Environmental Encyclopedia
Подождите немного. Документ загружается.


Environmental Encyclopedia 3
Biogeochemistry
include
carbon
,
nitrogen
,
phosphorus
, potassium, cal-
cium, magnesium, and sulfur. Some of these can occur vari-
ously as gases in the
atmosphere
, as ions dissolved in water,
in minerals in rocks and
soil
, and in a great variety of
organic chemicals in the living or dead biomass of organisms.
Ecologists study
nutrient
cycles by determining the quanti-
ties of the various chemical forms of nutrients in various
compartments of ecosystems, and by determining the rates
of transformation and cycling among the various compart-
ments.
The
nitrogen cycle
is particularly well understood,
and it can be used to illustrate the broader characteristics of
nutrient cycling. Nitrogen is an important nutrient, being
one of the most abundant elements in the tissues of organ-
isms and a component of many kinds of biochemicals, in-
cluding amino acids, proteins, and nucleic acids. Nitrogen
is also one of the most common limiting factors to
primary
productivity
, and the growth rates of plants in many ecosys-
tems will increase markedly if they are fertilized with nitro-
gen. This is a fairly common characteristic of terrestrial and
marine environments, and to a lesser degree of freshwater
ones.
Plants assimilate most of their nitrogen from the soil
environment, as nitrate (NO
3
-
) or ammonium (NH
4
+
) dis-
solved in the water that is taken up by roots. Some may also
be taken up as gaseous
nitrogen oxides
(such as NO or
NO
2
) that are absorbed from the atmosphere. In addition,
some plants live in a beneficial
symbiosis
with
microorgan-
isms
that have the ability to fix atmospheric dinitrogen gas
(N
2
) into ammonia (NH
3
), which can be used as a nutrient.
In contrast, almost all animals satisfy their nutritional needs
by eating plants or other animals and metabolically breaking
down the organic forms of nitrogen, using the products (such
as amino acids) to synthesize the necessary biochemicals of
the animal. When plants and animals die, microorganisms
active in the detrital cycle metabolize organic nitrogen in
the dead biomass into simpler compounds, ultimately to
ammonium.
The nitrogen cycle has always occurred naturally, but
in modern times some of its aspects have been greatly modi-
fied by human influences. These include the fertilization of
agricultural ecosystems, the dumping of nitrogen-containing
sewage into lakes and other waterbodies, the
emission
of
gaseous forms of nitrogen into the atmosphere, and the
cultivation of nitrogen-fixing legumes. In some cases, human
effects on nitrogen biogeochemistry result in increased pro-
ductivity of crops, but in other cases serious ecological dam-
ages occur.
Toxic Chemicals in Ecosystems
Some human activities result in the release of toxic
chemicals into the environment, which under certain condi-
tions can pose risks to human health and cause serious dam-
137
ages to ecosystems. These damages are called
pollution
,
whereas the mere presence of chemicals which cause no
damage in the environment is referred to as contamination.
Biogeochemistry is concerned with the emissions, transfers,
and quantities of these potentially toxic chemicals in the
environment and ecosystems.
Certain chemicals have a great ability to accumulate
in organisms rather than in the non-living (or inorganic)
components of the environment. This tendency is referred to
as bioconcentration. Chemicals that strongly bioconcentrate
include methylmercury and all of the persistent organochlo-
rine compounds, such as
dichlorodiphenyl-trichloroeth-
ane
(DDT),
pentachlorophenol
(PCBs), dioxins, and
fu-
rans
. Methylmercury bioconcentrates because it is rather
tightly bound in certain body organs of animals. The organo-
chlorines bioconcentrate because they are extremely insolu-
ble in water but highly soluble in fats and lipids, which are
abundant in the bodies of organisms but not in non-living
parts of the environment.
In addition, persistent organochlorines tend to occur
in particularly large concentrations in the fat of top predators,
that is, in animals high in the ecological food web, such as
marine mammals, predatory birds, and humans. This hap-
pens because these chemicals are not easily metabolized into
simpler compounds by these animals, so they accumulate in
increasingly larger residues as the animals feed and age.
This phenomenon is known as food-web magnification (or
biomagnification
). Food-web magnification causes chemi-
cals such as DDT to achieve residues of tens or more
parts
per million
(ppm) in the fatty tissues of top predators, even
though they occur in the inorganic environment (such as
water) in concentrations smaller than one part per billion
(ppb). These high body residues can lead to ecotoxicological
problems for top predators, some of which have declined in
abundance because of their exposure to
chlorinated hydro-
carbons
.
Because a few
species
of plants have an affinity for
potentially toxic elements, they may bioaccumulate them to
extremely high concentrations in their tissues. These plants
are genetically adapted ecotypes which are themselves little
affected by the residues, although they can cause toxicity to
animals that might feed on their biomass. For example, some
plants that live in environments in which the soil contains
a mineral known as serpentine accumulate
nickel
to concen-
trations which may exceed thousands of ppm. Similarly,
some plants (such as locoweed) growing in semi-arid envi-
ronments can accumulate thousands of ppm of selenium in
their tissues, which can poison animals that feed on the
plants.
[Bill Freedman Ph.D.]

Environmental Encyclopedia 3
Biogeography
R
ESOURCES
B
OOKS
Atlas, R.M., and R. Bartha. Microbial Ecology. Menlo Park, CA: Benjamin/
Cummings, 1987.
Freedman, B. Environmental Ecology, 2nd ed. San Diego, CA: Academic
Press, 1995.
Schlesinger, W. H. Biogeochemistry: An Analysis of Global Change. San
Diego, CA: Academic Press, 1991.
Smith, R. P. A Primer of Environmental Toxicology. Philadelphia, PA: Lea &
Febiger, 1992.
Biogeography
Biogeography is the study of the spatial distribution of plants
and animals, both today and in the past. Developed during
the course of nineteenth century efforts to explore, map,
and describe the earth, biogeography asks questions about
regional variations in the numbers and kinds of
species
:
Where do various species occur and why? What physical
and biotic factors limit or extend the range of a species? In
what ways do species disperse (expand their ranges), and
what barriers block their dispersal? How has species distribu-
tion changed over centuries or millennia, as shown in the
fossil record? What controls the makeup of a
biotic commu-
nity
(the combination of species that occur together)? Bioge-
ography is an interdisciplinary science: many other fields,
including paleontology, geology, botany, oceanography, and
climatology, both contribute to biogeography and make use
of ideas developed by biogeographers.
