Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

reaction will facilitate the displacement of Cu
0
with Ag
0
within the dendrimer
interior (Eq. 9).
Cu + 2 Ag
þ
! Cu
2þ
+2Agð9Þ
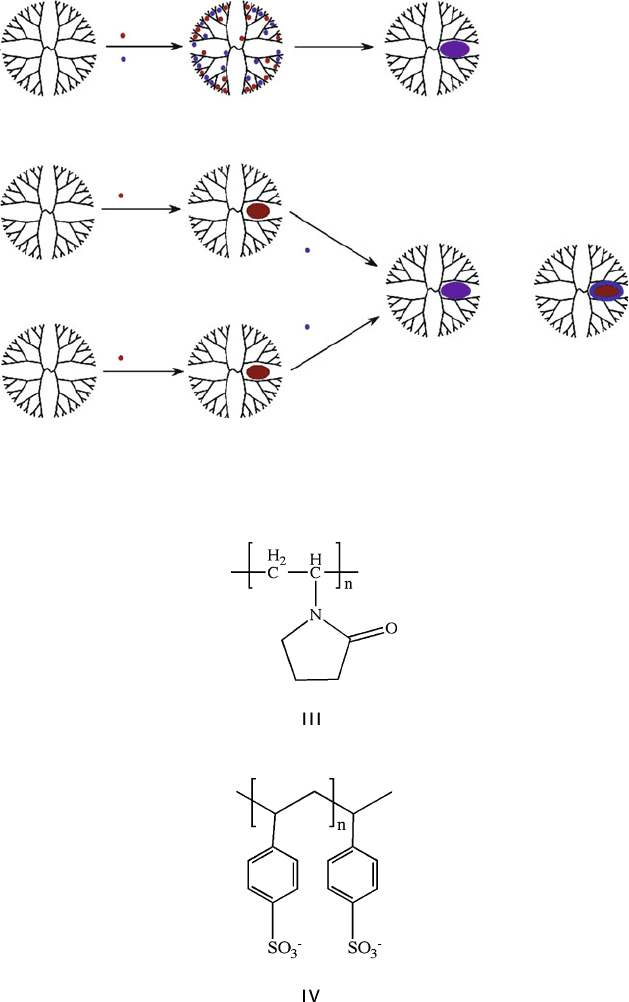
In addition to simple metallic nanostructures, more complex intermetallic species
have also been synthesized through the introduction of mor e than one metal. For
instance, bimetallic nanoclusters may be generated via three routes within a den-
dritic host (Figure 6.43). In addition to already bein g proven for core–shell nanoclus-
ters, this route should also be amenable for the growth of trimetallic nanostructures
for interesting catalytic applications.
[129]
Other polymers may also serve as effective stabilizing agents for nanostruc-
tural growth. For instance, poly(vinylpyrrolidone) (PVP, III), poly(styrenesulfo-
nic acid) sodium salt (PSS, IV), and poly(2-ethyl-2-oxazoline) (PEO, V)were
recently used to generate a number of intermetallic nanoalloys via amild
metallurgy in a beaker approach developed by Schaak and coworkers.
[130]
Since individual metal nanoparticles are in intimate contact with high surface
reactivity and low melting points, the use of high-temperature annealing is not
required, unlike bulk-scale alloy synth eses. The PVP architecture has al so been
shown to facilitate the growth of Au@Ag co re–shell nanostructures, as well as
Ag nanowires and nanocubes.
[131]
Interestingly, one is able to fine-tune the
LSPR frequency of core-shell nanoclusters by varying the shell thickness or
composition/size of the core.
