Fahim M.A., Sahhaf T.A., Elkilani A.S. Fundamentals of Petroleum Refining
Подождите немного. Документ загружается.

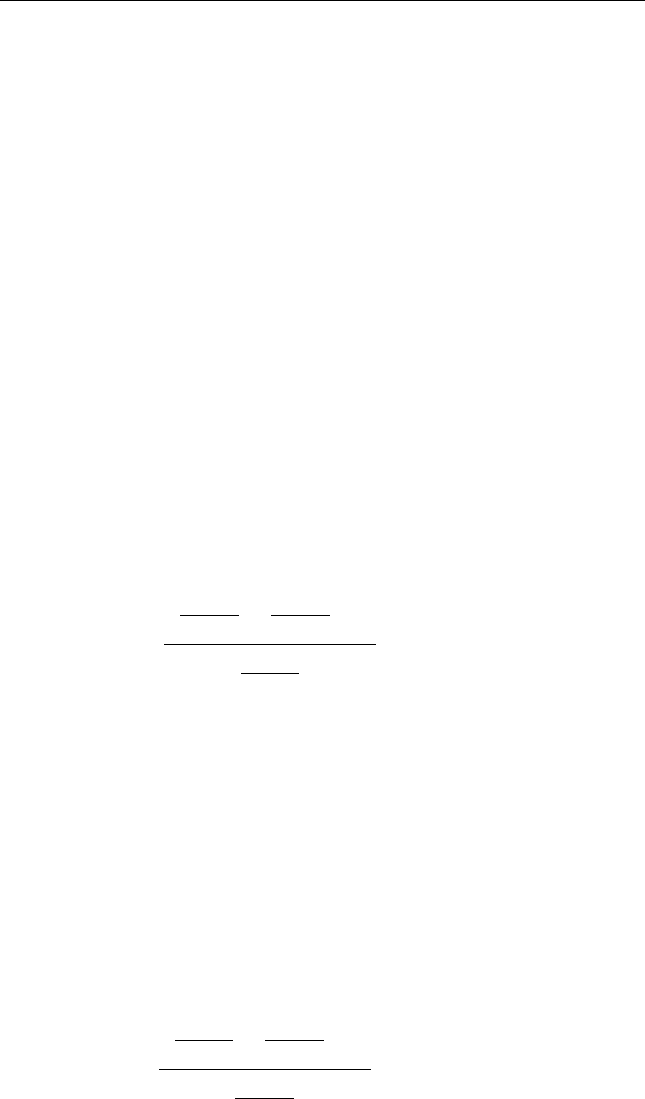
The constants a and b are given in the table above.
Assuming that the main reaction in reforming is the conversion of paraffin to the
corresponding aromatics:
(a) Calculate the composition of reformate produced at 500
C and
10 bar pressure.
(b) Repeat the calculations using the process simulator UNISIM with
three equilibrium reactors.
(c) Compare the results and comment.
Solution:
(a) Reaction 1
C
6
H
14
1x
C
6
H
6
x
þ4H
2
4x
where x is reaction conversion
K
eq
¼ exp a=T þ bðÞ
¼ exp 32:6 10
3
= 500 þ 273:15ðÞþ57:77ðÞ
¼ 8142:33
K
eq
¼ P
C
6
H
6
P
4
H
2
=P
C
6
H
14
¼ y
C
6
H
6
y
4
H
2
P
4
=y
C
6
H
14
K
eq
¼
x
1 þ 4x
4x
1 þ 4x
4
P
4
1 x
1 þ 4x
x ¼ 0.7266.