Because physical and biotic environments strongly in-
fluence species distribution, the study of
ecology
is closely
tied to biogeography. Precipitation, temperature ranges,
soil
types, soil or water
salinity
, and insolation (exposure to the
sun) are some elements of the physical
environment
that
control the distribution of plants and animals. Biotic limits
to distribution, constraints imposed by other living things,
are equally important. Species interact in three general ways:
competition
with other species (for space, sunlight, water,
or food), predation (e.g., an owl species relying on rodents
for food), and
mutualism
(e.g., an insect pollenizing a plant
while the plant provides nourishment for the insect). The
presence or absence of a key plant or animal may function as
an important control on another species’ spatial distribution.
Community ecology
, the ways in which an assemblage of
species coexist, is also important. Biotic communities have
a variety of niches, from low to high trophic levels, from
generalist roles to specialized ones. The presence or absence
of species filling one of these roles influences the presence
or survival of a species filling another role.
Two other factors that influence a region’s biotic
composition or the range of a particular species are dis-
persal, or spreading, of a species from one place to another;
138
and barriers, environmental factors that block dispersal.
In some cases a species can extend its range by gradually
colonizing adjacent, hospitable areas. In other cases a
species may cross a barrier, such as a mountain range, an
ocean, or a
desert
, and establish a colony beyond that
barrier. The cattle egret (Bubulcus ibis) exemplifies both
types of movement. Late in the nineteenth century these
birds crossed the formidable barrier of the Atlantic Ocean,
perhaps in a storm, and established a breeding colony in
Brazil. During the past one hundred years this small egret
has found suitable
habitat
and gradually expanded its
range around the coast of South America and into North
America, so that by 1970 it had been seen from southern
Chile to southern Ontario.
The study of dispersal has special significance in
island
biogeography
. The central idea of island biogeography,
proposed in 1967 by R. H. MacArthur and
Edward O.
Wilson
, is that an island has an equilibrium number of
species that increases with the size of the land mass and its
proximity to other islands. Thus species diversity should
be extensive on a large or nearshore island, with enough
complexity to support large carnivores or species with very
specific food or habitat requirements. Conversely, a small
or distant island may support only small populations of a
few species, with little complexity or
niche
specificity in the
biotic community.
Principles of island biogeography have proven useful
in the study of other “island” ecosystems, such as isolated
lakes, small mountain ranges surrounded by deserts, and
insular patches of forest left behind by clearcut
logging
.
In such threatened areas as the Pacific Northwest and the
Amazonian rain forests, foresters are being urged to leave
larger stands of trees in closer proximity to each other so
that species at high trophic levels and those with specialized
food or habitat requirements (e.g., Northern spotted owls
and Amazonian monkeys) might survive. In such areas as
Yellowstone National Park
, which national policy desig-
nates as an insular unit of habitat, the importance of adjacent
habitat has received increased consideration. Recognition
that clearcuts and farmland constitute barriers has led some
planners to establish forest corridors to aid dispersal, enhance
genetic diversity, and maintain biotic complexity in unsettled
islands of natural habitat.
[Mary Ann Cunningham Ph.D.]
R
ESOURCES
B
OOKS
Brown, J. H., and A. C. Gibson. Biogeography. St. Louis: Mosby, 1983.
MacArthur, R. H., and E. O. Wilson. The Theory of Island Biogeography.
Vol. 1, Monographs in Population Biology. Princeton: Princeton University
Press, 1967.

Environmental Encyclopedia 3
Bioindicator
Biohydrometallurgy
Biohydrometallurgy is a technique by which
microorgan-
isms
are used to recover certain metals from ores. The tech-
nique was first used over 300 years ago to extract
copper
from low-grade ores. In recent years, its use has been ex-
tended to the recovery of
uranium
and gold, and scientist
believe that it will eventually be applied to the recovery of
other metals such as
lead
,
nickel
, and zinc.
In most cases, biohydrometallurgy is employed when
conventional mining procedures are too expensive or ineffec-
tive in recovering a metal. For example, dumps of unwanted
waste materials are created when copper is mined by tradi-
tional methods. These wastes consist primarily of rock,
gravel, sand, and other materials that are removed in order
to reach the metal ore itself. But the wastes also contain
very low concentrations (less than 0.5%) of copper ore.
Until recently, the concentrations of copper ore in a
dump were too low to have any economic value. The cost
of collecting the ore was much greater than the value of the
copper extracted. But, as richer sources of copper ore are
used up, low grade reserves (like dumps) become more attrac-
tive to mining companies. At this point, biohydrometallurgy
can be used to leach out the very small quantities of ore
remaining in waste materials.
The extraction of copper by means of biohydrometal-
lurgy involves two types of reactions. In the first, microorgan-
isms operate directly on compounds of copper. In the second,
microorganisms operate on metallic compounds other than
those of copper. These metallic compounds are then con-
verted into forms which can, in turn, react with copper ores.
The use of biohydrometallurgical techniques on a cop-
per ore waste dump typically begins by spraying the dump
with dilute sulfuric
acid
. As the acid seeps into the dump,
it creates an
environment
favorable to the growth of acid-
loving microorganisms that attack copper ores. As the micro-
organisms metabolize the ores, they convert copper from an
insoluble to a soluble form. Soluble copper is then leached
out of the dump with sulfuric acid. It is recovered when the
solution is pumped out to a recovery tank.
A second reaction occurs within the dump. Microor-
ganisms also convert ferrous iron (Fe
2+
) in ores such as pyrite
(FeS
2
) to ferric iron (Fe
3+
). The ferric iron, in turn, oxidizes
copper in the dump from an insoluble to a soluble form.
The mechanism described here is a highly efficient
one. As microorganisms act on copper and iron compounds,
they produce sulfuric acid as a by-product, thus enriching
the environment in which they live. Ferric iron reduces and
oxidizes copper at the same time, making the copper available
for attack by microorganisms once again.
