Elsevier Encyclopedia of Geology - vol I A-E
Подождите немного. Документ загружается.


Phanerozoic Diversity Change
Marine biodiversity change The patterns of marine
biodiversity change at the family level during the Pha-
nerozoic emerging from the current databases consist-
ently show a rise from a handful of taxa at the
beginning of the Cambrian to some 1900 families at
the present day (e.g. Figure 1A). This rise included
steep increases in the Early to mid-Cambrian, the
Early and mid-Ordovician, and from the Triassic
to the Holocene. The diversity level attained in the
Ordovician established a plateau, which was essen-
tially maintained until the end Permian mass extinc-
tion event (see Palaeozoic: End Permian Extinctions).
Other mass extinctions in the latest Ordovician and
Late Devonian and at the ends of the Triassic and
Cretaceous periods (see Mesozoic: End Cretaceous
Extinctions) punctuated the overall pattern. The
genus-level curve (Figure 1B) shows a similar overall
pattern but, unsurprisingly, is much more saw-
toothed, and major extinction events (not just the
‘big five’ listed above) had a much more profound
effect at lower taxonomic levels.
The overall diversity curves have been resolved
into three so-called evolutionary faunas (Figure 1),
each characterized by the dominance of a set of
major clades and together representing an increase in
ecological complexity through time (Table 1). The
Cambrian Fauna dominated its eponymous period
but declined thereafter. The Palaeozoic Fauna had its
origins in the Cambrian but rose during the Ordovi-
cian biodiversification event to a dominance that was
largely maintained for the rest of the Palaeozoic. The
Palaeozoic Fauna on the low-latitude Laurentian plate
showed an onshore–offshore pattern of innovation
and expansion of communities during the Ordovician.
The Modern Fauna has been an important component
of the marine biota, initially in nearshore habitats,
since the Ordovician but did not become dominant
until after the end Permian extinction event.
Mass extinctions produced the major troughs in the
diversity curves but represent one extreme of a con-
tinuum of rates of species extinction per million years.
The end-Palaeozoic, end-Mesozoic, and, to a lesser
extent, Late Devonian mass extinctions did not
simply reduce overall biodiversity; they disrupted
the ecological patterns so severely that major changes
in overall community and even ecosystem structure
could occur. However, whilst some significant
changes in the proportions of marine taxa grouped
by fundamental differences in autecology and physi-
ology took place after mass extinctions, many of the
episodes of change or of relative stasis of such fea-
tures did not mirror the concurrent trajectory in
global diversity. Thus, for example, while there were
stepped increases in the proportion of predator taxa
following the end-Permian and end-Cretaceous ex-
tinction events, this proportion then fluctuated only
within a fairly narrow band during the subsequent
rises in overall diversity.
Non-marine biodiversity change The shape of the
non-marine biodiversity curve is markedly different
from that of the marine biodiversity curve (Figure 2),
and its principal components are the plants, insects,
and tetrapod vertebrates.
