach, whereas laser vaporization would be a ‘bottom-up’
technique, which proceeds via homogeneous nucleation/growth.
The particle size distribution may be controlled in both laser ablation/vaporization
techniques by manipulating the pulse energy/duration (usually in the timeframe of
10–50 ns/pulse). It should be noted that when laser ablation is used for thin-film
deposition, the technique is referred to as pulsed-laser deposition (PLD).
[123]
At lower operating pressures (ca. <0.1 Torr), thin films will be favored; however,
at higher pressures (ca. >1 Torr), nanoparticles will be formed due to a greater
opportunity for gas-phase nucleation to occur.
Physical vapor deposition (PVD) techniques such as evaporation or sputtering
may also be used to synthesize 0-D nanoparticles. One technique, known as inert gas
evaporation (Figure 6.36f), consists of evaporation of a precursor material within a
cooling inert gas at low pressures (ca. 100 Pa). Vaporization may be accomplished
via resistive heating, ion bombardment (sputtering), or laser irradiation. As one
Figure 6.37. Schematic (top) illustrating the selective etching of facets within a Si nanoparticle by
hydrogen atoms present in a plasma. The TEM image (bottom) illustrates the resultant cubic
morphology of Si nanoparticles, realized by faster etching of Si(100) facets relative to (110) planes.
Reproduced with permission from Bapat, A.; Gatti, M.; Ding, Y. -P.; Campbell, S. A.; Kortshagen, U.
J. Phys. D 2007, 40, 2247. Copyright 2007 The Institute of Physics.
6.3. Nanoscale Building Blocks and Applications 501
might expect, reactive gases such as O
2
or NH
3
may be plumbed into the system to
allow for the production of oxide or nitride nanoparticles, respectively.
In addition to evaporating solid sources, one may also utilize dilute liquid
suspensions of nanoparticles. However, simple evaporation of these solutions in
an oven will serve to concentrate any impurities that were present in the original
solvent. In order to circumvent this limitation, the nanoparticle suspensions must be
sprayed into a heat source as small droplets whose uniformity may be controlled
using a nebulizer or electrospray techniques (Figure 6.36g). The growth of nano-
particles via this synthetic technique is referred to as spray pyrolysis, and has been
used to synthesize a variety of metal and metal oxide 0-D nanoparticles/nanoclus-
ters.
[124]
As shown in Figure 6.38h, one may easily control the crystallinity/phase of
the developing nanoparticle by varying the annealing temperature.
Solution-phase syntheses of 0-D nanostructures
Most of our discussion thus far has involved some rather extreme synthetic envi-
ronments of lasers, plasmas, or high-temperature pyrolysis. However, a pref erred
route toward nanoclusters/nanoparticles of metals and their compounds is through
use of relatively mild conditions – often taking place at room temperature on the
bench-top. This is not possibl e for carbon nanoallotropes, since the precursor (e.g.,
graphite) contains strong covalent bonding, which requires a significant amo unt of
energy for dissociation prior to self-assembly. However, for metallic and compound
nanostructural growth, the simple reduction of metal salts (usually via NaBH
4
,H
2
,
or hydrazine as reducing agents), or combination of reac tive molecular precursors,
are amenable for mild, solution-phase growth. For instance, Eqs. 6 and 7 represent
the reduction of copper ions by sodium borohydride (and subsequent hydrolysis of
borane); Eq. 8 shows the side-reaction of NaBH
4
with the water, indicating its
relative instability in aqueous solutions. In theory, any metal with a larger standard
reduction potential (E
) than the reducing agent (e.g ., 1.33 V for sodium borohy-
dride; 0.23 V and 1.15 V for hydrazine in acidic and basic media, respectively) is
a candidate for reduction to its metallic form. This includes most of the first-row
transition metal ions, and many others from the main group/transition metal series.
However, it should be noted that solution pH and side-reactions (e.g., metal ions
being converted to borides by BH
4
rather than reduction) often provide a barrier
toward successful metal ion reduction.
CuCl
2
þ2NaBH
4
! Cu
0
þ 2 NaCl + B
2
H
6
þ H
2
ð6Þ
B
2
H
6
þ 6H
2
O ! 2 B(OHÞ
3
þ 6H
2
ð7Þ
NaBH
4
þ 2H
2
O ! NaBO
2
þ 4H
2
ð8Þ
If the above reduction were to be carried out as-is, a metallic film or bulk powder
would be formed rather than nanostructures. That is, as the metal ions are reduced,
they would instantly agglomerate with one another to form larger particulates.