[132]
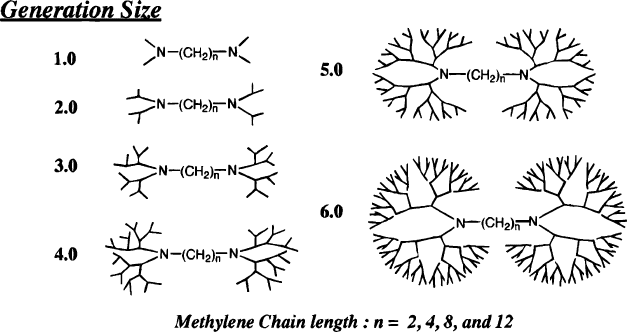
Figure 6.42. Molecular structures of dendrimers modified with long-chain aliphatic cores. Unlike
traditional dendritic structures with smaller cores, as the generation size increases, there is an available
channel for external species to enter the dendrimer interior. Reproduced with permission from
Watkins, D. M.; Sayed-Sweet, Y.; Klimash, J. W.; Turro, N. J.; Tomalia, D. A. Langmuir 1997, 13,
3136. Copyright 1997 American Chemical Society.
508 6 Nanomaterials
1. Co-complexation Method
2. Sequential Method
3. Partial Displacement Method
M
A
p+
M
A
p+
M
A
p+
NaBH
4
NaBH
4
NaBH
4
M
B
q+
M
B
q+
M
B
q+
Alloy
Alloy
Weak Reducing
Agent (i.e H
2
)
More Noble
Metal Salt
Gn-OH
Gn-OH
Gn-OH
or
Core/Shell
Figure 6.43. Schematic of the three methods used to generate bimetallic nanoclusters within a dendritic
host. Reproduced with permission from Scott, R. W. J.; Wilson, O. M.; Crooks, R. M. J. Phys. Chem. B
2004, 109, 692. Copyright 2004 American Chemical Society.
6.3. Nanoscale Building Blocks and Applications 509

For the synthesis of nanostructures within nonpolar solvents, one may use a
stabilizing agent that contains alkyl chains rather than —OH endgroups. One of
the first capping agents to be used for noble metal colloidal growth was alkylthiols
(CH
3
—[CH
2
]
X
—SH).
[133]
In this system , the —SH end is bound to the surface
of the nanostructure, and the long organic tail is responsible for dispersion within
the organic solvent. Though these capping stabilizers worked well for colloidal
growth to prevent agglomeration, even allowing solvent removal/redispersion into
organic solvents, it was relatively difficult to control the size dispersity of the
nanoparticles.
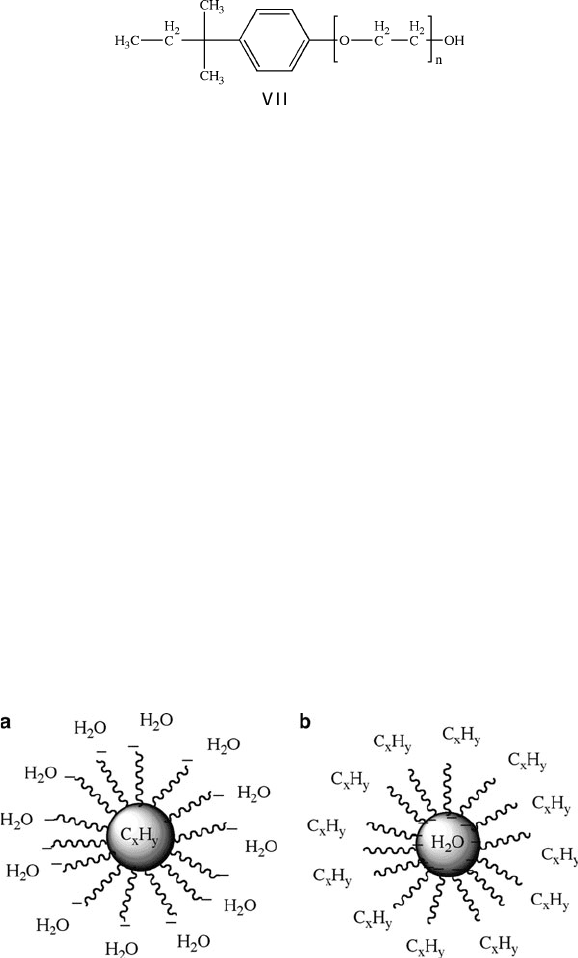
Accordingly, systems that contain a nanoreactor template have been used more
recently for controlled nanostructural growth. In fact, everyone who has washed
dishes or laundry already has some experience with these types of stabilizing agents,
known as micelles. These compounds contain both polar (—OH, cationic/anionic)
and nonpolar (aliphatic) ends. Soaps and surfactants work by surrounding the dirt
particle with the nonpolar ends, leaving the hydrophilic polar groups exposed to
the surrounding water molecules . This results in pulling the dirt particle from the
surface of the clothing fiber, forming an aqueou s suspension. In a similar fashion, an
oil-in-water microemulsion may be set up using common surfactants such as sodium
bis(2-ethylhexyl)sulfosuccinate (also referred to as Aerosol OT or AOT, VI), or
the nonionic surfactant Triton-X (VII) in the presence of the biphasic oil/water
mixture.