Whilst the land probably had a microbial flora
extending back into the Precambrian, spores and
phytodebris indicate that land plants were present
Table 1 The major components and ecological structures of the three marine evolutionary faunas
Evolutionary fauna
Community
diversity Food webs Tiering
Suspension
feeders
Detritivores
and
carnivores
Planktonic
food
Animals
in water
column
Modern evolutionary fauna
(demosponges, gastropods,
bivalves, gymnolaemate
bryozoans, malacostracans,
echinoids, vertebrates)
High Highly
complex
Complex Epifaunal
and
infaunal
Common Abundant Many
Palaeozoic evolutionary fauna
(anthozoans, cephalopods,
stenolaemate bryozoans,
‘articulated’ brachiopods,
ostracods, stelleroids,
crinoids, graptolites)
Intermediate Intermediate Develops Epifaunal
common
Common Common Many
Cambrian evolutionary fauna
(hyolithids, monoplacophorans,
‘inarticulate’ brachiopods,
trilobites, eocrinoids)
Low Simple Limited Few Dominant Limited Few
262 BIODIVERSITY
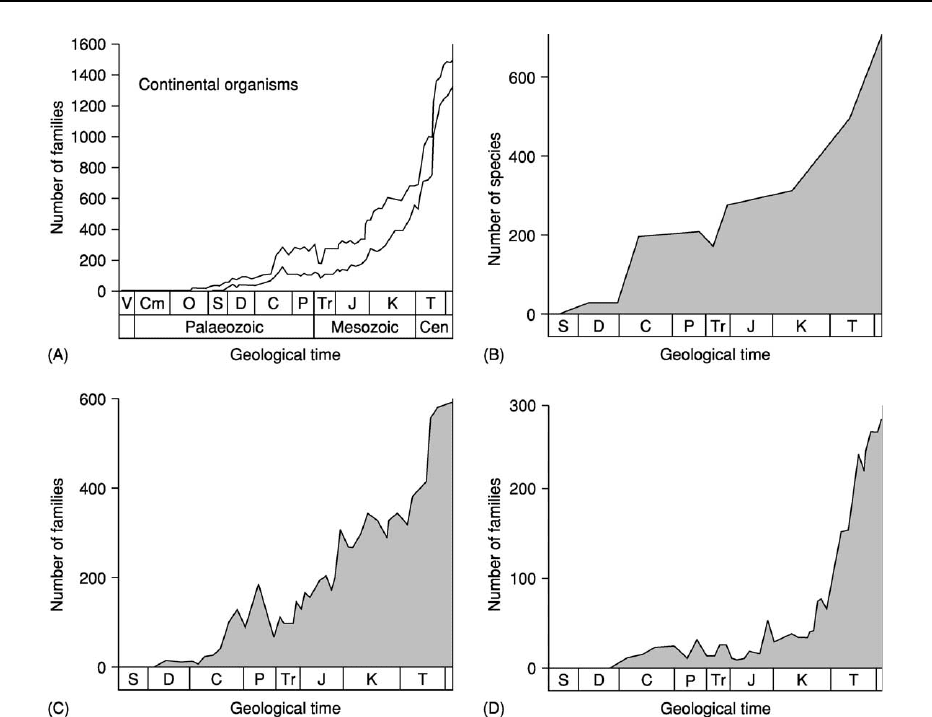
from the mid-Ordovician onwards. These earliest
plants were probably bryophytes; spore evidence sug-
gests that vascular plants or their immediate ancestors
appeared in the Early Silurian. The first unequivocal
land-plant megafossils are known from the mid-Silur-
ian (Wenlock). Following the Silurian and Devonian
development and radiation of early vascular-plant
groups, the plant biodiversity curve (Figure 2B)reflects
the successive acmes of the pteridophytes and early
gymnosperms in the Carboniferous–Permian and
the gymnosperms (see Fossil Plants: Gymnosperms) in
the Triassic–Jurassic and the rise of the angiosperms
from the Cretaceous onwards.
The diversity curve for the insects (Figure 2C)
shows a rather saw-toothed exponential rise from the
first appearance of the group in the Devonian. The
tetrapods (Figures 2D and 3A) also show an essen-
tially exponential pattern, with a long (Devonian to
mid-Cretaceous) period of weak diversity increase
followed by a steep rise. The curve primarily reflects
the radiations and acmes of three major sets of groups:
the basal tetrapods and synapsids in the Palaeozoic,
archosaurs in the Mesozoic, and lissamphibians,
lepidosaurs, birds, and mammals in the Cenozoic.
The colonization of freshwater and the increase
in diversity therein was achieved both by air-breathing
groups and by taxa from several major marine clades
that independently became adapted to reduced salinity
by movement upstream through estuarine environ-
ments. However, the occupation by invertebrates of
freshwater habitats, especially those within the sub-
strate, took a remarkably long time, and it was not
until the Mesozoic that a significant range of originally
marine groups developed the necessary osmoregula-
tory, reproductive, and dispersal capacities to occupy
freshwater environments.
