Fahlman B.D. Materials Chemistry
Подождите немного. Документ загружается.

laser and arc plasma systems), but may also result in lowering the cost due to a more
straightforward industrial scale-up.
Buckyballs represent the smallest fullerene that obeys the Isolated Pentagon Rule
(IPR) – i.e., an energetic requirement that pentagons be surrounded by hexagons, so
that adjacent pentagons do not share an edge. Calculations show that p bonds shared
between six-membered rings have large positive bond resonance energies (BREs)
Carbon Chains/
Rings
100
11
15
19
23
3
50
80
90
70
×10
60
0.7 nm
40
100 120
80
80
60
60
Cluster Size (Atoms)
Ion Signal
40
40
20
20
0
0
Fullerenes
“Forbidden
Zone”
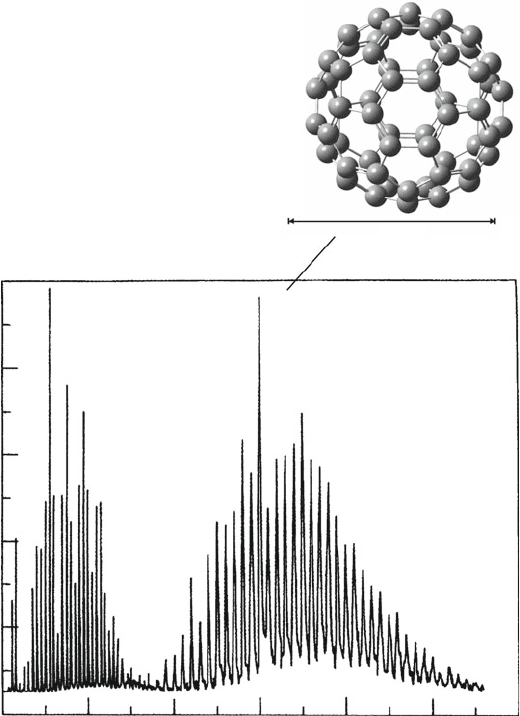
Figure 6.24. Mass spectrum of carbon clusters in a supersonic beam, originally observed by Nobel
Laureates Smalley and Curl. Reproduced with permission from J. Chem. Phys. 1984, 81, 3322. Also
shown is the molecular structure of Buckminsterfullerene, C
60
, containing alternating six- and five-
membered rings of sp
2
hybridized carbon atoms. This is only one isomer for C
60
, out of a staggering
total of 1,812 possible structures.
[100]
488 6 Nanomaterials
and bond orders, indicating a high degree of aromaticity and stability/unreactivity.
However, p bonds between adjacent five-membered rings have large negative BREs
with very small bond orders, indicating a much lower thermodynamic stability.
Most likely, this difference is due to the increased ring strain that would be imposed
in the fullerene structure as a result of two smaller rings directly adjacent to one
another. Theoretical calculations indicate that the strain energy of the icosohedral
Buckminsterfullerene structure is at least 2 eV lower than any other non-IPR isomer,
of which there are over 1,800 possibi lities.
Interestingly, it has recently been reported that adjacent pentagons containing at
least one N atom instead of C (e.g., C
58
N
2
rather than C
60
), may actually be more
stable than C
60
(Figure 6.27).
[102]
This apparent anomaly is a direct contradiction of
the IPR. The most plausible explanation is the reduction of ring strain due to sp
3
hybridization of N, as well as the addition of p-electron density (from the N lone-pair
electrons) to the pentagons, resulting in an enhanced aromaticity/stability. To date,
only short-lived azafullerenes C
59
NandC
58
N
2
–
have been identified experimentally;
the search continues for stable structures, since these will likely result in dramatically
different properties and associated applications relative to their C-only analogues.
