Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.


The equilibrium conversion is calculated as follows
moles of cyclohexane ¼ 1 bbl
42 gal
1 bbl
!
10
6
cm
3
264:17 gal
!
0:779 g
cm
3
!
1 mol
84:16 g
!
¼ 1471:63 mol
moles of hydrogen ¼
10;000 SCF
1 bbl
1 mol
379 SCF
1bbl of CHðÞ¼26:39mol
We will assume X moles is transformed to benzene (B) and 3X to hydrogen
(H
2
). The equilibrium constant can be expressed as:
K ¼
P
B
P
3
H
2
P
CH
The mole fractions of products are:
moles of B ¼ X
moles of CH¼ 1471.63 X
moles of H
2
¼ 3X þ 26.39
Total mole (1498 þ 3X)
The equilibrium constant can be expressed as:
K ¼
y
B
P
T
ðÞy
H
P
T
ðÞ
3
y
CH
P
T
6:55 10
5
¼
X
1498 þ 3X
3X þ 26:39
1498 þ 3X
3
20ðÞ
3
1471:63 X
1498 þ 3X
solving X ¼ 1464 mol
Equilibrium conversion of CH ¼
moles of CH reacted
moles of CH fed
¼
1464
1471:63
¼ 0:995
Barrels of benzene produced are:
¼ 1464 mol
78:11 g
1 mol
cm
3
0:879 g
264:17 gal
10
6
cm
3
1 bbl
42 gal
¼ 0:818 bbl
Catalytic Reforming and Isomerization 101

5.2.6. Reaction Kinetics and Catalysts
The catalyst used for reforming is a bifunctional catalyst composed of platinum
metal on chlorinated alumina. Platinum acts as the centre for the dehydrogena-
tion reaction, and chlorinated alumina acts as an acidic site to promote structure
changes, such as cyclization of paraffins and isomerization of the naphthenes.
Recently additional elements have been added to platinum to promote
additional properties for the catalyst. Iridium (Ir) is added to boost activity,
Rhenium (Re) is added to operate at lower pressures and Tin (Sn) is added
to improve yield at low pressures. The use of Pt/Re is now most common
in semi-regenerative (SR) processes with Pt/Sn is used in moving bed
reactors. The quantity of chlorine used is approximately 1 wt% of the
catalyst and the quantity of platinum is from 0.2 to 0.6 wt%. Impurities
that might cause deactivation or poisoning of the catalyst include: coke,
sulphur, nitrogen, metals and water. Because of these problems, the
reformer feed has to be severely hydrotreated to remove most of these
impurities, and the reformer should be operated at high temperature and
low pressure to minimize coke deposition.
Paraffin and naphthene dehydrogenation reactions are very rapid and
usually occur in the first reactor. The isomerization of paraffin and naphthenes
is fast, whereas hydrocracking is slow and takes place in the last reactor. The
effect of operating conditions on reaction rate and other properties is shown
in Table 5.1.
5.2.7. Process Technology
There are several commercial processes available for reforming. These
include Platforming (UOP), Powerforming (Exxon), Magna forming
(Engelhard), Catalytic reforming (IFP), Rheniforming (Chevron) and
Ultra forming (Amoco). The old technologies are fixed bed configuration.
Moving bed technology has also recently been introduced.