A number of microorganisms have been used in biohy-
drometallurgy. One of the most effective for the
leaching
139
of copper is Thiobacillus ferrooxidans. Research is now being
conducted on the development of genetically engineered
microorganisms that can be used in the recovery of copper
and other metals.
The two other metals for which biohydrometallurgy
seems to be most useful are uranium and gold. Waste dumps
in South Africa and Canada have been treated to convert
insoluble forms of uranium to soluble forms, allowing recov-
ery by a method similar to that used with copper. In the
treatment of gold ores, biohydrometallurgy is used in a pre-
treatment step prior to the conventional conversion of the
metal to a cyanide-complex. The first commercial plants for
the biohydrometallurgical treatment of gold ores are now in
operations in South Africa and Zimbabwe.
[David E. Newton]
R
ESOURCES
B
OOKS
McGraw Hill Encyclopedia of Science and Technology. 7th ed. New York:
McGraw-Hill, 1992.
Rossi, G. Biohydrometallurgy. New York: McGraw-Hill, 1990.
Bioindicator
A bioindicator is a plant or animal
species
that is known
to be particularly tolerant or sensitive to
pollution
. Based
on the known association of an organism with a particular
type or intensity of pollution, the presence of the organism
can be used as a tool to indicate polluted conditions relative
to unimpacted reference conditions. Sometimes a set of spe-
cies or the structure and function of an entire
biological
community
may function as a bioindicator. In assessing the
impacts of pollution, bioindicators are frequently used to
evaluate the “health” of an impacted
ecosystem
relative to
a reference area or reference conditions. Field-based, site-
specific environmental evaluations based on the bioindicator
approach generally are complemented with laboratory stud-
ies of toxicity testing and
bioassay
experiments.
The use of individual species or a community structure
as bioindicators involves the identification, classification and
quantification of biota in the affected area. While many
species are in use, the most widely used biological communi-
ties are the benthic macroinvertebrates. These are the seden-
tary and crawling worms and insect larvae that reside in the
bottom sediments of aquatic systems such as lake and river
bottoms. The bottom sediments usually contain most of the
pollutants introduced into an aquatic system. Since these
macroinvertebrates have limited mobility, they are continu-
ally exposed to the highest concentrations of pollutants in
the system. Therefore, this benthic community is an ideal

Environmental Encyclopedia 3
Biological community
bioindicator: stationary, localized and exposed to maximum
pollutant concentrations within a specific location.
Often, alterations in community structure due to pol-
lution include a change from a more diverse to a less diverse
community with fewer species or taxa. The indicator com-
munity may also be composed mostly of species that are
tolerant of or adapted to polluted conditions and pollution-
sensitive species that are present upstream may be absent in
the impacted zones. However, depending on the type of
pollutant, the abundance of the pollution-tolerant species
may be very high and, therefore, the size of the benthic
community may be similar to or exceed the reference com-
munity upstream. This is common in cases where pollution
from sewage discharges adds organic matter that provides
food for some of the tolerant benthic species. In the case of
toxic chemical (e.g.,
heavy metals
, organic compounds)
pollution, the benthic community may show an overall re-
duction both in diversity and abundance.
Tubificid worms are an example of pollution-tolerant
indicator organisms. These worms live in the bottom sedi-
ments of streams and lakes and are highly tolerant of the
kind of pollution that results from sewage discharges. In a
river polluted by
wastewater discharge
from a
sewage
treatment
plant, it is common to see a large increase in
the number of tubificid worms in the stream sediments
immediately downstream of the discharge. Upstream of the
discharge, the number of these worms is much lower, re-
flecting the cleaner conditions. Further downstream, as the
discharge is diluted, the number of tubificid worms again
decreases to a level similar to the upstream portions of the
river. Large populations of these worms dramatically demon-
strate that pollution is present, and the location of these
populations may also indicate the general area where the
pollution enters the
environment
.
Alternatively, pollution-intolerant organisms can also
be used to indicate polluted conditions. The larvae of may-
flies live in stream sediments and are known to be particularly
sensitive to pollution. In a river receiving wastewater dis-
charge, mayflies show a pattern opposite to that of the tubi-
ficid worms. The mayfly larvae are normally present in large
numbers above the discharge point, decrease or disappear at
the discharge point (just where the tubificid worms are most
abundant) and reappear further downstream as the effects
of the discharge are diluted. In this case, the mayflies are
pollution-sensitive indicator organisms and their absence
serves as the indication of pollution. Similar examples of
indicator organisms can be found among plants, fishes and
other biological groups. Giant reedgrass (Phragmites austra-
lis) is a common marsh plant that is typically indicative of
disturbed conditions in
wetlands
. Among fish, disturbed
conditions may be indicated by the disappearance of sensitive
species like trout which require clear, cold waters to thrive.
140
The usefulness of indicator organisms is unquestion-
able but limited. While their presence or absence provides
a reliable general picture of polluted conditions, it is often
difficult to identify clearly the exact sources of pollution,
especially in areas with multiple sources of pollution. In the
sediments of New York Harbor, for example, pollution-
tolerant insect larvae are overwhelmingly dominant. How-
ever, it is impossible to attribute the large larval populations
to just one of the numerous possible sources of pollution
in this area which include ship traffic, sewage discharge,
industrial discharge, and
storm runoff
. As more is learned
about the physiology and life-history of an
indicator orga-
nism
and its response to different types of pollution, it may
be possible to draw more specific conclusions.
Although the two terms are sometimes used inter-
changeably, indicator organisms should not be confused with
monitor organisms (also called biomonitors) which are
organisms that bioaccumulate toxic substances present in
trace amounts in the environment. For example, when it is
difficult to measure directly the low concentrations of a
pollutant in water, chemical analysis of shellfish tissues from
that location may show much higher, easily detected concen-
trations of that pollutant. In this case, the shellfish is used
to monitor the level of the long-term presence of that pollut-
ant in the area.
In the environmental field, bioindicators are com-
monly used in field investigations of contaminated sites to
document impacts on the biological community and ecosys-
tem. These studies are then followed up with focused labora-
tory tests to pinpoint the source of toxicity or stress. After
clean-up and remedial actions have been implemented at a
site, bioindicators are also used to track the effectiveness of
the
remediation
activity. In the future, bioindicators may
be used more widely as investigative and decision-making
tools from the initial pollution and impact assessment stage
to the remediation and post-remediation monitoring stages.