Hence, the most crucial component of nanostructure synthesis is the stabilizing
502 6 Nanomaterials
agent that isolates the growing nuclei from one another. In addition, shape control of
nanostructures may be afforded in solution by varying the stabilizing agent or other
experimental conditions.
[125]
Some desirable traits of a stabilizing (or entraining)
agent are:
(i) Chemically unreactive toward the growing nanocluster, rendering an unpassi-
vated nanocluster surface ;
(ii) Stru cturally well-defined (size/shape), which allows for the controlled growth
of the encapsulated nanocluster;
(iii) Comprised of light elements (organic-based), so its structure does not interfere
with the characterization of the entrained nanocluster. This will also facilitate
its sacrificial removal from the nanocluster by pyrolysis at relatively low
temperatures, if desired;
(iv) Surface-modifiable, to allow for tunable solubility and selective intera ctions
with external stimuli. In addi tion, to afford controllable self-assembly of
entrained nanoclusters on a variety of surfaces through chemisorption, if
desired.
Though some references cite the processing conditions (“continuous” gas-phase vs.
“batch” solution-phase) as being superior for gas-phase techniques, the resultant batch-
to-batch properties (e.g., monodispersity, morphology, purity) of solution-grown nano-
particles has greatly improved in recent years. With optimized nanoparticle synthetic
recipes now pervasive in the literature, it is now safe to assume that the nanoparticles
generated in one batch will be virtually identical to those in subsequent batches (though
one must always confirm this via appropriate characterization techniques). Whereas
the nanoparticles generated from individual gas-phase runs may exhibit very similar
diameters/morphologies, their overall monodispersity will be relatively poor due to
the lack of an entraining agent (Figure 6.38). Accordingly, systems such as a differen-
tial mobility analyzers (DMAs, Figure 6.39) are often used to improve the size/
morphological uniformity of gas-phase synthesized nanoparticles.
[126]
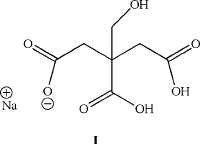
In aqueous solutions, the most common method used to stabilize nanostructures is
the use of organic “capping” ligands. For instance, the Turkevich process, which
dates back to early colloidal growth of the 1950s (and refined by Frens in the 1970s),
uses sodium citrate (I) to entrain the reduc ed gold nuclei. Particle diameters on the
order of 10–20 nm may be synthesized using this method. In this case, since gold is
easily reduced (E
¼ 1.00 V for AuCl
4
), the citrate reagent acts as both the reducing
and stabilizing agent.
The cationic stabilizing agent bis(11-trimethylammoniumdecanoylaminoethyl)
disulfide dibromide (TADDD, II) has also been utilized to grow metallic
6.3. Nanoscale Building Blocks and Applications 503
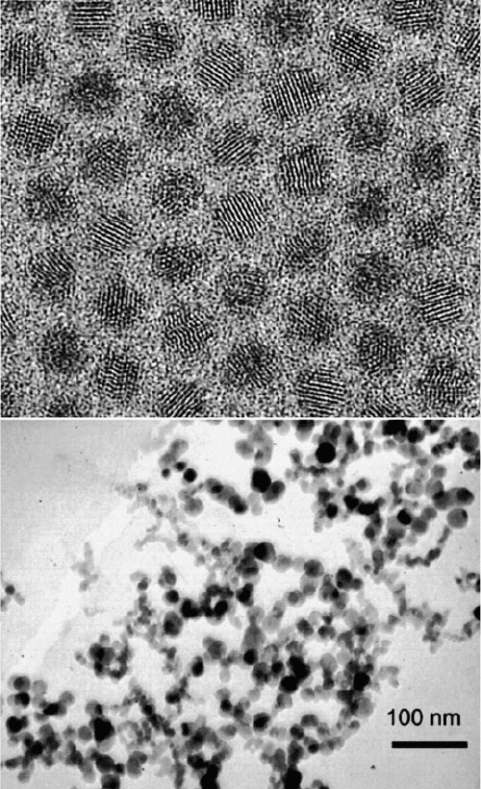
Figure 6.38. TEM images illustrating the common relative monodispersities of solvent-based (top) and
gas-phase (bottom) syntheses. The top image is of CdSe quantum dots synthesized using a solution-phase
mixture of TOPO/TOP/HDA at 300
C. Reproduced with permission from Talapin, D. V.; Rogach, A. L.;
Kornowski, A.; Haase, M.; Weller, H. Nano Lett. 2001, 1, 207. Copyright 2001 American Chemical
Society. The bottom TEM image shows silicon nanoparticles generated from the laser pyrolysis of silane
gas. Reproduced with permission from Swihart, M. T. Curr. Opin. Coll. Interf. Sci. 2003, 8, 127.