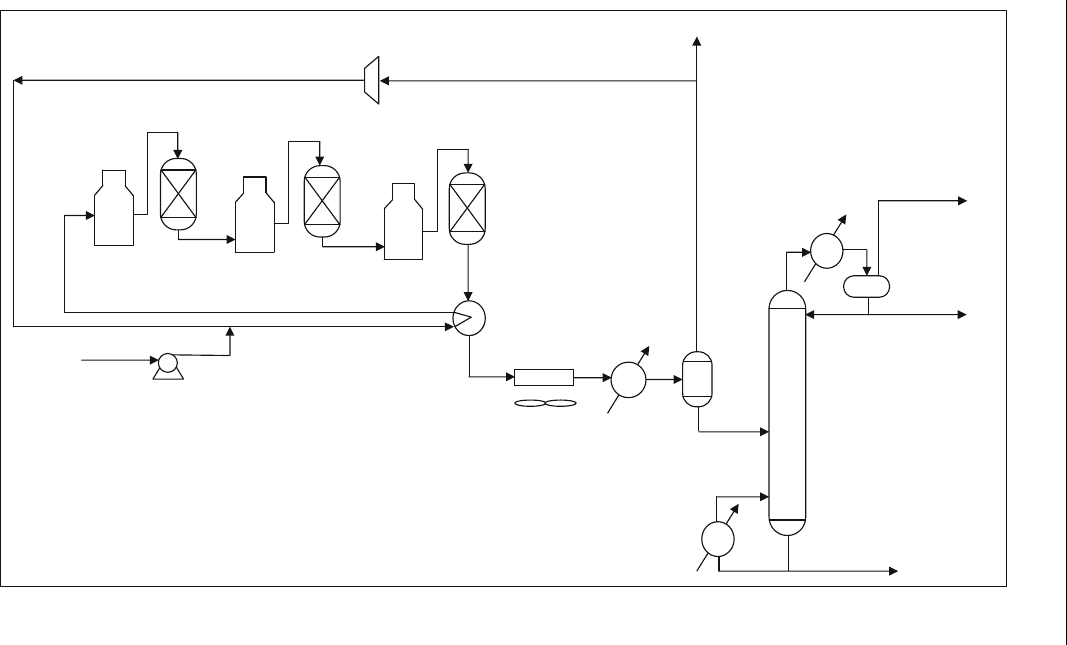
5.2.7.1. Semi-regenerative Fixed Bed Process
The schematic flow diagram of this process is shown in Figure 5.5. The
name semi-regenerative comes from regeneration of the catalyst in the fixed
bed reactors after shut down by burning off the carbon formed on the
catalyst surface.
Reactions such as dehydrogenation of paraffins and naphthenes which
are very rapid and highly endothermic (Table 5.1) occur in the first reactor,
with high temperature drop. Reactions that are considered rapid, such as
paraffin isomerization and naphthens dehydroisomerization, give moderate
temperature decline in the second reactor. Furthermore, slow reactions
such as dehydrocyclization and hydrocracking (Table 5.1) give low
temperature decline in the third reactor.
102 Chapter 5
Heavy
Naphtha
Heater
Heater
Heater
1
st
Reactor
2
nd
Reactor
3
rd
Reactor
Compressor
Recycle H
2
Net H
2
Stabilizer
Separator
Off gases
C3’s and C4’s
Reformate
Figure 5.5 Semi-regenerative (SR) fixed bed reforming process
Catalytic Reforming and Isomerization 103

Table 5.1 Thermodynamic and kinetic comparison and effect of operating condition on main reactions and products (Gary and
Handwerk, 1994)
Reaction
type
Reactive
rate
Heat
effect
Re action
equilibrium
Pressure
effect
Temperature
effect
H
2
production RVP
a
Product
density Yield Octane
Naphthene
dehydrogenation
Very
rapid
Very
endo
Yes þ Produce þ þ
Naphthene
dehydroisomerization
Rapid Very
endo
Yes þ Produce þ þ
Paraffin isomerization Rapid Slight
exo
Yes None Slight No þ Slight Slight þþ
Paraffin
dehydrocyclization
Slow Very
endo
No þ Produce þ þ
Hydrocracking Very slow exo No þþ þþ Consume þ þ
a
RVP is Reid vapour pressure.