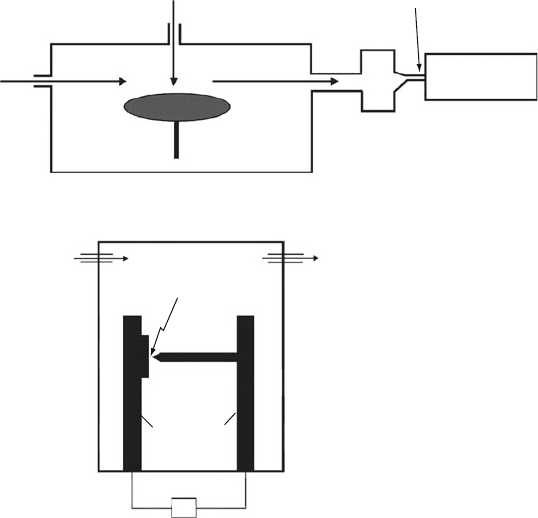
Vaporization laser
He Gas Pulse
a
b
He inlet
Rotating Graphite
Disk
Supersonic Nozzle
Mass
Spectrometer
"Integrating
Cup"
To Vacuum
Electric Arc
Graphite
Rod
Electrodes
Current Source
Figure 6.25. Schematic of apparati first used to synthesize fullerenes. Illustrated are (a) the Smalley/Curl
supersonic laser evaporation system, and (b) the Huffman/Kratschmer electric arc apparatus.
6.3. Nanoscale Building Blocks and Applications 489
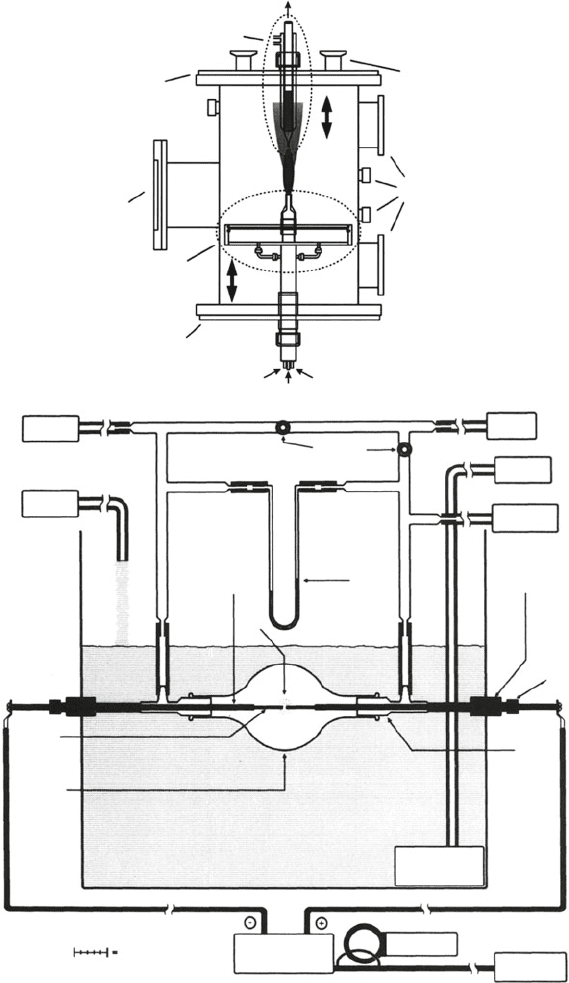
to pump
Watercooled Prode
Top Plate
Viewport
Burner
Bottom Plate
Oxygen Oxygen
Fuel/Argon
Vacuum Ports
VACUUM
PUMP
Auxiliary Ports
HELIUM
CYLINDER
WATER
IN
WATER
OUT
MERCURY
MANOMETER
flow control
valves
25 mm
rubber
tubing
rubber
septum
oil
manometer
copper
mounting rod
arc
graphite
electrode
1 L
Pyrex
reactor
vessel
SCALE: 5 cm
DC ARC WELDER
AC METER
220V/50 A
AC OUTLET
Pyrex
guide
arm
SUBMERSIBLE
PUMP
a
b
Figure 6.26. Cross-section schematics of reactors used for fullerene synthesis. Shown are (a) a reduced-
pressure fuel-rich pyrolytic chamber, and (b) a benchtop modified arc evaporation system. Reproduced
with permission from (a) Hebgen, P.; Goel, A.; Howard, J. B.; Rainey, L. C.; Vander Sande, J. B. Proc.