[134]
510 6 Nanomaterials
Since most precursors for solution-phase nanostructural growth are ionic metal
salts, a typical micelle would not be effective for since the precursor would not be
confined to the interior of the microemulsion. Hence, reverse micelles (or inverse
micelles, Figure 6.44) are used to confine the precursor ions to the aqueous interior,
which effectively serves as a nanoreactor for subsequent reduction, oxidation, etc.
en route to the final nanostructure. Not surprisingly, either PAMAMOS den-
drimers (see Chapter 5) or dodecyl-terminated (hydrophobic) PAMAM dendrimers
(Figure 6.45) have been recently employed for this application.
[135]
It should be noted that dendrimer-entrained nanoclusters synthesized within
aqueous solutions may also be phase-transferred into an organic solvent by mixing
with alkylthiols dissolved in a nonpolar solvent.
[136]
This also results in monodis-
perse nanoclusters, with much less polydispersity than early colloidal syntheses that
employed thiol-based entraining agents. That is, the nanocluster size has already
been controlled via intradendrimer stabilization. In contrast, the use of alkylthiols
from the initial stages of growth is not as effective toward preventing agglomeration
during the nucleation step.
Inorganic-based entraining agents have also been used to stabilize nanoclusters.
Perhaps the earliest example is the use of polyoxometalates of general composition
M
x
O
y
n
or X
x
M
y
O
z
m
(where: X ¼ P or Si; M ¼ W, Mo, Nb or V) for the
synthesis of nearly-monodisperse Ir nanoclusters (e.g.,P
2
W
15
Nb
3
O
62
9
,
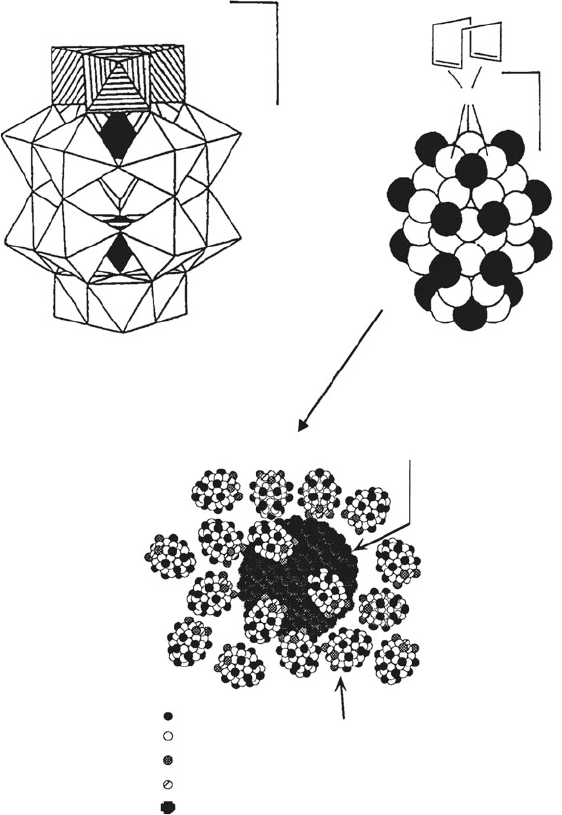
Figure 6.46).