Figure 2 Terrestrial biodiversity through the Phanerozoic. (A) All multicellular families (lower curve represents families undoubt-
edly present within each stage and restricted to the non-marine environment; upper curve includes less stratigraphically well-
attributed families and those that include environmentally equivocal that include marine or environmentally equivocal taxa); (B)
vascular land plants; (C) insects; and (D) non-marine tetrapods. (Adapted from Benton MJ (2001) Biodiversity through time. In: Briggs
DEG and Crowther P (eds.) (2001)
Palaeobiology II, pp. 211–220. Oxford: Blackwell Publishing.)
BIODIVERSITY 263
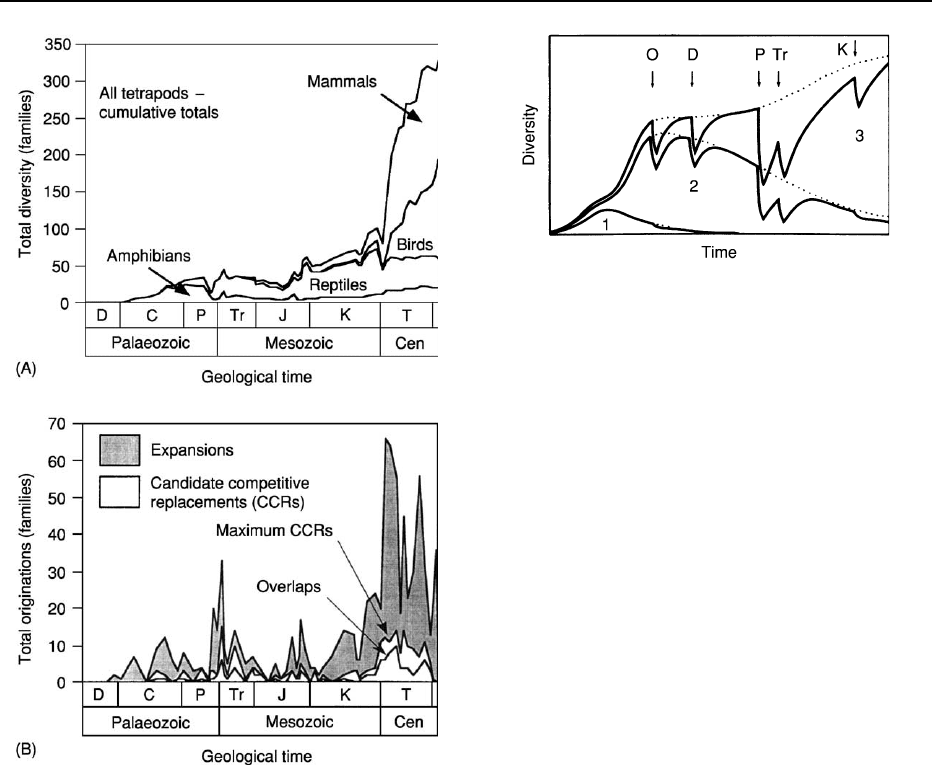
Understanding Biodiversity Curves
If the existing curves are even an approximation of
the true pattern, the great challenge is to interpret
them in terms of the processes leading to biodiversity
change through geological time. At the simplest level,
the curves reflect the complex interplay of species
originations, extinctions, and stasis, with the major
changes in trajectory representing episodes of radi-
ation punctuated by mass extinctions. Although the
traditional assumption has been that radiations were
‘adaptive’, representing the acquisition of new char-
acters by the radiating group that gave them superior-
ity over their competitors, there is increasing evidence
for the expansion of many clades into previously
unoccupied ecospace. This is perhaps most strikingly
illustrated by the tetrapods, for which it has been
calculated that a maximum of only 13% of families
could have originated by competitive replacement of
earlier taxa (Figure 3). It has also been argued that
clade radiations could arise randomly, without being
driven by any deterministic cause such as competition
or expansion associated with a new adaptation or
opportunity.
The shapes of biodiversity curves Several attempts
have been made to model mathematically the shapes
of global Phanerozoic diversity curves and therefore
to determine whether they reflect overarching global
processes and patterns of evolution.
Continental biodiversity, either as a whole or of
individual large clades (Figure 2), seems to show
an exponential rise without a sustained levelling off.
