Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.

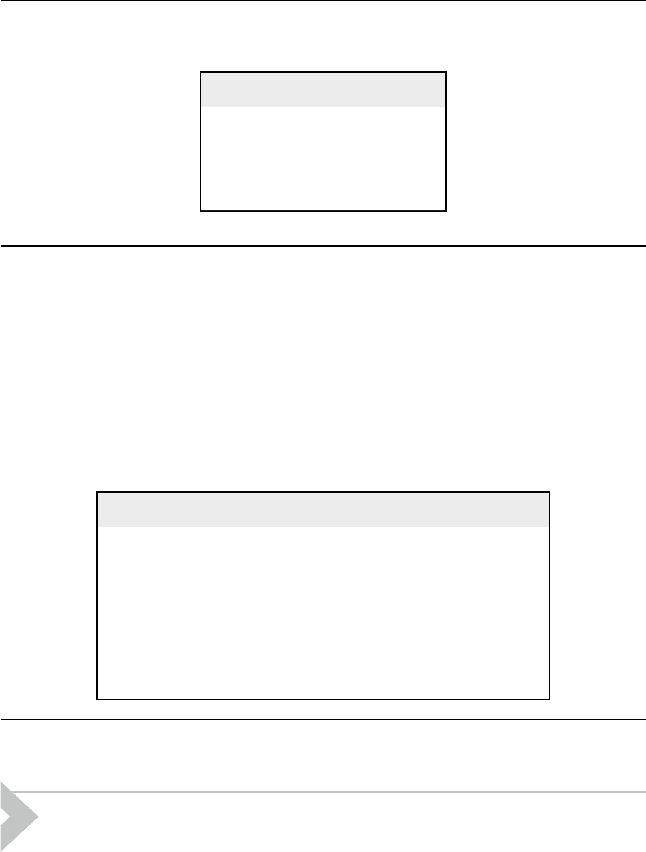
Example E5.6
Light naphtha with a specific gravity of 0.724 is used as a feed to the isomeriza-
tion unit at a rate of 100 m
3
/h. Find the product composition.
Solution:
Appling the yield guidelines of Table 5.7, the product composition is presented
in Table E5.6.1.
Questions and Problems
5.1. Why must C
6
hydrocarbons be removed from the reformer feed? How
are they removed?
5.2. Show the role of the reformer in the refinery.
5.3. List the types of reforming reactions and show their relative
importance.
5.4. Why was the CCR process developed as an alternative to the SR fixed
bed process?
Table E5.6.1 Isomerization yields
wt% kg/h
Feed 100 72,400
Product
C
3
0.348 251.9
iC
4
0.619 448.2
nC
4
1.770 1281.5
C
þ
5
97.261 70,417
Total 72,399
Table 5.7 Isomerization yield
Component Yield (wt%)
C
3
0.348
iC
4
0.619
nC
4
1.770
C
þ
5
97.261
Catalytic Reforming and Isomerization 121
5.5. The following feed of 5000 BPD of naphtha of 50 API was introduced
in a reformer:
Compound C
6
H
12
C
6
H
14
C
7
H
14
C
7
H
16
C
8
H
16
C
8
H
18
wt% 20 10 20 20 10 20
(a) How much hydrogen is produced?
(b) Calculate the composition of naphtha reformate.
5.6. Heavy naphtha of 1000 kg/h having a specific gravity of 0.75 is used as
a feed to a reformer. This feed has a boiling range of (190–380
F).
Make a material balance around the reformer knowing that the refor-
mate RON is 96.
5.7. Calculate the barrel of toluene formed from one barrel of methylcyclo-
hexane (MCH) at 1100
F and 500 psi. 80,000 SCF/bbl of hydrogen is
recycled and enters with the feed. Assume the free energy of the
reaction at this condition is 20,000 cal/gmol.
5.8. One thousand m
3
/h of light naphtha of API equaling 70 is fed into an
isomerization unit. Make a material balance around this unit.
5.9. Develop a UNISIM flowsheet simulation for three equilibrium bed
reactors at 550
C and 10 bar pressure.
The following feed is fed to the reformer:
Cycloheptane (C
7
H
14
): 25 mol%
Heptane (C
7
H
16
): 25 mol%
Dimethylcyclohexane (C
8
H
16
): 50 mol%
REFERENCES
Antos, G. J. et al. (1995). ‘‘Catalytic Naphtha Reforming.’’ Marcel Dekker, New York.