Combust. Inst. 2000, 28, 1397, Copyright 2000 Elsevier, and (b) Scrivens, W. A.; Tour, J. A. J. Org.
Chem. 1992, 57, 6932. Copyright 1992 American Chemical Society.
Although fullerenes have been actively investigated for more than two decades,
there is an ongoing debate regarding the growth mechanism of these nanoclusters.
Since the formation of fullerene s via laser/arc/combustion techniques occurs too
rapidly to isolate intermediate species, most of the mechanistic proposals are based
on theoretical techniques (quantum mechanical and molecular dynamics). It was
once thought that fullerenes were formed from the folding of preformed graphitic
sheets that emanated from the target following laser ablation. However, a variety of
experiments have shown that the growth process likely initiates from small linear
chains of carbon atoms. As the number of carbon atoms increases, the chains
preferentially connect into ring structures due to their greater stabilities. In particu-
lar, for C
n
where n < 10 (with the exception of C
6
as discussed below), linear
species are the preferred morphology rather than rings (Figure 6.28). The preference
for ring formation for n ¼ 6, and n 10 (especially for C
10
,C
14
,C
18
, etc.) is due to
the enhanced aromaticity/stability of planar rings when there are 4n +2p electrons
(where n ¼ 1, 2, 3, ... – the H
€
uckel rule).
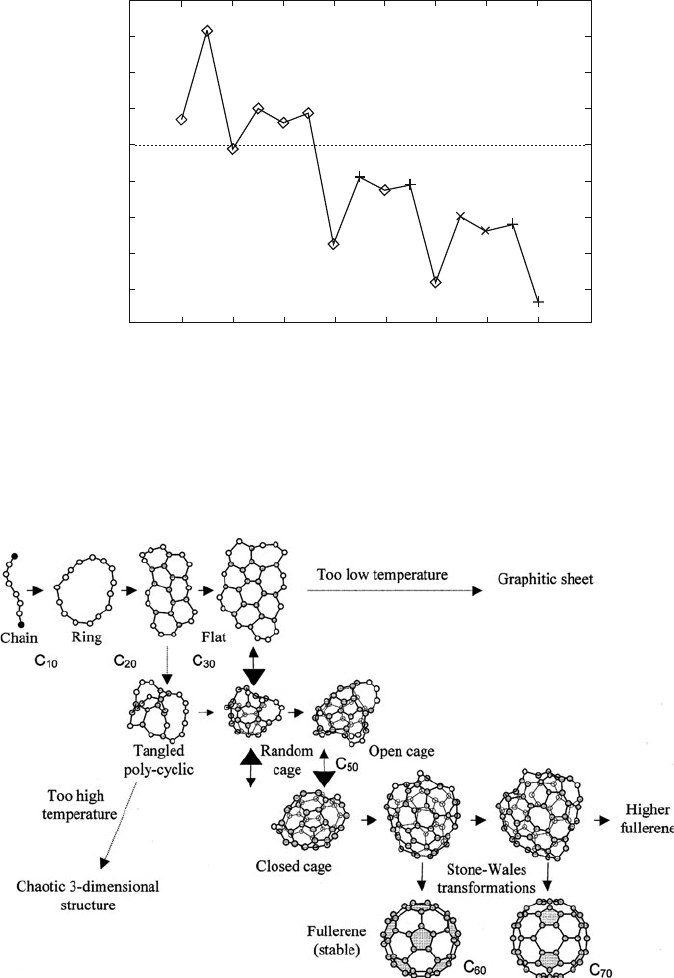
Figure 6.29 illustrates a proposed mechanism for the subsequent steps of fullerene
growth, involving the self-assembly of carbon rings. When n 30 or so, the
monocyclic rings are proposed to form graphitic sheets. The “pentagon road”
mechanism proposed by Smalley
[103]
assumes that the graphitic sheets contain
both hexagon and pentagon units. Closure of the sheets to form Buckyballs effec-
tively results in growth termination. In contrast, the “fullerene road” model assumes
the initial formation of smaller non-IPR fullerenes, which undergo thermal
rearrangement to yield C
60
and higher fullerenes.