[137]
In addition, one may also synthesize nanoparticulates by entrap-
ping the precursor within voids of a highly porous substrate. For instance, zeolites,
Figure 6.44. Comparison of a traditional micelle used to entrain organic oils/dirt using an anionic
surfactant, (a), and a reverse micelle used to stabilize aqueous nanoreactors within a nonpolar solvent, (b).
6.3. Nanoscale Building Blocks and Applications 511
layered solids, molecular sieves, gels and glasses have been used to sequester
reactive precursor species wherein hydrolysis, high temperature, or other chemical/
redox reactions are able to transform them into surface-immobilized nanostruc-
tures.
[138]
Of course, the encasing medium may then be removed by acid/base
dissolution, plasma/heat treatment, etc. if discrete nanostructures are desired.
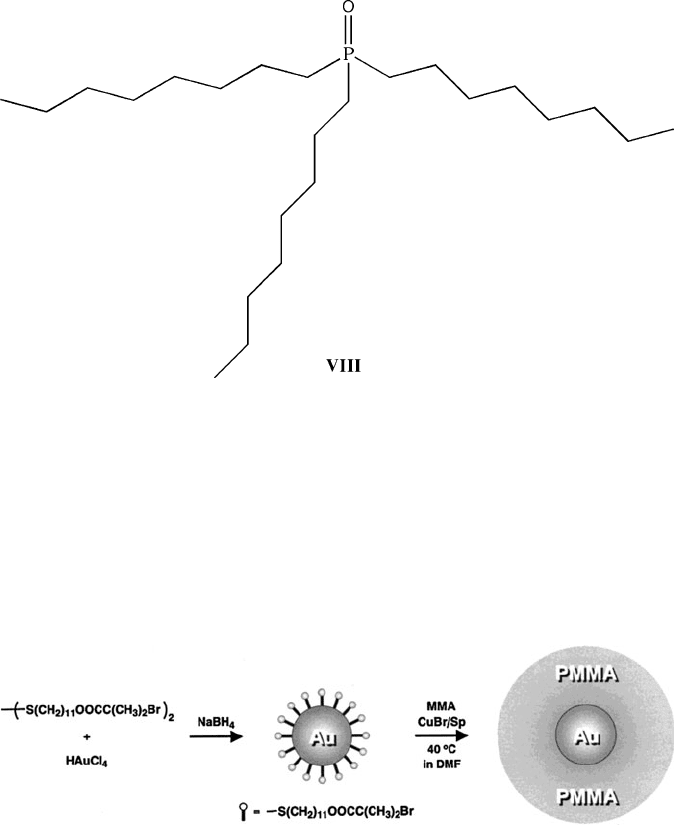
Tri-n-octylphosphine oxide (TOPO, VIII) is another common inverse-micelle
stabilizing agent used to control the sizes of semiconductor nanocrystals such as
CdE (E ¼ S, Se, Te) quantum dots. The synthesis is performed at elevated tem-
peratures since TOPO is a low-melting (53
C), moisture-sensitive solid. A typical
procedure consists of heating/degassing TOPO at ca. 360
C under an inert atmo-
sphere, quickly injecting a tri-n-octylphosphine (TOP) suspension of Me
2
Cd and
chalcogen powder at a reduced temperature (ca. 180
C), and re-heating to 250–
260
C.
[139]
The quick injection of precursors into the hot solvent generates homo-
geneous nucleation of CdE nanocrystals. Subsequent re-heating affords slow growth
N
NH
HN
HO CH HC OH
CH
2
CH
2
CH
3
CH
3
(CH
2
)
9
(CH
2
)
9
HN NH
O
O
G4-C
12
G5-PPI-C
12
CH
3
CH
3
(CH
2
)
11
(CH
2
)
11
N
O
O
HNNH
NH NH
Figure 6.45. Hydrophobic-functionalized PAMAM (G4–C
12
) and poly(propyleneimine) (PPI)
dendrimers, which serve as templates for Au nanocluster growth. Reproduced with permission from
Knecht, M. R.; Garcia-Martinez, J. C.; Crooks, R. M. Langmuir 2005, 21, 11981. Copyright 2005
American Chemical Society.