Copyright 2003 Elsevier B.V.
504 6 Nanomaterials
nanoclusters with diameters <10 nm. If NaBH
4
is used as the reducing agent, the
sulfide bond is cleaved resulting in a –SH capping group. The thiol is chemisorbed to
the surface of the growing nanostructure surface to prevent agglomeration (esp.
effective for “thiol-philic” noble metals such as Pt, Ag, and Au).
[127]
Recently, there has been much interest in the use of structurally perfect polymeric
dendrimers (see Chapter 5) as stabilizing templates for nanocluster growth. By
varying the peripheral groups and number of repeat branching units (known as
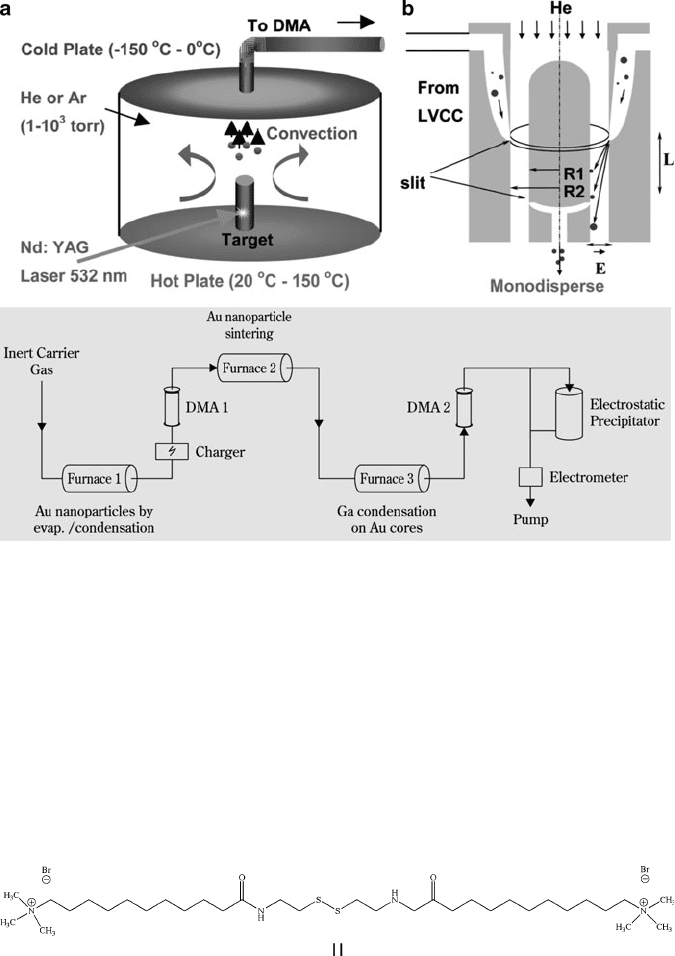
Figure 6.39. Schematic of nanoparticle growth via pulsed-laser vaporization with controlled condensation
(LVCC), coupled to a differential mobility analyzer (DMA). A DMA is used to control the size of gas-
phase synthesized nanoparticles by exploiting differences in the electrical mobility of nanoparticles under a
flow of an inert gas. Reproduced with permission from Glaspell, G.; Abdelsayed, V.; Saoud, K. M.;
El-Shall, M. S. Pur. Appl. Chem. 2006, 78, 1667. Copyright 2006 IUPAC. The bottom image shows how
gas-phase techniques may be used to synthesize Au/Ga core/shell nanoparticles with the assistance of
multiple DMAs. Reproduced with permission from Karlsson, et al. Aerosol Sci. Technol. 2004, 38, 948.
6.3. Nanoscale Building Blocks and Applications 505
“Generations”), one is able to fine-tune the size of the entrained nanocluster. Though
amine-terminated dendrimers and hyperbranched polymers may be used as a tem-
plate for M
n+
chelation and subsequent chemical reduction, the size of the resultant
nanoparticle is relatively large, with a greater degree of agglomeration possible.
This is especially the case for hyperbranched polymers that exhibit a random
structure, which results in a much greater nanoparticle polydispersity. On the
other hand, if the primary surface amines (NH
2
) are either protonated (NH
3
+
), or
replaced with hydroxyl groups (—OH), the prereduced metal ions are forced further
into the interior of the dendritic structure (Figure 6.40). This results in much smaller
diameters and narrow polydispersities for the reduced metal nanoclusters.