In contrast, the family-level marine curve has been
modelled fairly closely in terms of logistic curves rep-
resenting the Cambrian, Palaeozoic, and Modern
Faunas perturbed by the major mass extinction events
(Figure 4). In this coupled logistic model, each evolu-
tionary fauna showed a slow initial increase in diver-
sity, followed by a steep increase to a plateau and then
a slow decline corresponding to the rise of the follow-
ing evolutionary fauna (which had a lower initial rate
of diversification and a higher maximum diversity
level). Within such a model, the trajectory of the
curve for the Modern Evolutionary Fauna is currently
still in its exponential phase, but its roughly convex
shape suggests that an asymptotic stage could be pro-
jected in the future. The effects of the mass extinction
Figure 3 Biodiversity change in 840 non-singleton tetrapod
families, broken down into (A) the component groups and (B) the
styles of origination of new families. Overlap candidate competi-
tive replacements are those in which the range of an originating
family overlapped with that of another family rather than re-
placing it in the fossil record. (Adapted from Benton MJ (1999)
The history of life: large databases in palaeontology. In: Harper
DAT (ed.)
Numerical Palaeobiology, pp. 249–283. Chichester: Wiley.)
Figure 4 Three-phase coupled logistic model of changes in
family-level marine biodiversity during the Phanerozoic, includ-
ing perturbations simulating the mass extinctions at the end of the
Ordovician (O), in the late Devonian (D), and at the ends of
the Permian (P), Triassic (Tr), and Cretaceous (K). The numbers
1, 2 and 3 refer to the Cambrian, Palaeozoic and Modern evolu-
tionary faunas shown on Figure 1. Dotted lines represent the
trajectories of the unperturbed three-phase system. (Adapted
from Sepkoski JJ (1984) A kinetic model of Phanerozoic taxo-
nomic diversity. III. Post-Paleozoic families and mass extinctions.
Paleobiology 10: 246–267.)
264 BIODIVERSITY

events enhance the fit of the theoretical curves to the
measured global patterns.
By extending the theory of island biogeography
developed in the 1960s to the global scale, the logistic
curves applied to marine familial diversity have been
interpreted as reflecting evolution into new habitats
and empty niches until a level of dynamic equilibrium
is reached at the carrying capacity of the environ-
ment. This theory has received widespread, but not
universal, acceptance. It has been argued, for exam-
ple, that the shapes of the curves may represent not
global-scale biotic interactions but rather the total
effect of physical perturbations operating at a wide
range of geographical and temporal scales. Moreover,
the evidence for equilibrium, even on an ecological
rather than a geological time-scale, and the upper
limit on diversity that it would impose have also
been widely contested. The Palaeozoic diversity plat-
eau, taken to be strong evidence for equilibrium, may
have been maintained by factors other than biotic
interactions and may even be an artefact of the taxo-
nomic level of the data. Importantly, it is clear that
different clades or parts thereof may show very dif-
ferent patterns of biodiversity change.
The causes of biodiversity change Despite consider-
able research effort, there has been only limited success
in identifying ‘rules’ governing species and community
diversity and the relative abundances of species within
communities. It is highly unlikely, even for mass extinc-
tion events, that a single factor can be invoked to
explain global changes in biodiversity. In addition to
factors intrinsic to individual clades, a host of inter-
related extrinsic factors undoubtedly influence the evo-
lution, distribution, and diversity of organisms across
the spectrum of spatial and temporal scales. Crucially,
the factors operating at one scale may be very different
from those operating at another. Intriguingly though,
there is some evidence to suggest that the family-level
global diversity curve has properties of self-organized
criticality, and, thus, Phanerozoic diversity change may
be driven by the internal dynamics of life itself, as well
as responding to external factors.
The present-day concerns over biodiversity change
stem from the recognition of the deleterious conse-
quences of anthropogenic activities, including habitat
destruction, pollution, and influences on global cli-
mate. The fossil record provides a time perspective
not only on the patterns of biodiversity change but
also on the natural physical factors that drive it, from
local fluctuations in environmental conditions to plate
tectonics, eustasy, ocean circulation patterns, and cli-
mate changes. Heightened awareness of the quality
and detail of the data involved in the generation and
analysis of diversity curves at all spatial and temporal
scales should result in greater confidence in the
conclusions drawn from them.