[104]
As discussed earlier, pentagon units are essential to the fullerene structure, sinc e
they allow the planar graphitic sheet to curl. The driving force for this rearrangement
is likely the C—C bonding of edge carbons that satisfies their unfilled valences.
As noted in Figure 6.29, adequate annealing is required in order to incorporate a
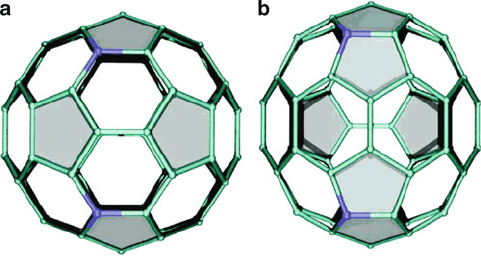
Figure 6.27. Illustration of C
58
N
2
that (a) satisfies the IPR and (b) violates the IPR with adjacent
pentagons. The structure with adjacent pentagons, (b), is more stable than (a) by 12.5 kcal mol
1
.
Reproduced with permission from Ewels, C. P. Nano Lett. 2006, 6, 890. Copyright 2006 American
Chemical Society.
6.3. Nanoscale Building Blocks and Applications 491
monocyclic rings
linear chains
Number of Carbon Atoms
Relative Stability [kcal/mol]
80
60
40
20
0
−20
−40
−60
−80
−100
2 4 6 8 10 12 14 16 18 20
Figure 6.28. The relative stabilities of linear-chain carbon clusters vs. monocyclic rings with changing
cluster size. Reproduced with permission from Hutter, J.; Luthi, H. P.; Diederich, F. J. Am. Chem. Soc.
1994, 116, 750. Copyright 1994 American Chemical Society.
Figure 6.29. Proposed mechanism for fullerene growth. Reprinted from Yamaguchi, Y.; Maruyama, S.
Chem. Phys. Lett. 1998, 286, 343. Copyright 1998, with permission from Elsevier. 87985734.
492 6 Nanomaterials
sufficient number of pentagons. For instance, if the cooling rate is too high,
amorphous soot particles will be preferentially formed rather than fullerenes.
In addition, an overall low growth temperature will not be sufficient to cause cage
formation, yielding planar graphitic fragments instead of fullerenes.
Regardless of the proposed mechanism, a final thermal annealing step is likely
required to organize the hexagon and pentagon subunits into the lowest-energy IPR
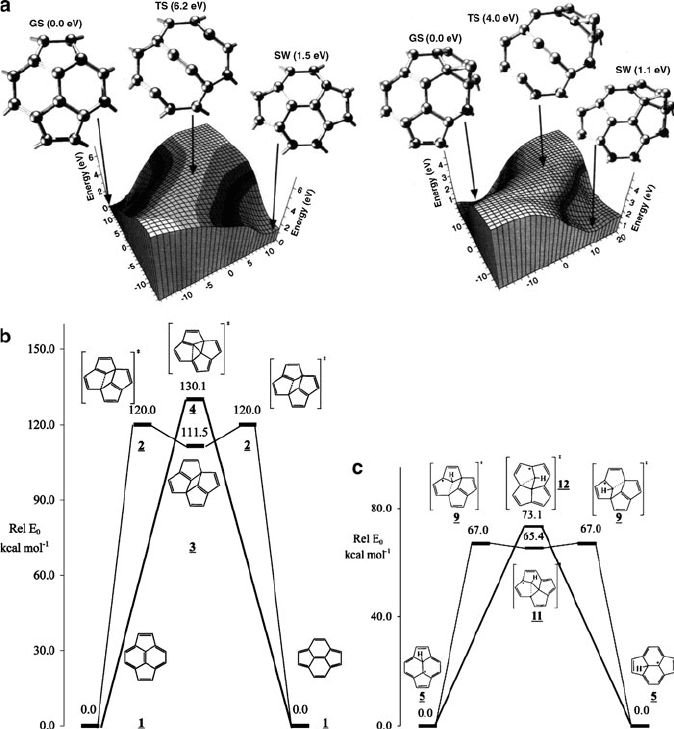
arrangement. This rearrangement step is known as the Stone–Wales (SW) transfor-
mation, and involves a concerted reorganization of the hexagon/pentagon units.