104 Chapter 5
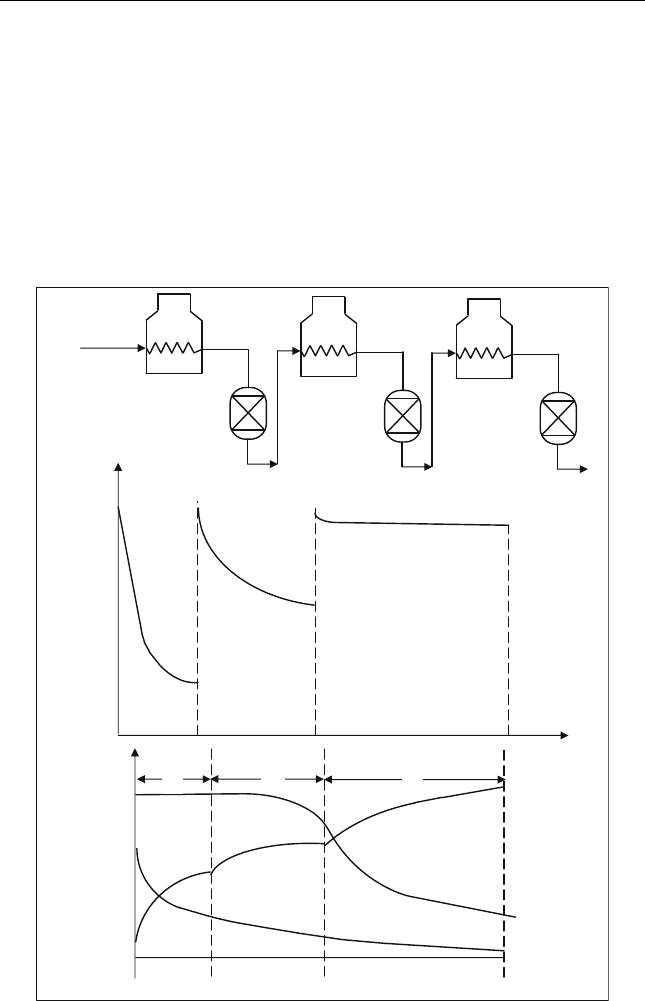
The temperature and concentration profile in each reactor is shown in
Figure 5.6. To prevent catalyst coking, the hydrogen partial pressure is
maintained at a level such that the hydrogen-to-hydrocarbon ratio by
weight (H
2
/HC) is greater than 25 for monometallic catalyst. This is
done by recycling some of the hydrogen produced (Figure 5.5). Some
light hydrocarbons (C
1
–C
4
) are separated from the reformate in the
stabilizer. At the top of t he stabilizer res idual hydrogen and C
1
to C
4
are
withdrawn as condenser products, which are then sent to gas processing,
and part of the liquid product (C
3
and C
4
)isreturnedfromthereflux
A
T
o
T
o
T
o
T
o
B
C
T
0
R
1
R
2
R
3
R
1
R
2
R
3
P
o
=60 vol%
N
o
=30 vol%
A
o
=10 vol%
Aromatics
Paraffins
Naphthenes
F
1
R
1
R
2
F
3
T
o
R
3
F
2
Figure 5.6 (A) Furnace and reactor layout for fixed bed reforme r (B) Variation of tem-
perature in the reactors. (C) Variation in effluent compositions; P
0
, initial Paraffins;
N
0
, initial Napthenes and A
0
, intial Aromatics (Martino, 2001)
Catalytic Reforming and Isomerization 105

drum back to the stabilizer (Figure 5.6). The main product of the column
is stabilized reformate, which is sent to the gasoline blending plant.
A slight modification to the semi-regenerative process is to add an extra-
reactor to avoid shutting down the whole unit during regeneration. Three
reactors can be running while the forth is being regenerated. This modified
process is called the ‘‘cyclic fixed bed’’ process.