512 6 Nanomaterials
9−
8−
6
5
4
12
13
14
15
11
10
17
16
18
9
7
8
ab
Ir
H
2
Ir(0) Metal
Core
W=O
W-O-W
Nb=O
Nb-O-Nb
Ir(0)
P
2
W
15
Nb
3
O62
9−
Stabilizing Matrix
1
3
2
Figure 6.46. Polyoxoanion-stabilized Ir
0
nanocluster formation. Shown is (a) Polyhedral representation
of the a-1,2,3-P
2
W
15
Nb
3
O
62
9
stabilizing anion, and (b) a space-filling representation of the [(1, 5 –
COD)Ir(P
2
W
15
Nb
3
O
62
)]
8
complex. In (a), the three Nb atoms are indicated by the striped octahedra in
positions 1–3. The WO
6
polyhedra occupy the 4–18 positions, and the internal PO
4
tetrahedral units are
illustrated in black. In (b), the black spheres represent M–O terminal groups, and the white spheres
represent M–O–M bridging groups. The Bu
4
N
+
and Na
+
counterions are omitted for clarity. Images (a)
and (b) reproduced with permission from Finke, R. G.; Lyon, D. K.; Nomiya, K.; Sur, S.; Mizuno, N.
Inorg. Chem. 1990, 29, 1784. The bottom image of the stabilized nanocluster is reproduced from Watzky,
M. A.; Finke, R. G. Chem. Mater. 1997, 9, 3083. Copyright 1997 American Chemical Society.
6.3. Nanoscale Building Blocks and Applications 513
of nanocrystals via Ostwald ripening
[140]
– the growth of large particles at the
expense of smaller ones, due to the relatively higher surf ace free energy (less
stability) of small nanoclusters. This is important in order to achieve a very high
degree of monodispersity.
It is not always necessary for the precursor metal ions to be encapsulated withi n a
stabilizing polymer during chemical reduction. For instance, the reduced metal may
be entrained by polymerization precursors, such as post-reduction living radical
polymerization that takes place on the surface of gold nanoparticles (Figure 6.47).
This results in a dense “polymer brush”
[141]
that encapsulates the metallic nanopar-
ticle, effectively stabilizing the structure against agglomeration. Subsequent align-
ment and surface reactivity of the resultant nanostructures may be fine-tuned by
varying the nature of the polymer coating.
Figure 6.47. Schematic of the formation of gold nanoparticles coated with free-radical polymerization
initiators that subsequently yield Au@polymer nanostructures through a surface-controlled living
polymerization process. Reproduced with permission from Ohno, K.; Koh, K.-M.; Tsujii, Y.; Fukuda,
T. Macromolecules 2002, 35, 8989. Copyright 2002 American Chemical Society.
514 6 Nanomaterials
Nanoshells
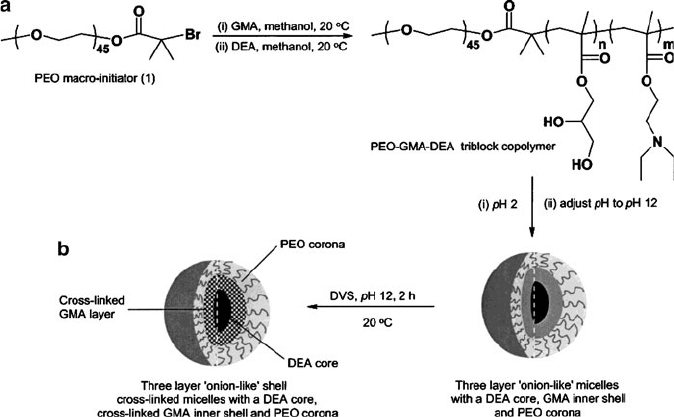
Increasingly complex stabilizing agents such as cross-linked micelles have also been
developed in recent years. These nanostructures consist of a polymeric core that is
cross-linked with surrounding polymer layer(s) (Figure 6.48). In contrast to solid
nanoparticles, a hollow nanoshell may be synthesized by sacrificially removing the
core material by chemical or thermal decomposition. Such intriguing nanobuilding
blocks will likely be of extreme importance for next-generation medical treatment/
sensing, hydrogen storage, ion- exchange, and microelectronics applications.