See Also
Biological Radiations and Speciation. Biosediments
and Biofilms. Evolution. Fossil Plants: Gymnosperms.
Mesozoic: End Cretaceous Extinctions. Palaeoecology.
Palaeozoic: End Permian Extinctions. Sequence Stra-
tigraphy.
Further Reading
Bambach RK, Knoll AH, and Sepkoski JJ (2002) Anatom-
ical and ecological constraints on Phanerozoic animal
diversity in the marine realm. Proceedings of the Na-
tional Academy of Sciences USA 99: 6854–6859.
Benton MJ (1999) The history of life: large databases in
palaeontology. In: Harper DAT (ed.) Numerical Palaeo-
biology, pp. 249–283. Chichester: Wiley.
Benton MJ (2001) Biodiversity on land and in the sea.
Geological Journal 36: 211–230.
Briggs DEG and Crowther P (eds.) (2001) Palaeobiology II.
Oxford: Blackwell Publishing.
Crame JA and Owen AW (eds.) (2002) Palaeobiogeography
and Biodiversity Change: The Ordovician and Mesozoic–
Cenozoic Radiations. Special Publication 194. London:
Geological Society.
Gaston KJ and Spicer JI (1998) Biodiversity: An Introduc-
tion. Oxford: Blackwell Science.
Hewzulla D, Boulter MC, Benton MJ, and Halley JM
(1999) Evolutionary patterns from mass originations
and mass extinctions. Philosophical Transactions of the
Royal Society of London Series B 354: 463–469.
Knoll AH (1994) Proterozoic and early Cambrian protists:
evidence for accelerating evolutionary tempo. Pro-
ceedings of the National Academy of Sciences USA 91:
6743–6750.
Levin SA (ed.) (2001) Encyclopedia of Biodiversity, 5 vols.
San Diego: Academic Press.
May RM (1992) How many species inhabit the Earth?
Scientific American October 1992: 18–24.
Miller AI (2000) Conversations about Phanerozoic global
diversity. Paleobiology 26(4 Suppl.): 53–73.
Sepkoski JJ (1984) A kinetic model of Phanerozoic taxo-
nomic diversity. III. Post-Paleozoic families and mass
extinctions. Paleobiology 10: 246–267.
Sepkoski JJ (1997) Biodiversity: past, present, and future.
Journal of Paleontology 71: 533–539.
Smith AB (2001) Large-scale heterogeneity of the fossil
record: implications for Phanerozoic biodiversity studies.
Philosophical Transactions of the Royal Society of
London, Series B 356: 351–367.
Ward BB (2002) How many species of prokaryotes are
there? Proceedings of the National Academy of Sciences
USA 99: 10234–10236.
BIODIVERSITY 265
a0005 BIOLOGICAL RADIATIONS AND SPECIATION
P L Forey, The Natural History Museum,
London, UK
Copyright 2005, Natural History Museum. All Rights Reserved.
Introduction
This entry discusses the evidence for speciation and
the patterns of species multiplication and associ-
ated morphological change as revealed in the fossil
record. Speciation is regarded as the key stage in
the generation of biological diversity as it is the
point at which reproductive isolation is achieved –
the hallmark of the biological species (see Evolution).
Many studies of modern species concern themselves
with the short-term genetic fluctuations between
populations of a single species, how those fluctu-
ations may come about and how they are maintained.
Such studies are not possible in the fossil record. On
the other hand, the fossil record documents extended
timescales and may, therefore, identify patterns of
long-term changes relevant to different theories of
speciation that are unavailable in the Recent world.
Irrespective of the mode of speciation, the fossil
record demonstrates repeated instances, geologically
speaking, of sudden increases in the numbers of species
and these are usually associated with rapid morpho-
logical divergence. Such events are commonly referred
to as radiations, more usually adaptive radiations, and
are inferred to have come about by causes intrinsic to
the organisms and/or extrinsic causes. Although stud-
ies of radiations involving populations and closely
related species are studied in the Recent world,
broad-scale patterns can only be studied through the
time dimension supplied by the fossil record.