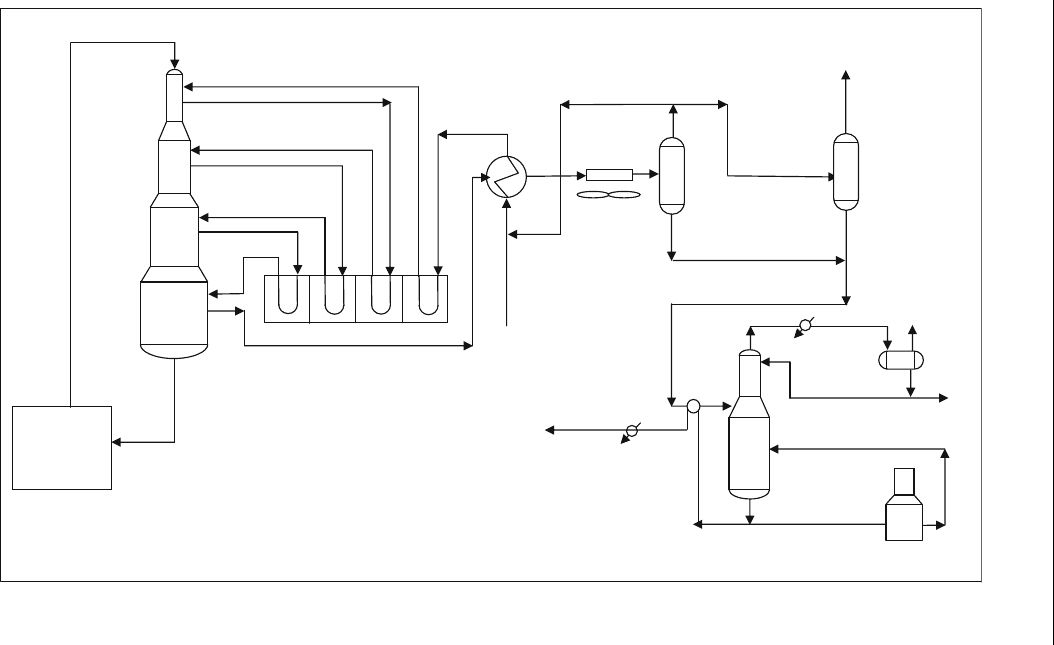
5.2.7.2. Continuous Regenerative (moving bed) CCR Platforming
UOP Process
In this process, three or four reactors are installed one on the top of the
other. The schematic flow diagram of the continuous regenerative process
(CCR) is shown in Figure 5.7 (Martino, 2001). UOP has licensed this
process under the CCR Platforming process (Martino, 2001). The effluent
from each reactor is sent to a common furnace for heating. The catalyst
moves downwards by gravity from the first reactor (R1) to the forth reactor
(R4). The catalyst is sent to the regenerator to burn off the coke and then
sent back to the first reactor R1. The final product from R4 is sent to the
stabilizer and gas recovery section.
The process can be operated at lower hydrogen partial p ressure
(P
H2
¼ 3 bar) compared to the semi-generative process (P
H2
¼ 35
bar), with a reformate yield gain of nearly 10 vol%. Table 5.2 gives a
comparison of the operating conditions for the three reforming
processes.
5.2.8. Material Balance in Reforming
5.2.8.1. Material Balance Using Emperical Correlations
Catalytic reforming data base compiled by Maples (1993) were correlated
using multiple regression. Yield correlations for the reformer were devel-
oped as given in Table 5.3. The correlation coefficients were in the range of
0.990 – 0.999.
5.2.8.2. Material Balance Using Conversion Criteria
If detailed analysis of the reformer feed is known, the feed conversion can be
calculated from the conversion data for each class of compounds as shown in
Tables 5.4 and 5.5.
5.2.9. Process Simulation of Reformer by Equilibrium
Reactions
The process simulator UNISIM can be used to calculate the material balance
around each of the three reactors. Energy balance can also be calculated for each
reactor, furnace and other units in the schematic diagram given in Figure 5.5.
Simulation of equilibrium of dehydrocyclizatio n and hydrocracking reactions
using the process simulator are illustrated in examples E5.4 and E5.5.