Reaction 2
C
7
H
16
1x
C
7
H
8
x
þ4H
2
4x
where x is reaction conversion
K
eq
¼ exp a=T þ bðÞ
¼ exp 31:24 10
3
= 500 þ 273:15ðÞþ53:36ðÞ
¼ 4:22 10
5
K
eq
¼ P
C
7
H
8
P
4
H
2
=P
C
7
H
16
¼ y
C
7
H
8
y
4
H
2
P
4
=y
C
7
H
16
K
eq
¼
x
1 þ 4x
4x
1 þ 4x
4
P
4
1 x
1 þ 4x
Catalytic Reforming and Isomerization 111
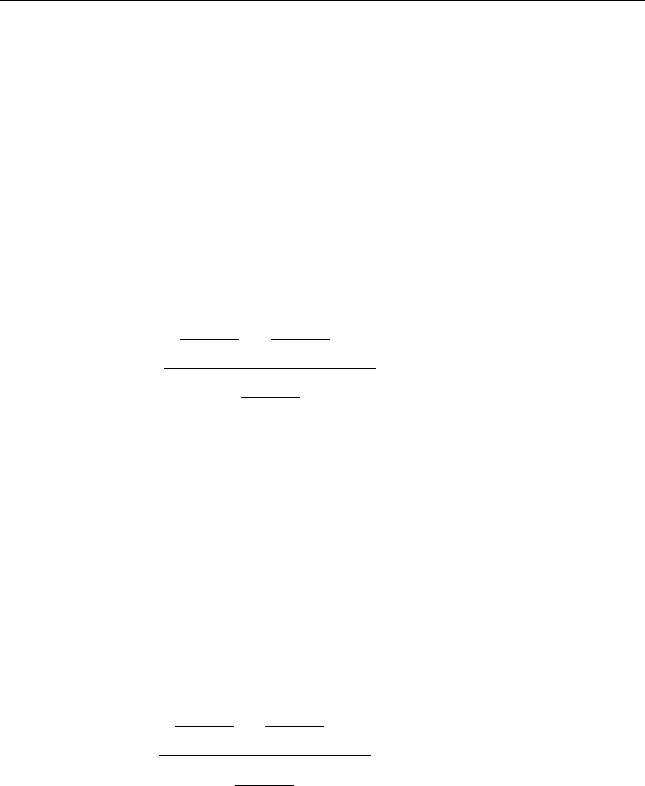
x ¼ 0.9905.
Reaction 3
C
8
H
18
1x
C
8
H
10
x
þ4H
2
4x
where x is reaction conversion
K
eq
¼ exp a=T þ bðÞ
¼ exp 29:7 10
3
= 500 þ 273:15ðÞþ53:36ðÞ
¼ 3:1 10
6
K
eq
¼ P
C
8
H
10
P
4
H
2
=P
C
8
H
18
¼ y
C
8
H
10
y
4
H
2
P
4
=y
C
8
H
18
K
eq
¼
x
1 þ 4x
4x
1 þ 4x
4
P
4
1 x
1 þ 4x
x ¼ 0.9987.
Reaction 4
C
9
H
20
1x
C
9
H
12
x
þ4H
2
4x
where x is reaction conversion
K
eq
¼ exp a=T þ bðÞ
¼ exp 28:94 10
3
= 500 þ 273:15ðÞþ53:78ðÞ
¼ 1:26 10
7
K
eq
¼ P
C
9
H
12
P
4
H
2
=P
C
9
H
20
¼ y
C
9
H
12
y
4
H
2
P
4
=y
C
9
H
20
K
eq
¼
x
1 þ 4x
4x
1 þ 4x
4
P
4
1 x
1 þ 4x
x ¼ 0.9997.
The composition of the reformate can be calculated from the equilibrium
conversions as shown in Table E5.4.1.
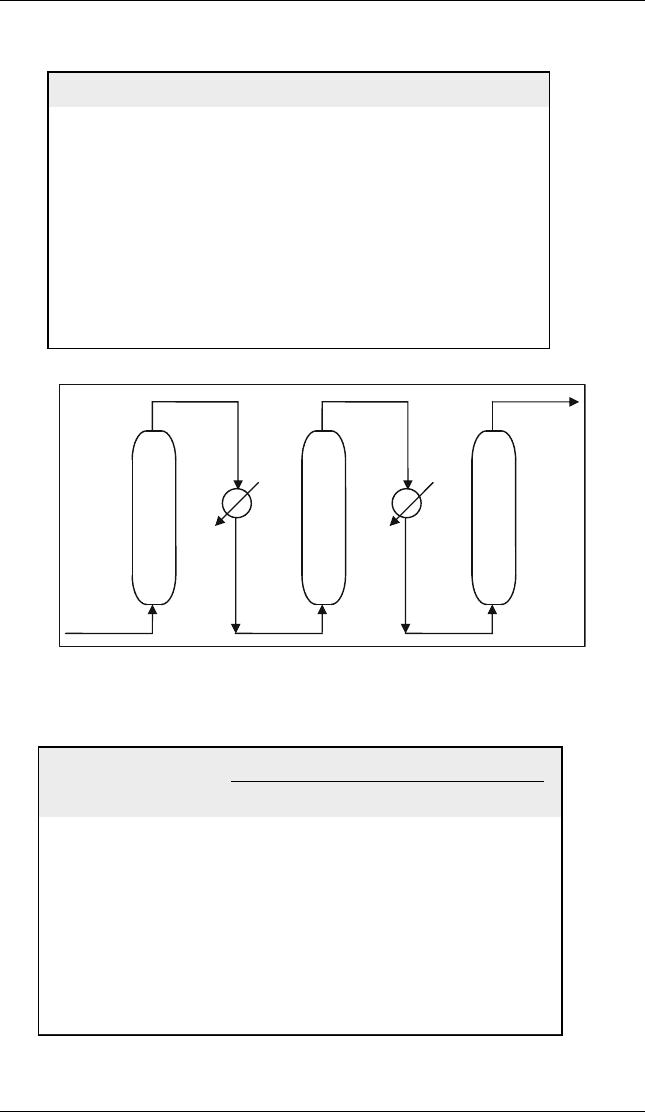
(b) The simulation of these reactions is carried out using equilibrium reactors in
the process simulator UNISIM. The feed is defined as the four components
with the given compositions, temperature and pressure. The reactions are
entered in the simulator with stoichiometry only and no conversion is required
since equilibrium reactors are used. In the calculations, adiabatic equilibrium
reactors are used. Since the reactions are endothermic the temperature in the
reactor decreases. The outlet from each reactor is heated back to the same inlet