We already saw an example of a rar e SW transformation where the non-IPR
N-containing species was actually lowest in energy (Figure 6.27). However, most
often this rearrangement occurs in the opposite direction – transforming adjacent
pentagons into a hexagon-isolated structure. It should be noted that the Stone–Wales
transformation is actually thermally forbidden via the Woodward–Hoffman rules;
calculations show an energy barrier of at least 5 eV for this pathway. However, it has
been shown that this rearrangement may likely be catalyzed by additional carbon
and/or hydrogen atoms that are present during laser/arc or thermal combustion
syntheses (Figure 6.30).
Interestingly, a fullerene structure may serve as a nucleation site for additional
layers of graphite en route toward multishell fullerenes (Figure 6.31). These are
denoted as “C
60
@C
240
” where the @ symbol represents the encapsulated species.
There are even triple-layered structures such as “C
60
@C
240
@C
560
.”
[105]
Though
very small quantities (<0.01%) of multilayered fullerenes are found in the soot
resulting from laser vaporization, the yield may be improved by in vacuo sub-
limation of the vapor phase at a high temperature (ca. 1,200
C).
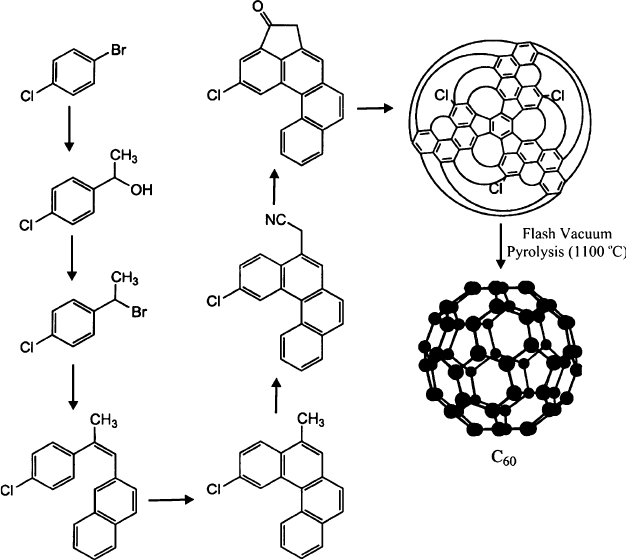
Although the “brute force” methods of laser/arc and high-temperature pyrolysis
represent the most common techniques for generating fullerenes, a goal of the
synthetic organic chemist has long been the solution-phase, stepwise synthesis of
C
60
. In 1999, a promising step in that direction was accomplished with the first
nonpyrolytic synthesis of “buckybowls.”
[106]
These structures had a bowl-shaped
structure, and consisted of the hexagon-isolated pentagon backbone exhibited by
fullerenes (Figure 6.32). In early 2002, a chlo rinated C
60
precursor was reported
using a traditional 12-step organic synthesis. This compound was subsequently
converted to Buckyballs using high-temperature vacuum pyrolysis (Figure 6.33).
The yield of C
60
was <1% – certainly not useful for commercial production of
Buckyballs! However, the novelty of this approach was that pyrolysis did not
decompose the precursor into smaller units, but rather served to stitch together
adjacent arms of the molecular precursor. Hence, this method provides a targeted
route toward individual fullerenes based on the structure of the precursor, rather than
high-energy methods that always result in a mixture of fullerene products.
In addition to pristine fullerene structures, it has been discovered that various
metal ions may be encapsulated inside the caged structure to yield endohedral
fullerenes. Thus far, a variety of alkali and lanthanide metals, Group V atoms,
noble gases, and neutral molecules such as H
2
,
[107]
CO, and H
2
O have been
sequestered inside the C
60
structure. Calculations have shown that the encapsulation
6.3. Nanoscale Building Blocks and Applications 493
of noble gas atoms and small ions (e.g., Li
+
,F
,Cl
) actually stabilize the fullerene
cage, whereas larger species (e.g., Rb
+
,Br
,I
) dest abilize the cage.