Species, Species Recognition and
Speciation in the Fossil Record
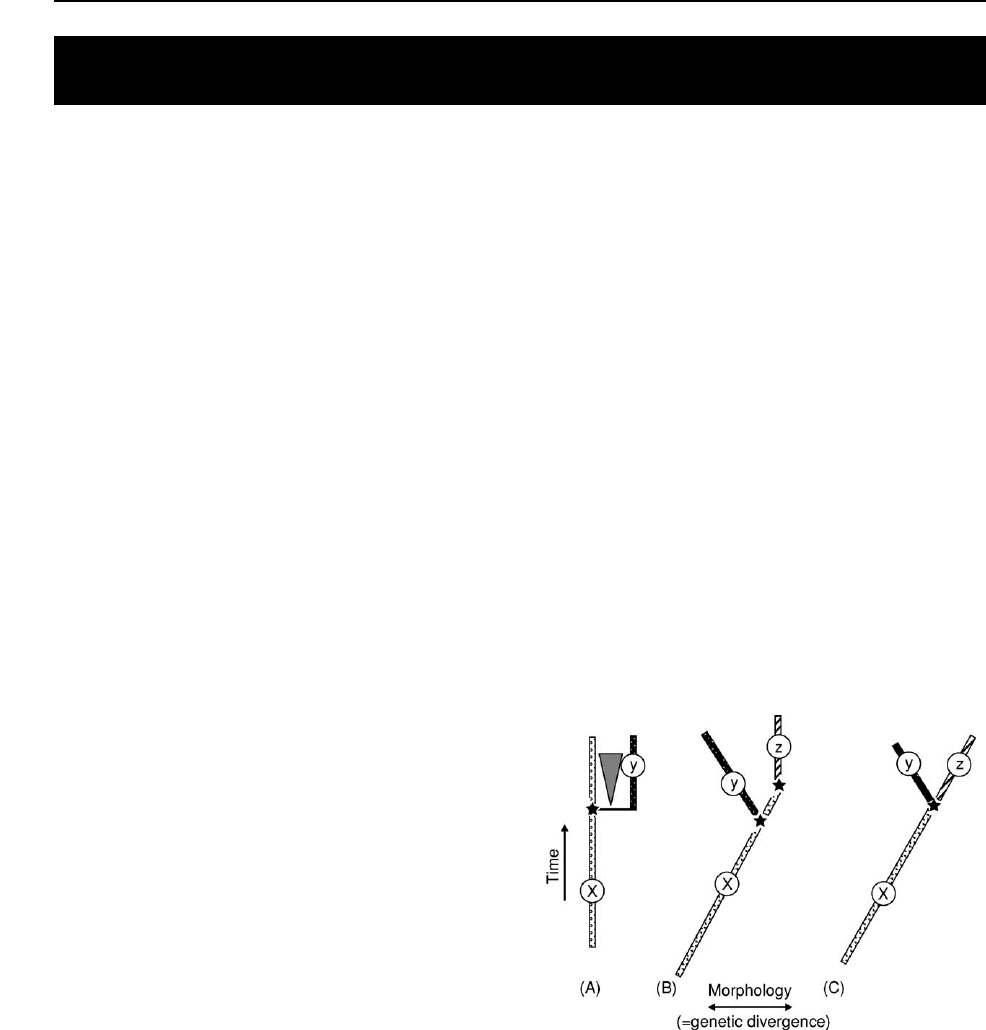
There is a plethora of species definitions and criteria
for recognizing species in the Recent world, but
few are applicable to the fossil record where only a
limited amount of morphological data, usually the
skeleton, is available for study. Three such definitions
that rely on some underlying process of speciation are
shown in Figure 1. The biological species concept of
Ernst Mayr (Figure 1A) is probably that most
favoured by people studying the Recent world.