temperature using a heater. The simulator performs the necessary energy
balances on the reactors and the heaters. Figure E5.4.1 shows the UNISIM
reactor flowsheet. In addition, UNISIM results are shown in Table E5.4.2.
112 Chapter 5
(c) From Table E5.4.2, it can be observed that the UNISIM results and
calculation in part (a) are in good agreement.
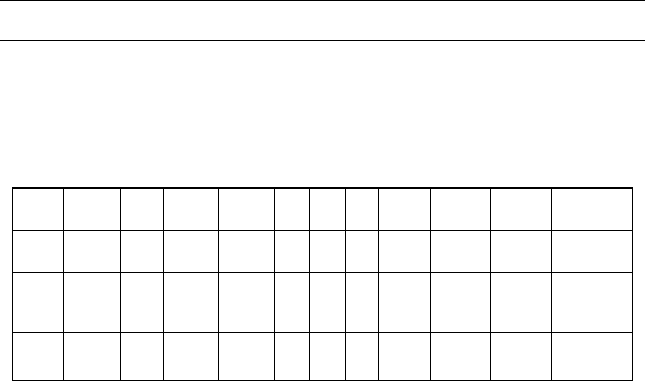
Table E5.4.1 Composition of reformate produced
Component Moles Composition
C
6
H
6
25 0.7266 ¼ 18.165 0.03852
C
7
H
8
25 0.9905 ¼ 24.763 0.05251
C
8
H
10
25 0.9987 ¼ 24.9675 0.05295
C
9
H
12
25 0.9997 ¼ 24.9925 0.05300
C
6
H
14
25 (1 0.7266) ¼ 6.835 0.01449
C
7
H
16
25 (1 0.9905) ¼ 0.2375 0.000504
C
8
H
18
25 (1 0.9987) ¼ 0.0325 0.0000689
C
9
H
20
25 (1 0.9997) ¼ 0.0075 0.0000159
H
2
371.55 0.7879
Total 471.55 1
Feed I
Equilibrium
reactor I
Feed II Feed III
Heater Heater
Equilibrium
reactor II
Equilibrium
reactor III
Figure E5.4.1 UNISIM flowsheet
Table E5.4.2 Comparison of hand calculation and UNISIM simulator
Composition
Calculations UNISIM
C
6
H
6
0.03852 0.048281
C
7
H
8
0.05251 0.05038
C
8
H
10
0.05295 0.05043
C
9
H
12
0.05300 0.05045
C
6
H
14
0.01449 0.00218
C
7
H
16
0.000504 0.000081
C
8
H
18
0.0000689 0.000036
C
9
H
20
0.0000159 0.00001
H
2
0.7879 0.79815
Catalytic Reforming and Isomerization 113
Example E5.5
Use UNISIM simulator to calculate the exit compositions and temperatures for
a series of three equilibrium reactors. The composition of the feed is shown in
the following table with the flow rate for each component in m
3
/h. The total
feed volumetric flow rate is 1325 m
3
/h at 93.3
C and 689.5 kPa.