[108]
Metallo-
fullerenes (M@C
x
) are typically grown by either laser ablation of metal-doped
graphite disks at high temperature (ca. 1,200
C), or carbon arc techniques with
metal-doped graphite rods. An example of a metallofullerene (Gd
3+
@C
60
) was
shown in Figure 6.22a; these structures are commonly employed as MRI contrast
agents.
Figure 6.30. The potential energy surface of the Stone–Wales transformation before/after the addition
of catalyzing moieties such as (a) carbon, and (b) hydrogen atoms. Reproduced with permission from
(a) Eggen, B. R.; Heggie, M. I.; Jungnickel, G.; Latham, C. D.; Jones, R.; Briddon, P. R. Science 1996,
272, 87, Copyright 1996 AAAS; and (b) Nimlos, M. R.; Filley, J.; McKinnon, J. T. J. Phys. Chem. A 2005,
109, 9896. Copyright 2005 American Chemical Society.
494 6 Nanomaterials
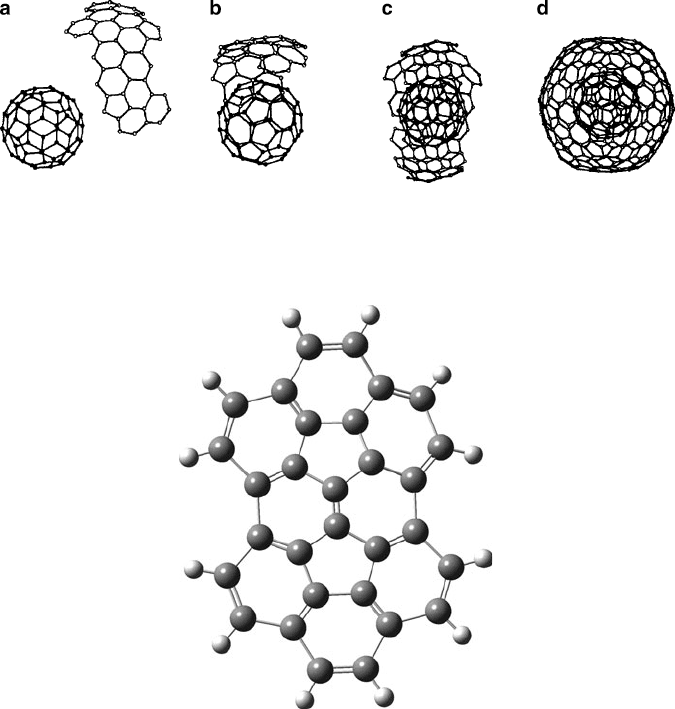
You may be wondering “how does the ion get inside the cage?” That is, does this
occur during the growth of the fullerene structure itself, or does the metal ion insert
after the cage is already formed? It has been shown that the latter likely occurs, with
the exact entrance pathway dependent on the size of the dopant species. Small
dopants such as He or H
+
may directly pass through either hexagon or pentagon
units of the cage toward the vacant core. However, for larger ions/atoms, some
framework C—C bonds must be reversibly broken in order to accommodate the
incoming species – aptly referred to as a window mechanism (Figure 6.34). Since
non-IPR fullerenes have relatively large strain ener gies due to fused pentagons, this
process should occur readily for these struct ures. Indeed, there has been much recent
interest in synthesizing “unconventional” metallofullerenes such as Sc
2
@C
66
.
[109]
Figure 6.31. Proposed scheme for the formation of the multishell fullerene C
60
@C
240
. Reproduced from
Mordkovich, V. Z.; Shiratori, Y.; Hiraoka, H.; Takeuchi, Y. Synthesis of Multishell Fullerenes by Laser
Vaporization of Composite Carbon Targets, found online at http://www.ioffe.rssi.ru/journals/ftt/2002/04/
p581–584.pdf.
Figure 6.32. Backbone structure of semibuckminsterfullerene, C
30
H
12
.
6.3. Nanoscale Building Blocks and Applications 495
Unlike the empty C
66
counterpart, these structures are stable since the incoming
metal atom donates electron density to the C—C bond between adjacent pentagons,
causing a decrease in the local bond strain.