Gary, J. H., and Handwerk, G. E. (2001). ‘‘Petroleum Refining.’’ Marcel Dekker, New
York.
Kaes, G. L. (2000). ‘‘Refinery Process Modeling.’’ Kaes Enterprises Inc., Colbert (Georgia).
Maples, R. E. (1993). ‘‘Petroleum Refining Process Economics.’’ PennWell Book, Tulsa.
Martino, G. (2001). ‘‘Catalytic Reforming’’ Chapter 4 in ‘‘Conversion Processes’’ Petro-
leum Refining, Vol. 3, Leprince, P., ed., TECHNIP, France.
Travers, C. (2001). ‘‘Isomerization of light paraffins’’ Chapter 6 in ‘‘Conversion Processes’’
Petroleum Refining, Vol. 3, Leprince, P., ed., TECHNIP, France.
UNISIM Design Suite R370, Honeywell Process Solutions, Calgary, Alberta, Canada.
122 Chapter 5

CHAPTER SIX
Thermal Cracking and Coking
6.1. Introduction
Thermal cracking is the cracking of heavy residues under severe
thermal conditions. The liquid products of this process are highly olefinic,
aromatic and have high sulphur content. They require hydrogen treatment
to improve their properties. Coking is the process of carbon rejection from
the heavy residues producing lighter components lower in sulphur, since
most of the sulphur is retained in the coke.
The thermal treatment of hydrocarbons follows a free radical mechanism
where cracking reactions take place in the initiation step. The reactions in
the final step result in the formation of heavy fractions and products like
coke.
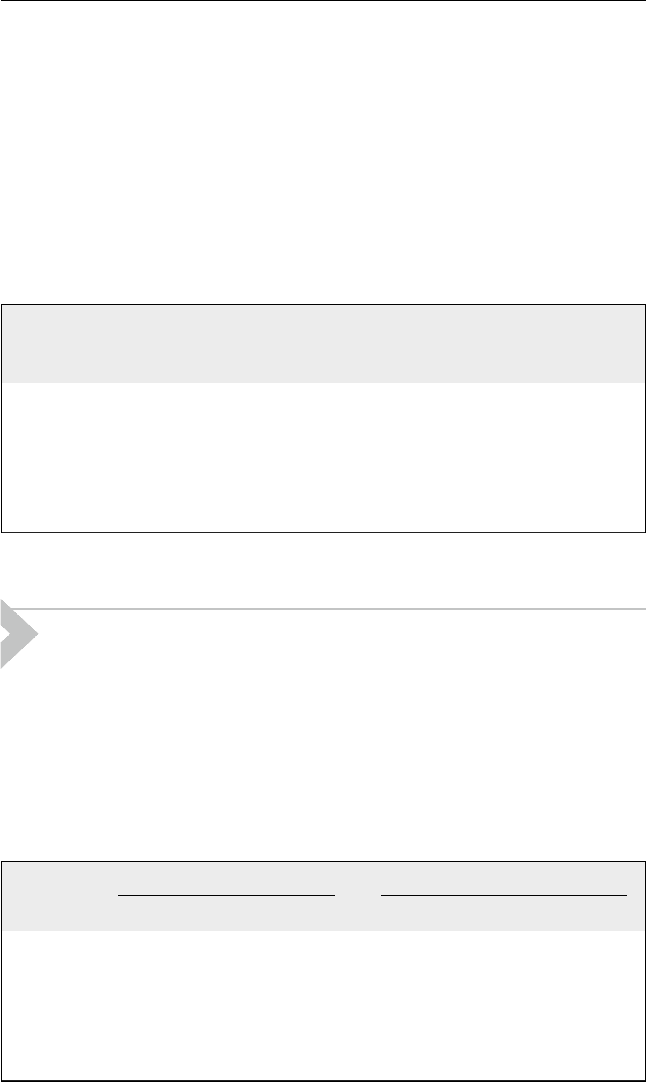
Reaction pathways of different fractions are expressed in Figure 6.1.
There are three classes of industrial thermal cracking processes. The first is
mild cracking (as in visbreaking) in which mild heating is applied to crack
the residue just enough to lower its viscosity and also to produce some light
products. The second process is delayed coking in which moderate thermal
cracking converts the residue into lighter products, leaving coke behind.
The third process involves severe thermal cracking: part of the coke is
burned and used to heat the feed in the cracking reactor, as in fluid coking.
In other version of the process, steam is used to gasify most of the coke
(flexicoking). More detailed operating conditions about the three processes
are given in Table 6.1 (Speight, 1991).