H
2
C
1
C
2
C
3
iC
4
C
4
iC
5
C
5
C
6
C
7
C
8
C
9
0 0 0 0 0 0 0 0 81.3 239.5 297 233.8
C
10
CC
6
CC
7
MCC
6
1,1-
MCC
6
B T X 1,3,5-
MB
PCUM 1,3,5-
MCC
6
1,2,3,4-
TMCC
6
33.4 28.8 0 73.5 103.7 4.6 36 77 34.7 0 74 7.1
Assume that the following equilibrium reactions take place in the three reactors
(Kaes, 2000):
C
6
B þ 4H
2
C
7
T þ 4H
2
C
8
X þ 4H
2
C
9
1; 3; 5-MB þ 4H
2
C
10
Para-cummene þ 4H
2
CC
6
B þ 3H
2
MCC
6
T þ 3H
2
1;1-MCC
6
X þ 3H
2
1;3;5-MCC
6
1; 3; 5-MB þ 3H
2
1;2;3;4-TMCC
6
Para-cummene þ3H
2
C
6
þH
2
iC
5
þ C
1
C
7
þH
2
iC
5
þ C
2
C
8
þH
2
iC
5
þ C
3
C
9
þH
2
iC
5
þ C
4
C
10
þH
2
2iC
5
Figure E5.5.1 shows the reformer flowsheet used in the UN ISIM simulation.
Compare the UNISIM simulation results with the correlations from Table 5.3.
114 Chapter 5
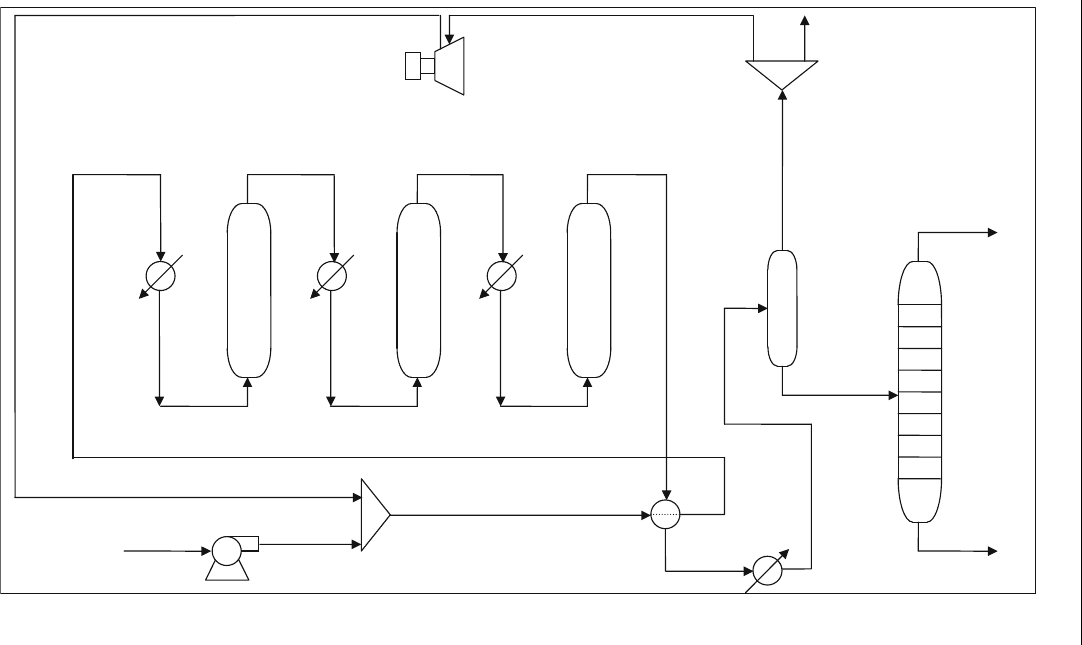
Feed
Equilibrium
reactor I
Heater Heater
Pump
Heater
CoolerHeat
exchanger
Separation
vessel
Hydrogen
recycle
Hydrogen
purge
Compressor
Gasses
Reformate
Distillation
Column
Equilibrium
reactor II
Equilibrium
reactor III
Figure E5.5.1 UNISIM simulation of reformer process
Catalytic Reforming and Isomerization 115

Solution:
The results of the UNISIM simulator are given in Table E5.5.1.