[Usha Vedagiri]
R
ESOURCES
B
OOKS
Connell, D. W., and G. J. Miller. Chemistry and Ecotoxicology of Pollution.
New York: Wiley-Interscience, 1984.
Biological community
A biological community is an association or assemblage of
populations of organisms living in a localized area or
habitat
.
The community is a level of organization incorporating indi-
vidual organisms,
species
, and populations. A population
is an assemblage of one species, and the community is a
collage of one or more populations. Communities may be

Environmental Encyclopedia 3
Biological methylation
large or small, ranging from the microscopic to the level of
biome
and
biosphere
.
“Community,” as contrasted conceptually to
ecosys-
tem
, does not necessarily include consideration of the
physical
environment
or the habitat of a particular group
of organisms, though it is of course impossible to under-
stand fully the dynamics of a community without reference
to the resources on which it exists. The term ecosystem
was coined to incorporate study of a community together
with its physical environment. Still, communities are adap-
tive systems, inseparable from and evolving in response
to changing environmental conditions. So, the supply and
availability of resources in the environment and also time
are considerations in the dynamics of community structure
and relationships. Individual communities may be relatively
stable or in constant flux. Actual equilibrium may never
exist but, even in approximation, it must be viewed as a
dynamic state—the community in constant, subtle flux
and change.
Biological communities are “interactional fields” char-
acterized by a complicated set of interactions among
complex assemblages, both within the locale and from
without—including trophic relationships, the “who eats
who” of energy exchanges. Interactions may be proximal
or between locales (close by or quite widely separated),
including intensive, extensive, or limited exchanges with
the outside world. Organisms in communities interact both
functionally and spatially or locationally, both “horizontally”
and “vertically,” interactions often independent of each
other and not reducible to one or the other. Not all
organisms necessarily interact with all the others, some
may be almost totally uncoupled from some of the others
and coexist relatively independently.
Most questions in
community ecology
focus on
the “existence, importance, looseness, transience, and
contingency of interactions.” The degree to which these
interactions result in meaningful biological patterns is
still an open question—though particular communities
are usually identified by some pattern of interactions, if
only to set them off from others for the purposes of
scientific study.
[Gerald L. Young]
R
ESOURCES
B
OOKS
Roughgarden, J. The Structure and Assembly of Communities. In Perspectives
in Ecological Theory, edited by J. Roughgarden, R. M. May, and S. A.
Levin. Princeton: Princeton University Press, 1989.
Strong, D. R., Jr., et. al., eds. Ecological Communities: Conceptual Issues and
the Evidence. Princeton: Princeton University Press, 1984.
141
Taylor, P. J. “Community.” In Keywords in Evolutionary Biology, edited by
E. Keller and E. Lloyd. Cambridge: Harvard University Press, 1991.
P
ERIODICALS
Drake, J. A. “The Mechanics of Community Assembly and Succession.”
Journal of Theoretical Biology 147 (1990): 213–233.
Richardson, J. L. “The Organismic Community: Resilience of an Embattled
Ecological Concept.” BioScience 30 (1988): 465–471.
Biological fertility
The number of offspring produced by a female organism.
In a population, biological fertility is measured as the general
fertility rate (the birth rate multiplied by the number of
sexually productive females) or as the total fertility rate (the
lifetime average number of offspring per female). General
dictionaries list fertility and
fecundity
as synonyms for re-
productive fruitfulness. In
population biology
, biological
fertility refers to the number of offspring actually produced,
while fecundity is merely the biological ability to reproduce.
Fecund individuals that fail to mate do not produce offspring
(that is, are not biologically fertile), and do not contribute
to
population growth
.
Biological integrity
see
Ecological integrity
Biological magnification
see
Biomagnification
Biological methylation
The process by which a methyl radical (-CH
3
) is chemically
combined with some other substance through the action of a
living organism. One of the most environmentally important
examples of this process is the
methylation
of
mercury
in
the sediments of lakes, rivers, and other bodies of water.
Elementary mercury and many of its inorganic compounds
have relatively low toxicity because they are insoluble. How-
ever, in sediments, bacteria can convert mercury to an organic
form, methylmercury, that is soluble in fat. When ingested
by animals, methylmercury accumulates in body fat and ex-
erts highly toxic, sometimes fatal, effects.
Biological oxygen demand
see
Biochemical oxygen demand

Environmental Encyclopedia 3
Biological Resources Division
Biological Resources Division
Created to assess, monitor, and research biological resources
in United States, the Biological Resources Division (BRD)
of the United States
Geological Survey
(USGS) is the
non-regulatory biological research component of the United
States Department of the Interior. First created in 1994 as
the National Biological Survey (NBS), an independent
agency within the
U.S. Department of the Interior
, the
NBS was merged with the USGS (also part of the Interior
Department) in 1996.
The BRD is the principal biological research and mon-
itoring agency of the federal government. It is responsible
for gathering, analyzing, and disseminating biological infor-
mation in order to support sound management and steward-
ship of nation’s biological and
natural resources
. It is also
directed to foster understanding of biological systems and
their benefits to society, and to make biological information
available to the public. Although it was created mainly on
the impetus of environmental and scientific organizations,
the BRD also supports commercial and economic interests
in that it seeks to identify opportunities for sustainable re-
source use. Agriculture and
biotechnology
are among the
industries that stand to benefit from BRD research on new
sources of food, fiber, and medicines.
Because it is independent of regulatory agencies—
which are responsible for enforcing laws—the BRD has
no formal regulatory, management, or enforcement roles.
Therefore the BRD does not enforce laws such as the
Endan-
gered Species Act
. Instead the BRD is responsible for gath-
ering data that are scientifically sound and unbiased. The
BRD also fosters public-private cooperation. For example,
it worked with the International Paper Company in Alabama
to develop a management plan for two
species
of pitcher
plants found on company land that were candidates for the
Endangered Species
List. If it succeeds, this management
plan will both preserve the pitcher plant populations—pre-
venting the legal complications of having it listed as a feder-
ally endangered species—and allow continued judicious use
of the land and resources.