106 Chapter 5
Feed
Gas Recovery
Furnace
Debutanizer
Reactor
Exchanger
Separator
R1
R2
R3
R4
Catalyst
Regenerator
Furnaces
Reformate
C
4
−
C
4
Figure 5.7 Continuous regenerative reformer (CCR), UOP Platform ing process
Catalytic Reforming and Isomerization 107
Table 5.2 Typical operating conditions of three reforming processes (Martino, 2001)
Catalyst P (bar)
H
2
/HC
(mol/mol)
Space
velocity
(h
1
)RON
Semi-
generative
fixed bed
Monometallic >25 >25 1–2 90–92
Bimetallic 12–20 4–6 2–2.5 81–98
Cyclic bed Bimetallic 15–20 4 2 96–98
Continuous
moving
bed
Bimetallic 3–10 2 2–3 100–102
>104 for
aromatic
production
Table 5.3 Reformer correlations
Correlation Equations
H
2
wt% ¼12.1641 þ 0.06134 C
þ
5
vol % þ 0.099482 RON
R
(5.8)
C
1
wt% ¼ 11.509 0.125 C
þ
5
vol % (5.9)
C
2
wt% ¼ 16.496 0.1758 C
þ
5
vol % (5.10)
C
3
wt% ¼ 24.209 0.2565 C
þ
5
vol % (5.11)
Total C
4
wt%
¼ 27.024 0.2837 C
þ
5
vol % (5.12)
nC
4
wt% ¼ 0.585 total C
4
wt% (5.13)
iC
4
wt% ¼ 0.415 total C
4
wt% (5.14)
C
þ
5
vol% ¼0:03806 RON
2
R
þ 6:292793 RON
R
14:4337 K (5.15)
C
þ
5
vol% ¼ 132.2483 þ 0.66472 RON
R
þ 0.297921 RON
F
(5.16)
C
þ
5
vol% ¼ 142.7914 0.77033 RON
R
þ 0.219122 (N þ 2A)
F
(5.17)
SCFB H
2
¼ 0.0002 þ 0.48848 H
2
wt% (5.18)
RON
F
¼ research octane number of feed; RON
R
¼ research octane number of reformate;C
þ
5
vol% ¼
volume percent of reformate yield; SCFB H
2
¼ standard cubic foot of H
2
produced/barrel of feed;
K ¼ characterization factor (T
B
)
1/3
/SG; T
B
¼ absolute mid-boiling of feed,
R;SG¼ specific gravity
of feed; N ¼ napthenes vol % and A ¼ aromatics vol %.
Example E5.2
100 m
3
/h of heavy naphtha (HN) with specific gravity of 0.778 has the
following composi tion: A ¼ 11.5 vol%, N ¼ 21.7 vol% and P ¼ 66.8 vol% is
to be reformed to naphtha reformate of RON ¼ 94. Calculate the yields of each
product for that reformer.
Solution:
Given RON
R
¼ 94 and N þ 2A ¼ 44.7%, equation (5.18) gives the following
reformer yield:
108 Chapter 5
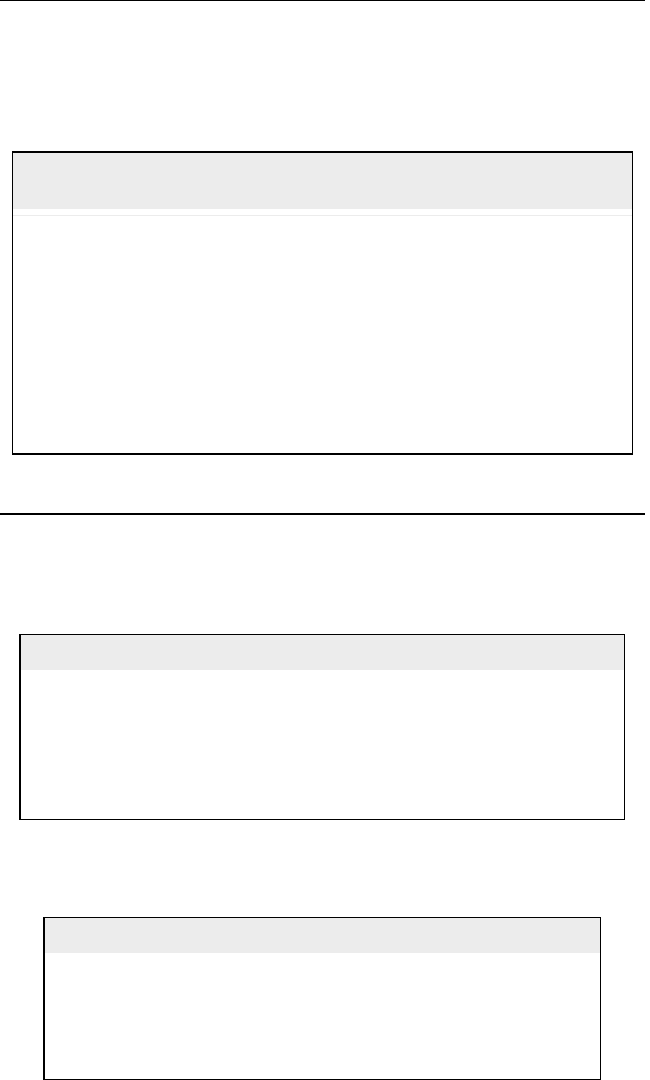
Table 5.4 Naphthenes conversion to aromatics by dehydrogenation (Gary and
Handwerk, 2001)
Feed (naphthenes) Conversion Products (aromatics)
Methylcylohexane (MCC
6
) 0.98 Toluene
Cyclohexane (CC
6
) 0.98 Benzene
Dimethylcyclopentane (DMCC
5