Another strategy for nanoshell growth consists of applying a thin metallic coating
onto surface-functionalized silica or polystyrene templating spheres. Such gold
nanoshells were pioneered by Naomi Halas at Rice University, and have far-reaching
applications in photonics and biomedicine such as cancer diagnosis/treatment.
[142]
If desired, one may also subsequently remove the template by treating with hydro-
fluoric acid (HF) or toluene for silica or polystyrene cores, respectively. The reverse
case of a polymer/ceramic coating onto removable metallic nanoparticles has also
been applied to yield nonmetallic nanoshells.
[143]
However, the removal of a rela-
tively large core (i.e., typicall y >200 nm) from a nanoscale coating generally results
in significa nt deformation of the resultant nanoshell.
Figure 6.48. Synthetic scheme for three-layer cross-linked micelles. Shown is the micellation of poly
[(ethylene oxide)-block-glycerol monomethacrylate-block-2-(diethylamino)ethyl methacrylate] (PEO-
GMA-DEA) triblock copolymers to the final “onion-like” layered nanostructure. Reproduced with
permission from Liu, S.; Weaver, J. V. M.; Save, M.; Armes, S. P. Langmuir 2002, 18, 8350.
Copyright 2002 American Chemical Society.
6.3. Nanoscale Building Blocks and Applications 515
The structural robustness of nanoshells has been recently improved through the
use of sacrificial cores of smaller diameters. An example of this strategy is the
growth of silver nanoshells from nanostructural Co templates (Figure 6.49). It should
be noted that NaBH
4
reduction of ligand-capped Co
2+
ions preferentially yields
Co
2
B rather than metallic cobalt. However, a post-exposure of oxygen converts the
boride into metallic cobalt nanostructures (Eq. 10).
[144]
Once the Co core was
formed, silver ions were introduced into the system through addition of AgNO
3
.
Since Ag
+
(E
red
¼ 0.799 V) is preferentially reduced relative to Co
2+
(E
red
¼
–0.377 V), the exchange of Ag for Co occurs spontaneously as Ag
+
/Co
0
boundaries
are formed. This process continues as Ag
+
ions diffuse through the growing Ag
0
layer, until all of the metallic Co is consumed from the core.
4Co
2
B þ 3O
2
! 8Coþ 2B
2
O
3
ð10Þ
Rather than redox-governed nanoshell growth, diffusion-governed routes are also
possible. For example, the exposure of cobalt nanoparticles to oxygen, sulfur, or
selenium does not simply form a Co-chalcogenide coating, but rather hollow nano-
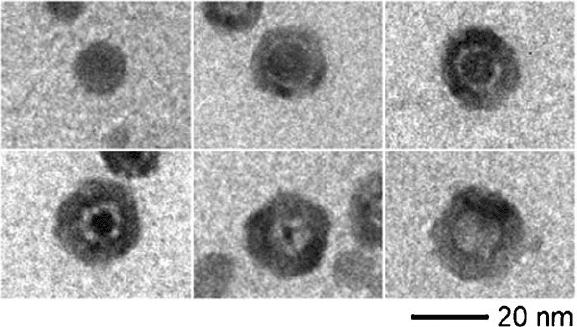
shells of the cobalt chalcogenide (Figure 6.50).