The Biological Resources Division of the USGS was
created in 1994 as an independent agency with the name
National Biological Survey. The Survey was established on
November 11, 1994, on the recommendations of President
Bill Clinton and Interior Secretary Bruce Babbit. Renamed
the National Biological Service (NBS) shortly after its cre-
ation, the agency was created by combining the biological
research, inventory, and monitoring programs of seven agen-
cies within the Department of the Interior. Built on the
model of the Geological Survey, the NBS was established
to provide accurate baseline data about ecosystems and spe-
cies in United States territory. The mission of the NBS
142
was to provide information to support sound stewardship of
natural resources on public lands under the administration
of the Department of the Interior. Part of its mission was
also to foster cooperation among other entities involved in
managing, monitoring, and researching natural resources.
On October 1, 1996, the NBS was renamed the Biological
Resources Division and merged with the United States Geo-
logical Survey. The USGS-BRD retains the research and
information provision mandates of the NBS. Appointed as
the first NBS/BRD director was H. Ronald Pulliam, a pro-
fessor and research ecologist from of the Institute of Ecology
and the University of Georgia in Athens.
The National Biological Service had a predecessor in
the Bureau of Biological Survey, which operated as part
of the Department of Agriculture from 1885–1939. The
Bureau’s first director, C. Hart Merriam, revolutionized bio-
logical collection techniques and in 15 years nearly quadru-
pled the number of known American mammal species. In
1939, the Bureau was transferred to the Department of the
Interior, where it was the predecessor to the
Fish and Wild-
life Service
. After its transfer to the Department of the
Interior, the Bureau of Biological Survey’s baseline data gath-
ering and survey functions gradually diminished. The Fish
and Wildlife Service now has regulatory and management
responsibilities as well as research programs.
In recent years the need for a biological survey, and
especially for the production of reliable baseline data, has
resurfaced. The resurgence of interest in baseline data results
in part from an interest in managing ecosystems, with a
focus on stewardship and
ecosystem
restoration, in place
of previous emphasis on individual species management.
Managing and restoring ecosystems requires basic data and
information on biological indicators, which are often un-
available. Interest in threatened and endangered species, and
public participation in environmental organizations probably
also contributed to the widespread support for establishing
a biological survey.
The BRD is important because it is the only federal
agency whose principal mission is to perform basic scientific,
biological, and ecological research. As an explicitly scientific
research body, the BRD works to incorporate current ecolog-
ical theory in its research and monitoring programs. Central
to the BRD’s mission are ideas such as long-term steward-
ship of resources, maintaining
biodiversity
, identifying eco-
logical services of biological resources, anticipating
climate
change, and restoring populations and habitats. Because of
its emphasis on basic research the BRD can address current
scientific themes that pertain to public policy such as fire
ecology,
endocrine disruptors
(
chemicals
such as penta-
chlorophenols [PCBs] that interfere with endocrine and re-
productive functions in animals), ecological roles of
wet-
lands
, and
habitat
restoration. The primary purpose of

Environmental Encyclopedia 3
Bioluminescence
this research activity is to provide land managers, especially
within the Department of the Interior, with sound informa-
tion to guide their management policies.
The BRD has two broad categories of research activi-
ties. One focus is on species for which the Department
of Interior has trust responsibilities, including endangered
species, marine mammals, migratory birds, and anadromous
fish such as
salmon
. Research on these organisms involves
studies of physiology, behavior, population dynamics, and
mortality
. The second general focus is on ecosystems. This
class of research is directed at using experimentation,
model-
ing
, and observation to produce practical information con-
cerning the complex interactions and functions of ecosys-
tems, as well as human-induced changes in ecosystems.
In addition to these ecological research questions, the
BRD is mandated to perform basic classification, mapping,
and description functions. The division is responsible for
classifying and mapping species within the United States,
as well as prospecting for new species. It is also directed to
develop a standard classification system for ecological units,
biological indicators for
ecosystem health
, protocols for
managing
pollution
, and guidelines for ecosystem resto-
ration.
Part of this basic assessment function is a series of
National Status and Trends Reports, to be released by the
BRD every two years. These reports are to assess the health
of biological resources and to report trends in their decline
or improvement. The first two National Status and Trends
Reports were released in 1995 and 1997. The 1995 report
included more than 200 contributions on monitoring and
population trends for plant, invertebrate, and vertebrate spe-
cies, as well as summaries on the status of several biological
communities and ecosystems. The 1997 report, published
in two volumes, discusses factors such as natural processes,
harvest exploitation, contaminants,
land use
, water use,
nonindigenous species, and climate change that affect eco-
systems in the United States. This status report also includes
a major section discussing marine biological resources.
The USGS-BRD also provides a nationwide coordi-
nated research agenda and a set of priorities for biological
research. For example, it has undertaken a set of coordinated
regional studies of the wide-ranging biological and ecological
impacts of wetland distributions in Colorado, California,
Texas, and other regions of the United States. Collectively
these studies can provide a picture of nationwide trends and
conditions, as well as producing coordinated information
on restoration techniques, biodiversity issues, and biological
indicators, at the same time as regional wetland problems
are being addressed. Similar sets of coordinated studies have
begun concerning the effects of endocrine-disrupting chemi-
cals in the
environment
and on the use of prescribed fire
as a management technique in a variety of ecosystems.
143
One of the principal mandates of the BRD is to distrib-
ute information. Free sharing of information is intended to
maximize the usefulness of research findings. Part of the
information sharing program includes substantial use of the
Internet to distribute publications. Even the book-size Na-
tional Status and Trends Reports are being published online
to facilitate easy public access to their findings.
In addition to performing its own research the BRD
provides coordination and a network of communication be-
tween federal agencies, state governments, universities, mu-
seums, and private
conservation
organizations. The Na-
tional Partnership for Biological Survey, coordinated by the
BRD, provides a network for sharing information and tech-
nology between federal, state, and other agencies. Partici-
pants in the Partnership include the United States
Forest
Service
, the Natural Resources Conservation Service, the
National Oceanic and Atmospheric Administration
, the
Environmental Protection Agency
, the National Science
Foundation, the Department of Defense, and the
Army
Corps of Engineers
, as well as the Natural Heritage data
centers and programs maintained by many states. The net-
work of Natural Heritage programs is coordinated by
The
Nature Conservancy
, a private organization dedicated to
preserving habitat and species diversity. Also included in the
Partnership are museums and universities nationwide that
serve as repositories of information on biological resources
and that conduct biological research, a range of non-govern-
mental organizations, international conservation and re-
search groups, Native American groups, private land holders,
and resource user groups.