The complexity of the mixtures that represent petroleum fractions and
their vacuum residues makes the identification of the reaction pathways of
individual hydrocarbon compound a very difficult task.
6.2. Coke Formation
Coke can be formed from the condensation of polynuclear aromatics
(such as n-butylnapthalene) as shown in equation (6.1)
Fundamentals of Petroleum Refining
#
2010 Elsevier B.V.
DOI: 10.1016/ B978-0-444-52785-1.00006-1 All rights reserved.
123
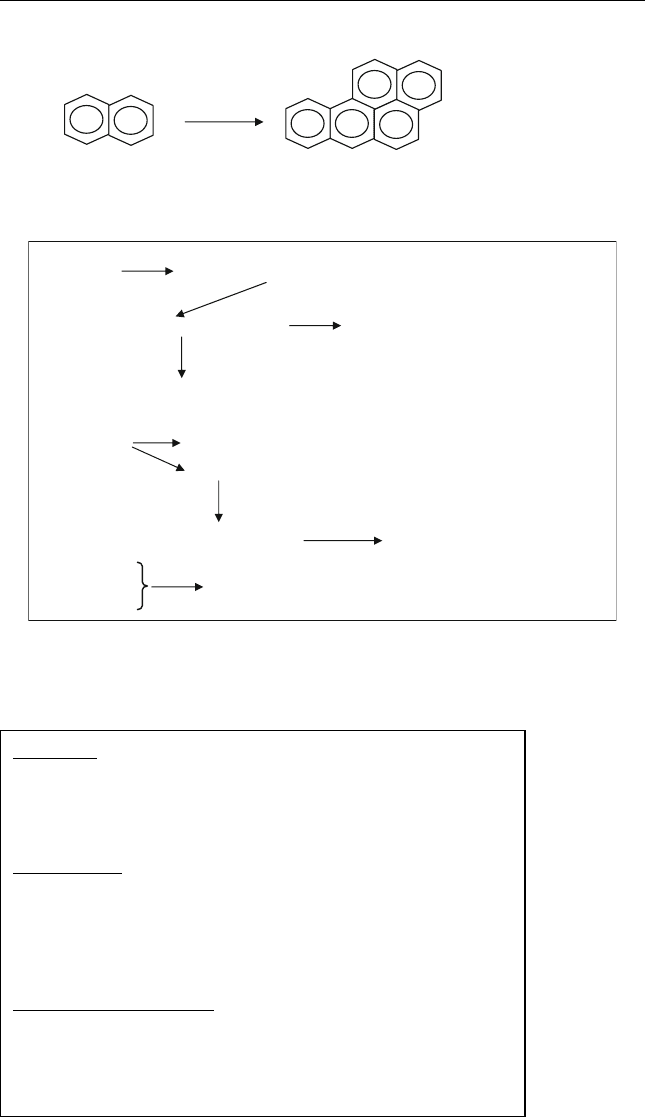
−C
4
H
9
−CH
3
CH
3
|
+ 2C
3
H
8
C
14
H
16
C
22
H
16
(Coke precursor)
H/C=0.73
Propane
2
ð6:1Þ
Table 6.1 Thermal cracking process
Visbreaking
Mild heating 471–493
C (880–920
F) at 50–200 psig
Reduce viscosity of fuel oil
Low conversion (10%) at 221
C (430
F)
Heated coil or soaking drum
Delayed coking
Moderate heating 482–516
C (900–960
F) at 90 psig
Soak drums 452– 482
C (845–900
F)
Residence time: until they are full of coke
Coke is removed hydraulically
Coke yield 30 wt%
Fluid coking and flexicoking
Severe heating 482–566
C (900–1050
F) at 10 psig
Fluidized bed with steam
Higher yields of light ends
Less coke yield (20% for fluid coking and 2% for flexicoking)
Saturates
Saturates + Unsaturates and light gas
Heat
Heat
Unsaturates free radicals
Lower boiling unsaturates and gas
Aromatics + lower unsaturates and gas
Lower boiling aromatics + unsaturates and gas
Heat
Heat
Aromatic free radicals + unsaturates and gas
Aromatics
Condensation
CokeHigher boiling aromatics
Coke + lower boiling aromatics + unsaturates and gas
Heat
Resins and
Asphaltenes
Figure 6.1 Thermal cracking mechanism
124 Chapter 6
The coke formed from the condensation of polynuclear aromatics has a
H/C ratio of 0.73 (Chang and Robinson, 2006). The coke formed through
other reactions might have the formula CH
a
where a ¼ 0.2–0.8. Coke
formation can occur through the condensation of olefins and butadiene
with aromatics to yield low hydrogen content coke. Thermal cracking of
C
6
hydrocarbons may yield certain amount of coke (CH
0.8
) as shown in
Table 6.2. These reactions also yield unsaturated hydrocarbons which might
react with aromatics to yield coke precursors (Zhorov, 1987).