[145]
This interesting result is a
modern extension of an effect that has been studied since the late 1940s – the
Kirkendall Effect. This phenomenon describes the differential diffusion rates of
two species in direct contact with one another at elevated temperatures (e.g., Cu
and Zn in brass). During the growth of oxide or sulfide films, pores are formed in the
solid due to a vacancy-exchange mechanism. That is, the outward move ment of
metal ions through the oxide layer is balanced with an inward migration of lattice
vacancies that settle near the metal/oxide boundary. Due to the large number of
defects and volume of bulk solids, the pores at the metal–oxide interface do not
coalesce into an ordered array. However, for a nanostructural system that is rela-
tively free of defects and exhibits a large surface/volume ratio, the pores readily
aggregate into a single hollow core.
Not surprisingly, 0-D nanostructural growth need not be limited to metallic
structures, but may also include other compounds such as metal oxides, sulfides,
Ag nanoparticles
Co core Ag nanoshell
Co
2+
Co
2+
Co
2+
Co
2+
Co
2+
Co
2+
Co
2+
Ag
+
Ag
+
Ag
+
Ag
+
Ag
+
Ag
+
Ag
+
Figure 6.49. Schematic of Ag nanoshell formation from a nanostructural Co core. Due to favorable redox
couples between Ag and Co, a nanoshell of metallic silver forms at the expense of the inner Co core.
Reproduced with permission from Chen, M.; Gao, L. Inorg. Chem. 2006, 45, 5145. Copyright 2006
American Chemical Society.
516 6 Nanomaterials
etc. There are a copious number of applications for nanostructural oxide s such as
high-density magnetic storage, heterogeneous catalysis, gas sensors, electrolytes for
lithium batteries, and fuel cells. Sun and coworkers recently describ ed a polyol
[146]
route for the synthesis of hexane-suspended Fe
3
O
4
(magnetite) nanoclusters through
the reaction between Fe(acac)
3
, oleylamine, oleic acid, and 1,2-hexadecanediol at
ca. 200
C within phenyl ethe r.
[147]
This one-pot synthesis results in the thermal
replacement of the acetylacetonate ligands (recall Chapter 4 – CVD precursors)
from the iron center, followed by oxide formation from reaction with the alcohol.
The oleylamine and oleic acid condense in situ to form a long-chain inverse micelle
that is capable of suspending the nanoclusters in nonpolar media. For purification,
one simply adds an excess of polar solvent (methanol or ethanol) to precipitate
the nanostructures. Following centrifugation, the nanocl usters are re-suspended in
hexane, with this precipitation/resuspension procedure repeated three to four times
to afford monodispersed, chemically pure Fe
3
O
4
nanoclusters. Another analogous
procedure uses oleic acid/trimethylamine N-oxide in trioctylamine (b.p. 365
C) to
decompose Fe(CO)
5
to yield monodispersed g-Fe
2
O
3
nanoclusters.
[148]
Interest-
ingly, shape control of nanoparticles may be afforded by altering the nature of the
solvent or injection timing of surfactants.
[149]
Another more recent strategy that we developed is the synthesis of metal oxide
nanoparticles from the simple reaction of dendritic-stabilized basic metalate salts
with CO
2
(Figure 6.51).
[150]
This strategy should work for any metalate salt that is
typically formed from the reaction of the metal hydroxide with a strong base (Eq. 11,
for sodium hafnate). Metal/nonmetal oxides may also be synthesized using a sol-gel
technique, analogous to that described in Chapter 4 for thin film growth. Fo r
Figure 6.50. Hollow nanoshell formation via the Kirkendall Effect. Shown is the evolution of the hollow
nanoshell after reaction times of (left–right): 0 s, 10 s, 20 s, 1 min, 2 min, 30 min. An interesting feature is
the formation of “bridges” that connect the core to the sulfide shell, facilitating the fast outward migration
of Co. Reproduced with permission from Yin, Y.; Rioux, R. M.; Erdonmez, C. K.; Hughes, S.; Somorjai,
G. A.; Alivisatos, A. P. Science 2004, 304, 711. Copyright 2004 AAAS.
6.3. Nanoscale Building Blocks and Applications 517