[Mary Ann Cunningham Ph.D.]
R
ESOURCES
O
THER
Biological Resources Division. Biological Resources Strategic Science Plan.
Washington, D.C.: Government Printing Office, 1996.
National Biological Service. Our Living Resources. Washington, DC: Gov-
ernment Printing Office, 1995.
O
RGANIZATIONS
US Geological Survey (USGS), Biological Resources Division (BRD),
Western Regional Office (WRO), 909 First Ave., Suite #800, Seattle, WA
USA 98104 (206) 220-4600, Fax: (206) 220-4624, Email:
brd_wro@usgs.gov, <http://biology.usgs.gov>
Biological treatment
see
Bioremediation
Bioluminescence
Bioluminescence ("living light") is the production of light
by living organisms through a biochemical reaction. The

Environmental Encyclopedia 3
Biomagnification
general reaction involves a substrate called luciferin and an
enzyme
called luciferase, and requires oxygen. Specifically,
luciferin is oxidized by luciferase and the chemical energy
produced is transformed into light energy. In
nature
, biolu-
minescence is fairly widespread among a diverse group of
organisms such as bacteria,
fungi
, sponges, jellyfish, mol-
lusks, crustaceans, some worms, fireflies, and fish. It is totally
lacking in vertebrate animals. Fireflies are probably the most
commonly recognized examples of bioluminescent organ-
isms, using the emitted light for mate recognition.
Biomagnification
The
bioaccumulation
of
chemicals
in organisms beyond
the concentration expected if the chemical was in equilibrium
between the organism and its surroundings. Biomagnifica-
tion can occur in both terrestrial and aquatic environments,
but it is generally used in relation to aquatic situations. Most
often, biomagnification occurs in the higher trophic levels
of the
food chain/web
, where exposure to chemicals takes
place mostly through food consumption rather than water
uptake.
Biomagnification is a specific case of bioaccumulation
and is different from bioconcentration. Bioaccumulation de-
scribes the accumulation of contaminants in the tissue of
organisms. Typical examples of this include the elevated
levels of many chlorinated pesticides and
mercury
in fish
tissue. Bioconcentration is used to describe the concentration
of a chemical in an organism from water uptake alone. This
is quantitatively described by the bioconcentration factor, or
BCF, which is the chemical concentration in tissue divided
by the chemical concentration in water, expressed in equiva-
lent units, at equilibrium. The vast majority of chemicals that
bioaccumulate are aromatic organic compounds, particularly
those with
chlorine
substituents. For organic compounds,
the mechanism of bioaccumulation is thought to be the
partitioning or solubilization of chemical into the lipids of
the organism. Thus the BCF should be proportional to
the lipophilicity of the chemical, which is described by the
octanol-water partition coefficient, Kow. The latter is a
physical-chemical property of the compound describing its
relative solubility in an organic phase and is the ratio of its
solubility in octanol to its solubility in water at equilibrium.
It is constant at a given temperature. If one assumes that a
chemical’s solubility in octanol is similar to its solubility in
lipid, then we can approximate the lipid-normalized BCF
as equal to the Kow. This assumption has been shown to
be a reasonable first approximation for most chemicals accu-
mulation in fish tissue.
144
However, animals are exposed to contaminants by
other routes in addition to passive partitioning from water.
For instance, fish can take up chemicals from the food they
eat. It has been noted in field collections that for certain
chemicals, the observed fish-water ratio (BCF) is signifi-
cantly greater than the theoretical BCF, based on Kow. This
indicates that the chemical has accumulated to a greater
extent than its equilibrium concentration. This is defined as
biomagnification. This condition has been documented in
aquatic animals, including fish, shellfish,
seals and sea
lions
,
whales
, and otters, and in birds, mink, rodents, and
humans in both laboratory and field studies.
The biomagnification factor, BMF, is usually de-
scribed as the ratio of the observed lipid-normalized BCF
to Kow, which is the theoretical lipid-normalized BCF.
This is equivalent to the multiplication factor above the
equilibrium concentration. If this ratio is equal to or less
than one, then the compound has not biomagnified. If the
ratio is greater than one, then the chemicals biomagnified
by that factor. For instance, if a chemical’s Kow were
100,000, then its lipid normalized BCF should be 100,000
if the chemical were in equilibrium in the organism’s lipids.
If the fish tissue concentration (normalized to lipids) were
500,000, then the chemical would be said to have biomagni-
fied by a factor of five.
Biomagnification in the aquatic food chain often leads
to biomagnification in terrestrial food chains, particularly in
the case of bird and
wildlife
populations that feed on fish.
Consider the following example that demonstrates the re-
sults of biomagnification. The concentrations of the insecti-
cide dieldrin in various trophic levels are determined to be
the following: water, 0.1 ng/L;
phytoplankton
, 100 ng/g
lipid;
zooplankton
, 200 ng/g lipid; fish, 600 ng/g lipid;
terns, 800 ng/g lipid. If the Kow were equal to one million,
then the phytoplankton would be in equilibrium with the
water, but the zooplankton would have magnified the com-
pound by a factor of 2, the fish by a factor of 6, and the
terns by a factor of 8.
The mechanism of biomagnification is not completely
understood. To achieve a concentration of a chemical greater
than its equilibrium value indicates that the elimination rate
is slower than for chemicals that reach equilibrium. Transfer
efficiencies of the chemical would affect the relative ratio of
uptake and elimination. There are many factors that control
the uptake and elimination of a chemical from the consump-
tion of contaminated food, and these include factors specific
to the chemical as well as factors specific to the organism.