6.3. Thermodynamics of Coking of Light
Hydrocarbons
Thermal cracking reactions are highly endothermic and require heat
which is either provided by heating furnaces or generated by burning some
of the produced coke. The enthalpies and Gibbs free energies of the six
reactions listed in Table 6.2 are given in Table 6.3 (Zhorov, 1987).
Table 6.3 Enthalpies and Gibbs energies of reaction
Reaction
DH (kJ) at T (K) DG (kJ) atT (K)
300 500 1000 300 500 1000
1 1.8 1.4 2.0 2.1 4.7 9.9
2 3.3 4.9 2.7 8.1 16.8 37.7
3 266 274 276 161 90 96
4 267 280 288 54 29 261
5 141.4 146 146.1 74.9 29.5 88.5
6 21. 1 20.3 12.0 36.3 47.3 78.3
Table 6.2 Model reactions of coke formation
Reaction
No. Reaction
Coke yield
mass
fraction
Type of
light-end
product
1C
6
H
14
!
1.15 C
5
H
12
þ 0.34 CH
0.8
0.05 Alkane
2C
6
H
14
!
1.33 C
4
H
10
þ 0.68 CH
0.8
þ 0.8 H
2
0.10 Alkane
3C
6
H
14
!
1.32 C
2
H
4
þ 3.36 CH
0.8
þ 3.01 H
2
0.50 Alkene
4C
6
H
14
!
0.66 C
4
H
6
þ 3.36 CH
0.8
þ 3.68 H
2
0.50 Diene
5C
6
H
12
!
1.36 C
2
H
4
þ 3.28 CH
0.8
þ 2.97 H
2
0.50 Alkene
6C
6
H
6
!
1.48 C
2
H
4
þ 3.04 CH
0.8
þ 2.82 H
2
0.50 Alkene
Thermal Cracking and Coking 125
Data shown in Table 6.3 indicates that coking is enhanced by high
temperatures. For example, reactions 3 and 5 become thermodynamically
possible only close to 1000 K, while at 300–500 K, they cannot proceed.
An aromatic coke forms due to poly-condensation with the evolution of
hydrogen. The reaction for producing coke from benzene (reaction 6) with
the simultaneous separation of a light-end aliphatic hydrocarbon (C
2
H
4
)is
thermodynamically improbable.
The evolution of substantial amounts of methane and hydrogen in
coking can be explaine d by thermodyna mic consideration s. The formati on
of light-end product from diene cracking (reaction 4) becomes thermody-
namically probable at high temperat ures, which is consistent with experi-
mental data. The formation of coke from alkanes, alkenes and cycloalkanes
is ge nerall y endothermic, while it i s exothermic for aromatics.
When determining the composition of the equilibrium mixture for a
coke formation reaction. It is important that the equilibrium constant K
P
is
expressed in terms of the partial pressures of only the gaseous products.
For example, for reaction 3, in Table 6.2:
K
P
¼
P
1:32
C
2
H
4
P
3:01
H
2
P
C
6
H
14
¼ exp
DG
RT
ð6:2Þ
At T ¼ 500 K
K
P
¼ exp
90; 000
8:31 500
¼ 3:92 10
10
At T ¼ 1000 K
K
P
¼ exp
96; 000
8:31 1000
¼ 1:04 10
5
Thus, while the reaction does not proceed at 500 K, it proceeds favour-
ably at 1000 K.
6.4. Visbreaking
Visbreaking is a mild thermal cracking of vacuum or atmospheric
residues to produce light products and 75–85% cracked material of lower
viscosity that can be used as fuel oil.
126 Chapter 6

6.4.1. Feed Sources
The feed to visbreaker can be either
Atmospheric residue (AR)
Vacuum residue (VR)
Vacuum residue is the heaviest distillation product and it contains two
fractions: heavy hydrocarbons and very heavy molecular weight molecules,
such as asphaltene and resins.