[128]
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
M
n+
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
NH
NH
NH
NH
NH
NH
NH
NH
NH
NH
NH
2
H
2
N
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
NH
NH
NH
NH
NH
NH
NH
H
NH
HN
NH
NH
HN
NH
NH
NH
NH
NH
NH
NH
NH
NH
NH
NH
HO
HO
HO
HO
HO
HO
HO
HO
OH
HO
HO
HO
HN
HN
HN
HN
HN
HN
HN
HN
HN
HN
HN
HN
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
HO
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
NH
2
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
N
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
N
1⬚
3⬚
3⬚
3⬚
1⬚
1⬚
1⬚
1⬚
3⬚ amines 1⬚ amines
3⬚
3⬚
1⬚
1⬚
1⬚
N
H
N
N
H
N
H
H
N
N
H
N
N
N
N
N
N
2-SBD
2-SBD 14 16
4-SBD 62 64
6-SBD 254 256
6-SBD
pH 6
pH 6
pH 8
Repeating unit:-(CH
2
CH
2
(CO)NHCH
2
CH
2
N)-
pKa(1⬚) ~ 7-9
p
Ka
(
3⬚
)
~ 3-6
Figure 6.40. Molecular structure of a second Generation (G2) amine-terminated PAMAM dendrimer,
illustrating the positions of the metal ions chelated to the primary amine groups (prereduction). In
comparison, a G2 hydroxyl-terminated PAMAM dendrimer is shown, with the metal ions now
preferring to chelate to the interior tertiary amine groups. Shown on the bottom is the effect of
protonation on G2/G6 amine-terminated PAMAM dendrimers. A schematic on the lower right illustrates
the positions of the protonated amines at varying pH values. As the generation size increases, the surface
density also increases which limits the access of protons (or chelating metal ions) to interact with the
interior tertiary amine groups. Reproduced with permission from Kleinman, M. H.; Flory, J. H.; Tomalia,
D. A.; Turro, N. J. J. Phys. Chem. B 2000, 104, 11472. Copyright 2000 American Chemical Society.
506 6 Nanomaterials
In addition to controll ing the surface moieties and solution pH, the generation
size of the dendrimer is also paramount for successful nanocluster growth. As the
degree of branching increases, s o does the s urfa ce d ens it y, whi ch pre v ents
the incoming metal ion (or H
+
during protonation) from entering t he interior of
the dendritic architecture. Alternatively, for smaller generations, the entrained
species becomes easily dislodged from the interior due t o its open structure.
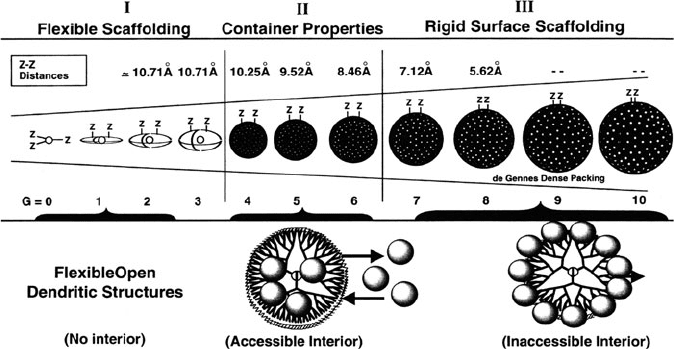
Hence, the most effective size range for nanocluster growth using polyamidoa-
mine (PAMAM) is between the fourth and sixth generations (G4–G6), which
exhibit strong container properties (Figure 6.41). As an illust ration of the extrem e
flexibility of the dendritic architecture, the core may also be altered to change its
solubility characteristics, or allow t he penetration of species through the periphery
at high generations (Figure 6.42). Since dendrimers contai ning an alm ost unlimi ted
range of cores and peripheral groups have been synthesized, it is now possible to
easily control nanocluster properties such as composition, size, morphology, solu-
bility, and encapsulation (e.g., control the release rate of entrained medicinal
agents/sensors based on structural or environmental changes, for targeted drug
delivery or in situ monitoring).
It should be noted that metal nanocluster growth using dendritic templates is
strongly governed by the degree of complexation of the precursor metal ions. For
instance, silver nanoclusters are not possible using hydroxyl-terminated PAMAM
dendrimers since Ag
+
is not strongly chelated to tertiary amine groups. However, if
Cu
0
nanoclusters are first generated within the structure, followed by Ag
+
, a redox
Figure 6.41. Relative sizes and surface densities of PAMAM dendrimers, showing the most suitable
range for nanocluster growth as Generation 4 to Generation 6. Reproduced with permission from
Dendrimers and other Dendritic Polymers, Frechet, J. M. J.; Tomalia, D. A. eds., Wiley: New York, 2001.
6.3. Nanoscale Building Blocks and Applications 507