The chemical properties include solubility, Kow, molecular
weight and volume, and diffusion rates between organism
gut, blood, and lipid pools. The organism properties include
the feeding rate, diet preferences, assimilation rate into the
gut, rate of chemical’s
metabolism
, rate of egestion, and
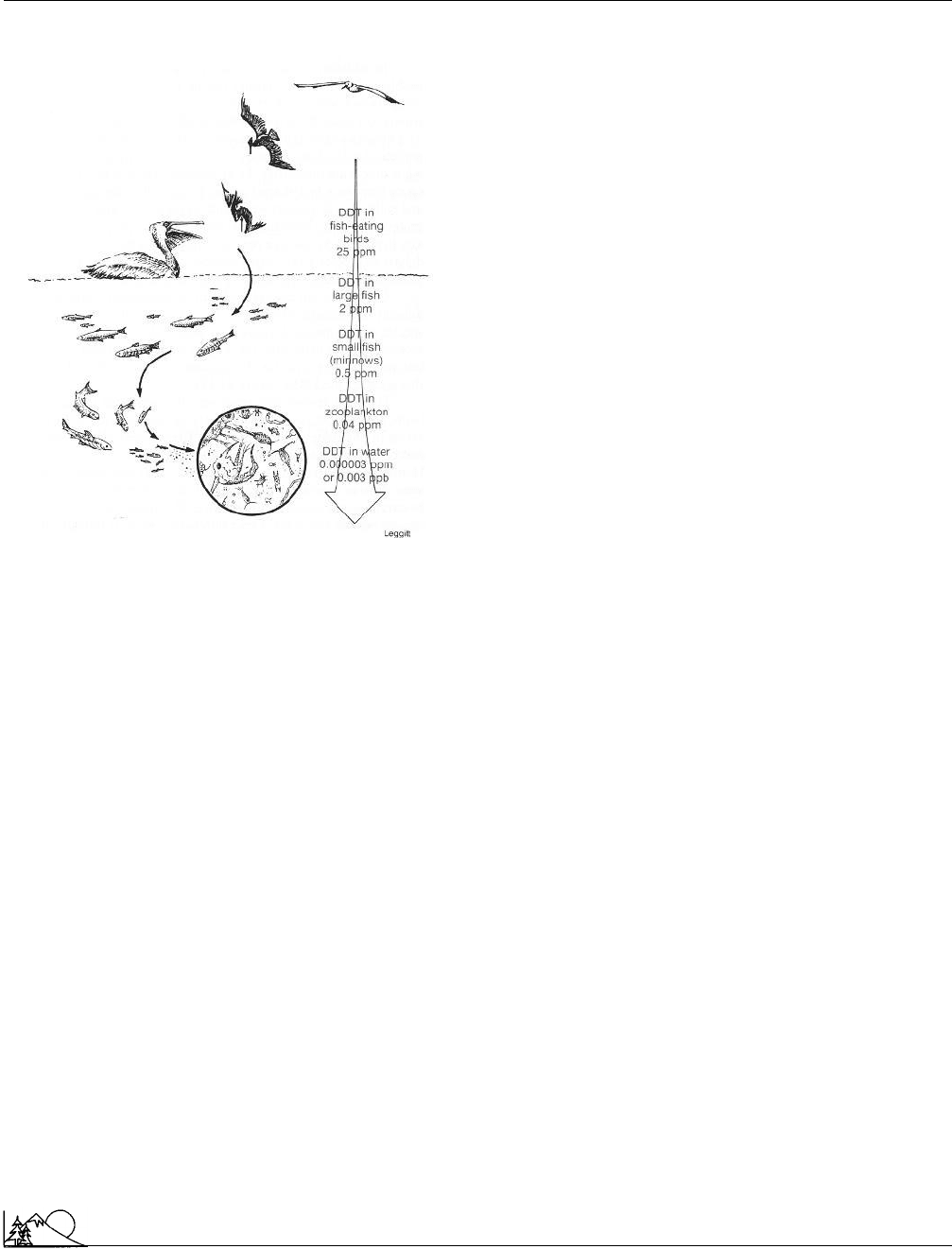
Environmental Encyclopedia 3
Biomass
Biomagnification of the pesticide DDT in the
food chain. DDT travels from runoff up through
the food chain, accumulating in all exposed
species. (McGraw-Hill Inc. Reproduced by permission.)
rate of organism growth. It is thought that the chemical’s
properties control whether biomagnification will occur, and
that it is the transfer rate from lipid to blood that allows
the chemical to attain a lipid concentration greater than its
equilibrium value. Thus it follows that the chemicals that
biomagnify have similar properties. They typically are or-
ganic; they have molecular weights between 200 and 600
daltons; they have Kows between 10,000 and 10 million;
they are resistant to metabolism by the organism; they are
non-ionic, neutral compounds; and they have molecular vol-
umes between 260 and 760 cubic angstroms, a cross sectional
width of less than 9.5 angstoms and a molecular surface
area between 200 and 460 square angstroms. The latter
dimensions allow them to more easily pass through lipid
bilayers into cells but perhaps do not allow them to leave
the cell easily due to their high lipophilicity. Since this dis-
equilibrium would occur at each
trophic level
, it results in
more and more biomagnification at each higher trophic level.
Because humans occupy a very high trophic level, we are
particularly vulnerable to adverse health effects as a result
of exposure to chemicals that biomagnify.
[Deborah L. Swackhammer]
145
R
ESOURCES
B
OOKS
Connell, D. W. Bioaccumulation of Xenobiotic Compounds. Boca Raton:
CRC Press, 1990.
P
ERIODICALS
Bierman Jr., V. J. “Equilibrium Partitioning and Biomagnification of Or-
ganic Chemicals in Benthic Animals.” Environmental Science and Technology
24 (September 1990); 1407–12.
Sijm, D., W. Seinen, and A. Opperhuizen. “Life Cycle Biomagnification
Study in Fish.” Environmental Science and Technology 26 (November 1992):
2162–74.