) 0.98 Toluene
Dimethylcyclohexan e (DMCC
6
) 0.98 Xylene
Cycloheptane (CC
7
) 0.98 Toluene
Methylcycloheptane (MCC
7
) 0.98 Xylene
Table 5.5 Paraffin conversion to aromatics by dehydrocyclization (Gary
and Handwerk, 2001)
Feed (paraffi ns) Conversion Products (aromatics)
Hexane 0.05 Benzene
Heptane 0.10 Toluene
Octane 0.25 Xylene
Nonane 0.45 1,3,4-Trimethylbenzene (TMB)
Decane 0.45 1,2,3,4Teramethyl benzene
C
þ
5
vol% ¼ 142.7914 0.77033 (94) þ 0.219122 (44.7) ¼ 80.17%
The yields for the other products can then be calculated from the correlations in
Table 5.3. The material balance for the reformer is presented in the following
table:
vol%
Volume
(m
3
/h)
Density
(kg/m
3
)wt%
Yield
(kg/h)
Feed HN 100 100 778 100 77,800
Products
H
2
wt% 2.1051 1637
C
1
wt% 1.4871 1157
C
2
wt% 2.4012 1868
C
3
wt% 3.6441 2835
Total C
4
wt% 4.2784 3328
nC
4
wt% 2.5029
iC
4
wt% 1.7755
C
þ
5
vol% 80.17 80.17 835 (86.086) (66,975)
Values in brackets are calculated by material balance between feed and products.
Catalytic Reforming and Isomerization 109

Example E5.3
Heavy naphtha, which has the following detailed analysis in mol%, is fed to a
reformer unit.
Cycloheptane (C
7
H
14
): 20%
Heptane (C
7
H
16
): 40%
Dimethylcyclohexan e (C
8
H
16
): 30%
1,3,5-Trimethylbenzene (C
9
H
12
): 10%
Find the composition of the products.
Solution:
The data, given in Tables 5.4 and 5.5, are used to estimate the composition of
the products as follows:
For 100 mol of feed:
^ Naphthenes
20 mol of cycloheptane (C
7
H
14
) will give 0.98 (20) ¼ 19.6 mol Toluene
30 mol of dimethy lcyclohexane (C
8
H
16
) will give 0.98 (30) ¼ 29.4 Xylene
^ Paraffins
40 mol of heptane (C
7
H
16
) will give 0.10 (40) ¼ 4.0 mol Toluene
^ Aromatics
1,3,5-trimethylbenzene stays as it is.
Final composition (mol%)
Cycloheptane Heptane Toluene DMCC
6
Xylene 1,3,5-TMB
C
7
H
14
C
7
H
16
C
7
H
8
C
8
H
16
C
8
H
10
C
9
H
12
0.4% 36% 23.6% 0.6% 29.4% 10%
Example E5.4
The following feed of 100 lb mol/h of naphtha was introduced to a reformer
Compound C
6
H
14
C
7
H
16
C
8
H
18
C
9
H
20
mol% feed 25 25 25 25
a 32.6 10
3
31.24 10
3
29.7 10
3
28.94 10
3
b 51.17 53.36 53.36 53.78
Knowing that:
ln K
eq
¼ a=T þ b
K
eq
is the equilibriu m constant and T in K.
110 Chapter 5