Table E5.5.1 Feed and product composition from each reactor
Main feed
(m
3
/h)
Feed Out
R1 (m
3
/h) R2 (m
3
/h) R3 (m
3
/h) R3 (m
3
/h) R3 (kg/h)
Reformate
(m
3
/h)
H
2
0.0000 15.1417 84.0284 128.4116 154.1731 11,207.0368 0.0000
C
1
0.0000 5.0571 55.5408 55.5034 55.4811 16,615.5011 0.0000
C
2
0.0000 12.3100 94.9343 87.5736 83.4081 24,923.6757 0.0000
C
3
0.0000 6.9888 94.2487 85.4533 60.1856 30,795.9545 0.0000
iC
4
0.0000 0.0563 0.0563 0.0563 0.0563 0.6784 0.0000
C
4
0.0000 1.3736 92.3584 89.3048 86.8241 40,382.7662 0.2572
iC
5
0.0000 3.2338 501.0640 455.3109 501.0640 264,459.0124 173.6960
C
5
0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
C
6
81.2637 81.2637 0.0049 0.0044 0.0041 2.6826 0.0035
C
7
239.4952 239.4952 0.0421 0.0354 0.0316 21.3128 0.0302
C
9
233.8160 233.8160 0.0139 0.0118 0.0105 7.4043 0.0105
C
10
33.4063 33.4063 0.0393 0.0327 0.0288 20.6296 0.0288
C
8
297.0433 297.0433 0.0491 0.0405 0.0359 24.7825 0.0354
CC
6
28.8036 28.8036 0.0001 0.0001 0.0001 0.0618 0.0001
CC
7
0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
MCC
6
73.5187 73.5187 0.0130 0.0102 0.0090 6.7627 0.0086
1,1-MCC
6
103.7000 103.7000 0.0046 0.0034 0.0029 2.2108 0.0028
B 4.5504 4.5508 0.1489 0.2110 0.2481 227.1990 0.2213
x
116 Chapter 5
Main feed
(m
3
/h)
Feed Out
R1 (m
3
/h) R2 (m
3
/h) R3 (m
3
/h) R3 (m
3
/h) R3 (kg/h)
Reformate
(m
3
/h)
T 36.0111 36.0437 42.3245 51.5524 56.7741 50,470.6546 55.0311
X 77.0384 77.0654 127.0019 139.4240 146.8633 128,367.6313 146.1156
135-MB 34.7444 34.7542 154.0493 158.2945 161.7427 140,948.5347 161.6444
pCum 0.0000 0.0086 178.8872 192.6322 199.4044 172,667.3075 199.3185
1,3,5-MCC
6
74.3908 74.3908 0.0019 0.0014 0.0012 0.8981 0.0012
1,2,3,4-
TMCC
6
7.1145 7.1145 0.0436 0.0283 0.0229 18.0522 0.0229
Total 1324.8964 1369.1361 1424.8551 1443.8961 1506.3719 881,170.7496 736.4281
Temperature
(
C)
93.3 523.9 523.9 523.9
Pressure
(atm)
6.8 22.46 21.77 21.1
Catalytic Reforming and Isomerization 117
5.3. Isomerization of Light Naphtha
Isomerization is the process in which light straight chain paraffins of
low RON (C
6
,C
5
and C
4
) are transformed with proper catalyst into
branched chains with the same carbon number and high octane numbers.
The hydrotreated naphtha (HTN) is fractionated into heavy naphtha
between 90–190
C (190–380
F) which is used as a feed to the reforming
unit. Light naphtha C
5
80
C(C
5
180
F) is used as a feed to the
isomerization unit. There are two reasons for this fractionation: the first is
that light hydrocarbons tend to hydrocrack in the reformer. The second is
that C
6
hydrocarbons tend to form benzene in the reformer. Gasoline
specifications require a very low value of benzene due to its carcinogenic
effect (Travers, 2001).
5.3.1. Thermodynamics of Isomerization
The isomerization reactions are slightly exothermic and the reactor works in
the equilibrium mode. There is no change in the number of moles and thus
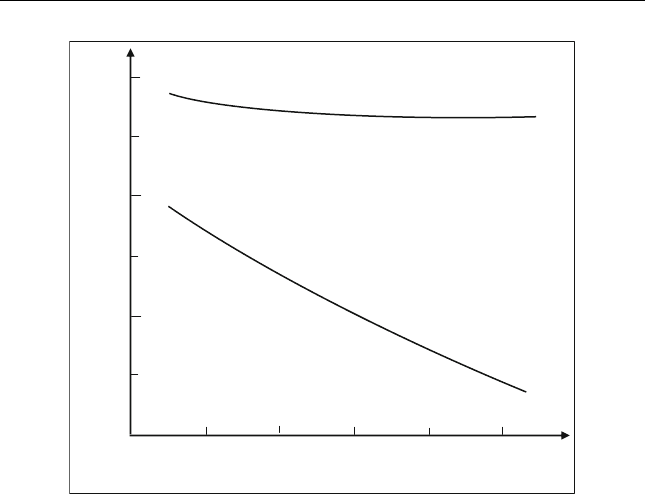
the reaction is not affected by pressure change. Better conversions are
achieved at lower temperature as shown in Figure 5.8. Operating the
Now let us compare these results with those given by the reformer correlations
of equations (5.9)–(5.19). The feed has (N þ 2A) equal to 44.7. The reformate
yield is calculated to be 80.17 at RON ¼ 94. The comparison between
UNISIM and correlations is presented in Table E5.5.2.