6.4.2. Visbreaking Reactions
Themainreactioninvisbreakingisthermal cracking of heavy hydrocarbons,
since resins are holding asphaltene and keep them attached to the oil. The
cracking of resin will result in precipitation of asphaltene forming deposits in the
furnace and will aslo produce unstable fuel oil. The cracking severity or
conversion is limited by the storage stability of the final residual fuel. The
possible reactions in visbreaking are:
Paraffinic side chain breaking which will also lower the pour point;
Cracking of naphthens rings at temperature above 482
C (900
F);
Coke formation by polymerization, condensation, dehydrogenation and
dealkylation; and
Further cracking will be the result of asphaltene and coke leaving the
liquid phase (delayed coking).
6.4.3. Visbreaking Severity
The severity of visbreaking can be defined according to the following
considerations:
Stability of residual fuel on storage
Material produced that boils below 160
C (330
F) (conversion)
Percent reduction in product viscosity (25–75%)
6.4.4. Kinetics of Visbreaking
The thermal decomposition of hydrocarbons having lower molecular
weight than resins is rather fast and highly endothermic. The mechanism
of the thermal cracking reactions is the result of chain reactions by radicals.
The kinetics can be described as first order:
kt ¼lnð1 xÞð6:3Þ
and
k ¼ k
o
exp
E
RT
ð6:4Þ
Thermal Cracking and Coking 127
where x is the weight fraction converted, t is the time, k is the reaction rate
constant, T is the reaction temperature, and the activation energy (E ) varies
according to feed from 150 to 315 kJ/mol. The corresponding activation
energies for asphaltene and coke are 250 and 380 kJ/mol, respectively.
6.4.5. Product Yield and Properties
Four products are produced i n the visbrea king process: gases (C
4
), Naph-
tha C
5
166
C(C
5
33 0
F), gas oil 166–350
C (330–660
F) and
residue or tar 350þ
C(660þ
F). Typical yields are given in Table 6.4.
Visbreaking results in an increase of API of 2–5 for the vacuum residue
feed and a reduction of viscosity of 25–75%. A specific example for
Kuwait residue vi sbreaking with product yields and pr operties is given
in Table 6.5.
6.4.6. Prediction of Visbreaking Yields
The following correlations are generated from plant operation data compiled
by Maple (1993). The correlation coefficients range from 0.981 to 0.999.
Products yields:
Conversion ¼ % Conv ¼
Gas wt% þ Naphtha wt%
FeedðVRÞwt%
100 ð6:5Þ
Table 6.5 Visbreaking of Kuwait residue
Feed Gases
Gasoline
(naphth a)
Gas oil
(GO)
Ta r
(residue)
API 14.4 65.0 32.0 11.0
Sulphur (wt%) 4.1 1.0 2.5 4.3
Viscosity cSt
at 50
C
720 – – – 250
Yield (wt%) – 2.5 5.9 13.5 78.1
Table 6.4 Typical yields of visbreaking process
Product wt% of charge
Gases (C
4
) 2–4
Naphtha (C
5
–330
F) 5–7
Gas oil (330–660
F) 10–15
Tar (660þ
F) 75–85
128 Chapter 6

Gas wt% ¼ 0:189825 % Conv þ 0:677163 ð6:6Þ
Gasoline wt% ¼ 0:738321 % Conv þ 0:260174 ð6:7Þ
Residue wt% ¼0:146668 ð% ConvÞ
2
2:203644 % Conv þ 98 :677947
ð6:8Þ
H
2
S in Gas wt% ¼ 0:02023 % Conv þ 0: 06043 wt%S
f
0:156
ð6:9Þ
where S
f
refers to sulphur in feed
Gas oil wt% ¼ 100 Gas wt% Gasoline wt% Residue wt% H
2
Swt%
ð6:10Þ
Sulphur(S) in visbreaker products:
SinH
2
S in Gas ¼ðH
2
S 32=34Þð6:11Þ
wt% S in Gasoline ¼ 0:260112 wt%S
f
ð6:12Þ
wt% S in Gas oil ¼ 0:539924 wt%S
f
ð6:13Þ
S in Residue ¼ S in Feed S in gasoline SinGOSinH
2
S ð6:14Þ
Gravity of visbreaking products:
Gasoline API ¼0:26215 % Conv þ 0 :315121 API
f
þ 56:83723
ð6:15Þ
Gas oil API ¼0:052919 % Conv þ 0:52228042 API
f
þ 12:9318914
ð6:16Þ
Residue API ¼0:7462183 % Conv þ 1:29131825 API
f
2:6831388
ð6:17Þ
where API
f
is feed API gravity and S
f
is the sulphur content in feed.