Biomass
Biomass is a measure of the amount of biological substance
minus its water content found at a given time and place on
the earth’s surface. Although sometimes defined strictly as
living material, in actual practice the term often refers to
living organisms, or parts of living organisms, as well as waste
products or non-decomposed remains. It is a distinguishing
feature of ecological systems and is usually presented as
biomass density in units of dry weight per unit area. The
term is somewhat imprecise in that it includes autotrophic
plants, referred to as phytomass, heterotrophic
microbes
,
and animal material, or zoomass. In most settings, phytomass
is by far the most important component. A square meter of
the planet’s land area has, on average, about 22.05 – 26.46
lb (10 – 12 kg) of phytomass, although values may vary
widely depending on the type of
biome
. Tropical rain forests
average about 45 kg/m
2
while a
desert
biome may have a
value near zero. The global average for heterotrophic biomass
is approximately 0.1 kg/m
2
, and the average for human bio-
mass has been estimated at 0.5 g/m
2
if permanently glaciated
areas are excluded.
The nature of biomass varies widely. Density of fresh
material ranges from a low of 0.14 g/cm
3
for floats of aquatic
plants to values greater that 1 g/cm
3
for very dense hardwood.
The water content of fresh material may be as low as 5% in
mature seeds or as high as 95% in fruits and young shoots.
Water levels for living plants and animals run from 50 to
80%, depending on the
species
, season, and growing condi-
tions. To insure a uniform basis for comparison, biomass
samples are dried at 221°F (105°C) until they reach a con-
stant weight.
Organic compounds typically constitute about 95% by
weight of the total biomass, and nonvolatile residue, or ash,
about 5%.
Carbon
is the principle element in biomass and
usually represents about 45% of the total. An exception
occurs in species that incorporate large amounts of inorganic
elements such as silicon or calcium, in which case the carbon
content may be much lower and nonvolatile residue several
times higher. Another exception is found in tissues rich in

Environmental Encyclopedia 3
Biomass fuel
lipids (oil or fat), where the carbon content may reach values
as high as 70%.
Photosynthesis
is the principle agent for biomass
production. Light energy is used by chlorophyll-containing
green plants to remove (or fix)
carbon dioxide
from the
atmosphere
and convert it to energy rich organic com-
pounds or biomass. It has been estimated that on the face
of the earth approximately 200 billion tons of carbon dioxide
are converted to biomass each year. Carbohydrates are usually
the primary constituent of biomass, and cellulose is the single
most important component. Starches are also important and
predominate in storage organs such as tubers and rhizomes.
Sugars reach high levels in fruits and in plants such as sugar
cane and sugar beet. Lignin is a very significant non-carbohy-
drate constituent of woody plant biomass.
[Douglas C. Pratt]
R
ESOURCES
B
OOKS
Lieth, H. F. H. Patterns of Primary Production in the Biosphere. Stroudsburg,
PA: Dowden, Hutchinson, and Ross, distributed by Academic Press, 1978.
Smil, V. Biomass Energies: Resources, Links, Constraints. New York: Plenum
Press, 1983.
Biomass fuel
A
biomass
fuel is an energy source derived from living
organisms. Most commonly it is plant residue, harvested,
dried and burned, or further processed into solid, liquid, or
gaseous fuels. The most familiar and widely used biomass
fuel is wood. Agricultural waste, including materials such as
the cereal straw, seed hulls, corn stalks and cobs, is also a
significant source. Native shrubs and herbaceous plants are
potential sources.
Animal waste
, although much less abun-
dant overall, is a bountiful source in some areas.
Wood accounted for 25% of all energy used in the
United States at the beginning of this century. With in-
creased use of
fossil fuels
, its significance rapidly declined.
By 1976, only 1–2% of United States energy was supplied
by wood, and burning of tree wastes by the forest products
industry accounted for most of it. Although the same trend
has been evident in all industrialized countries, the decline
has not been as dramatic everywhere. Sweden, for instance,
still meets 8% of its energy needs with wood, and Fin-
land, 15%.
Globally, it is estimated that biomass supplies about 6
or 7% of total energy, and it continues to be a very important
energy source for many developing countries. In the last 15–
20 years, interest in biomass has greatly increased even in
countries where its use has drastically declined. In the United
States rising fuel prices led to a large increase in the use of
146
wood-burning stoves and furnaces for space heating. Im-
pending fossil fuel shortages have greatly increased research
on its use in the United States and elsewhere. Because bio-
mass is a potentially renewable resource, it is recognized as
a possible replacement of
petroleum
and
natural gas
.
Historically, burning has been the primary mode for
using biomass, but because of its large water content it must
be dried to burn effectively. In the field, the energy of the
sun may be all that is needed to sufficiently lower its water
level. When this is not sufficient, another energy source may
be needed.
Biomass is not as concentrated an energy source as
most fossil fuels even when it is thoroughly dry. Its density
may be increased by milling and compressing dried residues.
The resulting briquettes or pellets are also easier to handle,
store, and transport. Compression has been used with a
variety of materials including crop residues, herbaceous na-
tive plant material, sawdust, and other forest wastes.
Solid fuels are not as convenient or versatile as liquids
or gases, and this is a drawback to the direct use of biomass.
Fortunately, a number of techniques are known for con-
verting it to liquid or gaseous forms.
Partial
combustion
is one method. In this procedure,
biomass is burned in an
environment
with restricted oxygen.
Carbon monoxide
and
hydrogen
are formed instead of
carbon dioxide
and water. This mixture is called synthetic
gas or “syngas.” It can serve as fuel although its energy
content is lower than natural gas (
methane
). Syngas may
also be converted to
methanol
, a one carbon-alcohol that
can be used as a
transportation
fuel. Because methanol is
a liquid, it is easy to store and transport.
Anaerobic digestion
is another method for forming
gases from biomass. It uses
microorganisms
, in the absence
of oxygen, to convert organic materials to methane. This
method is particularly suitable for animal and human waste.
Animal
feedlots
faced with disposal problems may install
microbial gasifiers to convert waste to gaseous fuel used to
heat farm buildings or generate electricity.
For materials rich in starch and sugar, fermentation
is an attractive alternative. Through
acid
hydrolysis or enzy-
matic digestion, starch can be extracted and converted to
sugars. Sugars can be fermented to produce
ethanol
, a liquid
biofuel with many potential uses.
Cellulose is the single most important component of
plant biomass. Like starch, it is made of linked sugar compo-
nents that may be easily fermented when separated from the
cellulose polymer. The complex structure of cellulose makes
separation difficult, but enzymatic means are being devel-
oped to do so. Perfection of this technology will create a
large potential for ethanol production using plant materials
that are not human foods.