Table E5.5.2 Comparison between UNISIM and correlations
Correlation
A vol% 11.499
N vol% 21.702
P vol% 66.800
(N þ 2A) vol% 44.69905
Correlation UNISIM
C
þ
5
vol% 80.17 80.4775
H
2
wt% 2.1051 1.1465
C
1
wt% 1.4871 1.6997
C
2
wt% 2.4012 2.5496
C
3
wt% 3.6441 3.1504
C
4
wt% 4.2784 4.1312
118 Chapter 5
reactor at 130
C (260
F) will give good results. In this figure, the degree of
conversion to iso-paraffins is measured by the increase of the RON. Paraffin
recycle substantially increases the conversion (Travers, 2001).
5.3.2. Isomerization Reactions
Isomerization is a reversible and slightly exothermic reaction:
n-paraffin
i-paraffin
The conversion to iso-paraffin is not complete since the reaction is
equilibrium conversion limited. It does not depend on pressure, but it can
be increased by lowering the temperature. However operating at low
temperatures will decrease the reaction rate. For this reason a very active
catalyst must be used.
5.3.3. Isomerization Catalysts
There are two types of isomerization catalysts: the standard Pt/chlorinated
alumina with high chlorine content, which is considered quite active, and
the Pt/zeolite catalyst.
Isomerization without recycle
Isomerization with recycle
92
88
84
80
100
200
300
Temperature (°C)
RON (at equilibrium)
Feed
C
5
Paraffins 60%
C
6
Paraffins 30%
C
6
Cyclos 10%
Figure 5.8 Thermodynamic equilibrium with and without recycling normal paraffin
(Tr ave rs, 2 0 01)
Catalytic Reforming and Isomerization 119
5.3.3.1. Standard Isomerization Catalyst
This bi-functional nature catalyst consists of highly chlorinated alumina
(8–15 w% Cl
2
) responsible for the acidic function of the catalyst. Platinum
is deposited (0.3–0.5 wt%) on the alumina matrix. Platinum in the
presence of hydrogen will prevent coke deposition, thus ensuring high
catalyst activity. The reaction is per formed at low temperature at about
130
C(266
F) to improve the equilibrium yield and t o lower chlorine
elution.
The standard isomerization catalyst is sensitive to impurities such as
water and sulphur traces which will pois on the c atalyst a nd lower i ts
activity. For this reason, the feed must be hydrotreated before isomeriza-
tion. Furthermore, carbon tetrachloride must be injected int o the feed to
activate the catalyst. The pressure of the hydrogen in the reactor will result
in th e elution of chlorine from the catalyst as hydrogen c hloride. For all
these reasons, the zeolite catalyst, which is resistant to impurities, was
developed.
5.3.3.2. Zeolite Catalyst
Zeolites are crystallized silico-aluminates that are used to give an acidic
function to the catalyst. Metallic particles of platinum are impregnated on
the surface of zeolites and act as hydrogen transfer centres. The zeolite
catalyst can resist impurities and does not require feed pretreatment, but it
does have lower activity and thus the reaction must be performed at a higher
temperature of 250
C (482
F). A comparison of the operating conditions
for the alumina and zeolite processes is shown in Table 5.6.
5.3.4. Isomerization Yields
The reformate yield from light naphtha isomerization is usually very high
(>97 wt%). Typical yields are given in Table 5.7.
Table 5.6 Comparison of operating conditions of isomerization
Pt/Chlorine
Operating condition Alumina catalyst Pt/Zeolite catalyst
Temperature (
C) 120–180 250–270
Pressure (bar) 20–30 15–30
Space velocity (h
1
) 1–2 1–2
H
2
/HC (mol/mol) 0.1–2 2–4
Product RON 83–84 78–80
120 Chapter 5