Example E6.1
A vacuum residue is fed into a coil visbreaker at a rate of 200,000 lb/h. It has an
API ¼ 8.5 and sulphur content of 3%. Assume 6 wt% conversion.
Make a material balance for the visbreaker.
Solution:
The solution of this example is summarized in Table E6.1.
Thermal Cracking and Coking 129
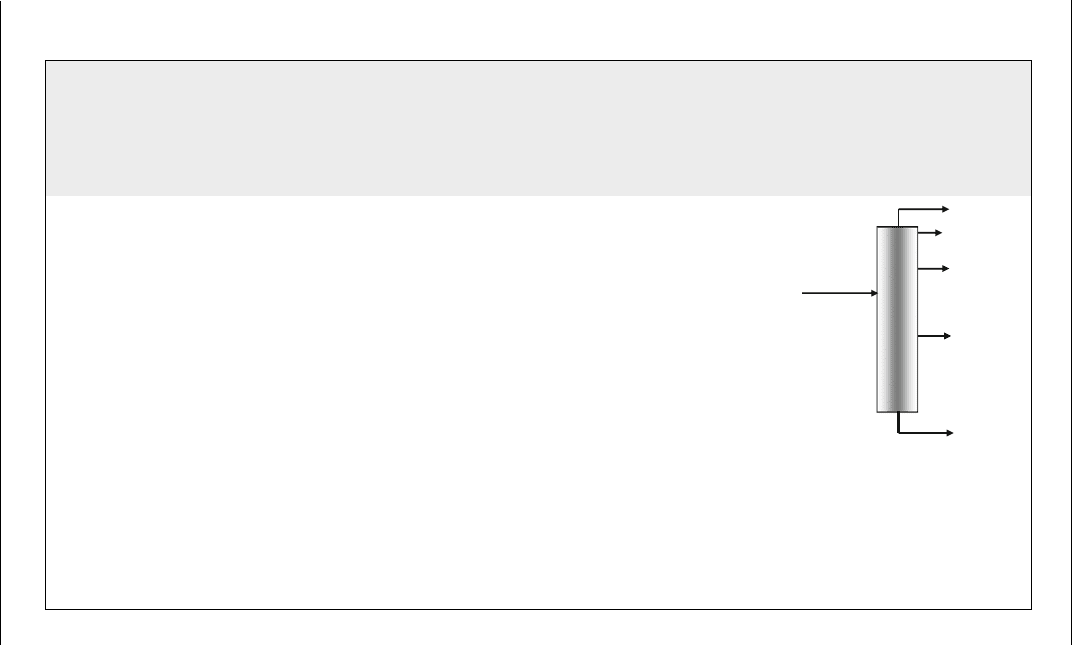
Table E6.1 Results of example E6.1
Feed rate ¼ 200,000 lb/h
Visbreaking
S
f
¼ 3wt%
Conversion ¼ 6wt%
API
f
¼ 8.5
Products yield wt% lb/h
Gas ¼ 0.189825 %Conv þ 0.677163 1.82 3632
Gasoline ¼ 0.738321 %Conv þ 0.260174 4.69 9380
Residue ¼0.146668 %Conv
2
2.203644 %Conv þ 98.677947 79.58 159,152
H
2
S ¼ 0.02023 %Conv þ 0.06043 wt% S 0.156 0.15 300
Gas oil ¼ 100 Gas Gasoline Residue H
2
S 13.76 27,536
VR
Residue
159,152
Gas 3632
Gasoline
9380
H
2
S 300
GO
27,536
200,000
100.00 200,000
Sulphur in visbreaker products
S in H
2
S ¼ H
2
S in gas (32/34)) 94.12 282
S in Gasoline ¼ 0.260112 S
f
0.78 73
S in GO ¼ 0.539924 S
f
1.62 444
S in Residue ¼ S in Feed S in gasoline S in GO S in H
2
S 3.48 5200
6000
Gravity of visbreaking products API SG
Gasoline ¼0.26215 %Conv þ 0.315121 API
f
þ 56.83723) 57.9 0.75
Gas oil ¼0.052919 %Conv þ 0.52228042 API
f
þ 12.9318914 17.1 0.95
Resid ¼0.74621 83 %Conv þ 1.29131825 API
f
2.6831388 3.8 1.05
130 Chapter 6