As you might imagine, relatively large ions such as Cs
+
,Y
3+
,orSc
3+
are likely not
encapsulated through a simple reversible windowing mechanism. In order for this to
occur, more than one C—C bond would need to be broken (Figure 6.34b), which
increases the energetic barrier for this to occur. More recently, a “hole-repairing
mechanism” was proposed for Y@C
82
metallofullerenes, in which calculations
predict the combina tion of a large C
76
open-cage fullerene and a smaller C
6
Y
fragment that effective ly repairs the framework hole (Figure 6.35).
[110]
Vapor-phase syntheses of 0-D nanostructures
In addition to fullerenes, a variety of metallic and compound (oxides, nitrides,
chalcogenides) nanoclusters/nanoparticles may be synthesized using vapor techni-
ques. The primary benefits of this approach relative to solution-phase methods
Figure 6.33. Synthesis of a C
60
precursor. Reproduced with permission from Scott, L. T.; Boorum, M. M.;
McMahon, B. J.; Hagen, S.; Mack, J.; Blank, J.; Wegner, H.; de Meijere, A. Science 2002, 295, 1500.
Copyright 2002 AAAS.
496 6 Nanomaterials
discussed in the next section is a much higher rate of production and a greater purity/
compositional control. That is, gas-phase generated nanoparticles will be free of
impurities acquired from solvents and added reagents such as surfactants, reducing
agents, etc. Further, complex compositions and (meta)stable phases (e.g., high-
temperature supercondu ctors, intermetallics, etc.) are often more easily synthesized
using gas-phase decomposition techniques rather than low-temperature solvent
processing.
[111]
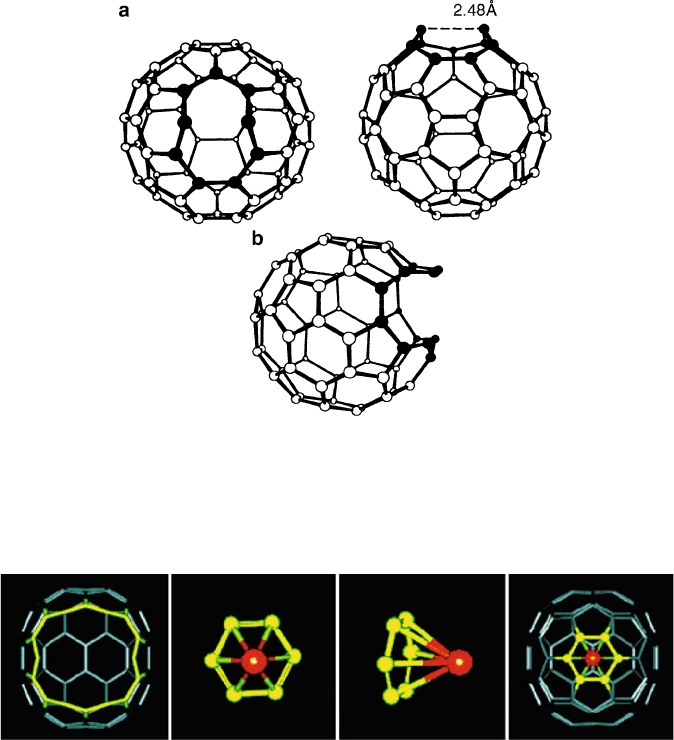
Figure 6.35. Images of the calculated structures involved in the “hole-repairing” mechanism for
endohedral fullerene growth. Shown from left to right are: the C
76
open cage, top and side views of the
C
6
Y fragment, and the final Y@C
82
metallofullerene. Reproduced with permission from Gan, L.-H.;
Wang, C.-R. J. Phys. Chem. A 2005, 109, 3980. Copyright 2005 American Chemical Society.
Figure 6.34. Theoretical intermediates during endohedral fullerene formation. Image (a) shows the nine-
membered ring formed by a single pentagon–hexagon bond. By comparison, image (b) illustrates the
formation of a larger 13-membered ring by breaking two framework bonds. Reproduced with permission
from Murry, R. L.; Scuseria, G. E. Science 1994, 263, 791. Copyright 1994 AAAS.
6.3. Nanoscale Building Blocks and Applications 497