Species status is achieved when populations diverge
genetically to such an extent that reproductive
cohesion is broken. There may be several reasons for
fragmentation of reproductive continuity (geography,
ecology, and behaviour are most commonly cited),
although Mayr stressed geographical isolation. The
parental species may continue to live alongside the
daughter species. In practice, most modern species
are not recognized on reproductive criteria: instead
some measure of morphological, genetic, behavioural
or ecological difference is used as a surrogate for
reproductive incompatibility. As such, palaeonto-
logical species, recognized almost exclusively on
morphological differences, are equally as valid as
modern species. In fact, there has been empirical jus-
tification for equating genetic differences with mor-
phological differences between modern species of
some groups such as bryozoans and Darwin’s finches.
It is, however, recognized that morphological differ-
ence may not always indicate species differences (e.g.,
sibling species or polymorphic species including
mimetic species).
The evolutionary concept of George Gaylord
Simpson (Figure 1B) defines a species as an an-
cestor-descendent sequence of populations changing
through time with their own trends and tendencies.
Figure 1 Three concepts of species. (A) Mayr’s biological
species. Populations reach species distinction when reproduct-
ive cohesion is broken, usually by the imposition of some isolat-
ing mechanism such as geographic separation of populations
(triangle). The original species (X) may or may not continue to
live contemporaneously with the daughter species (Y). (B) Simp-
son’s evolutionary species concept. Species are ancestor-
descendent sequences of populations changing through time.
A species may change gradually into another (X ! Z) through a
process of anagenesis or the lineage may split (X ! Y) through
cladogenesis. (C) The Hennigian species concept recognizes
species as lineage segments. As soon as a cladogenetic
event occurs there are automatically two new species (Y and
Z), regardless of any perceived morphological change. Stars
represent speciation events.
266 BIOLOGICAL RADIATIONS AND SPECIATION

This definition embraces asexual species. Such a def-
inition allows for one species to change gradually
into another (anagenesis) as well as instances where
a species is budded off or where one species splits into
two or more (cladogenesis).
The Hennigian species concept (Figure 1C) (named
after the German entomologist Willi Hennig), like the
biological species concept, is based on the criterion
of reproductive isolation. However, the species limits
are recognized only at the point where one species
splits to two or more and the interbreeding pattern of
gene flow is disrupted, at which point the ancestral
species cannot by definition live alongside the des-
cendants. This species definition is strictly dependent
upon the shape of the phylogenetic tree.
Recognition of Species
Species in the fossil record are nearly always recog-
nized on morphological criteria. There are some
exceptions, such as instances where species are distin-
guished from one another purely on the basis of
stratigraphic occurrence or geographic location, but
these are usually accepted as stopgap measures; to be
revised when more information about morphology
becomes available.
Monophyletic species are recognized on the basis
of possessing one or more unique morphological
characters and this corresponds most closely with
the Hennigian species concept. Species viewed in
this light have the same ontological status as higher
taxa such as genera, families, orders, etc. In reality,
very few species can be recognized in this way and
logically it denies the existence of ancestors since
ancestors have no unique features.
Typological species were once commonly recog-
nized, whereby a single specimen (usually the first dis-
covered or the most complete) was chosen as the
Linnean type and this formed the nucleus of morpho-
logical variation considered worthy of species status.
How much variation was to be allowed before a new
species was recognized was never stated. Such species
are usually frowned upon now as being steeped in
the philosophy of pre-Darwinian essentialism. Yet,
there remain many instances where such species are
still used.
Phenetic species arose out of numerical taxonomy.
Here species are measured for as many morphologic-
ally continuous variables as possible. The variables
are then subjected to multivariate analysis, and clus-
ters – (the species) – are then recognized. Although
this may appear to be objective and theory free, it is
heavily dependent upon the original samples analyzed
and the multivariate analysis algorithms used. It is
not always easy to identify which of the individual
variables is contributing to the differences and, there-
fore, it is difficult to diagnose the species.
Phylogenetic species are the smallest diagnosable
assemblages of specimens showing a unique combin-
ation of characters. Combination is the operative
word here since there is no requirement for identifying
unique characters (cf. monophyletic species recogni-
tion). Of all the recognition criteria this most faith-
fully agrees with all of the process-based definitions
of species, as well as agreeing with the day-to-day
practice of palaeontologists.
Estimates of species longevity may depend on which
of the above ways the species have been recognized.
Surveys across various fossil groups give average figures
that range from 1 million years (for small mammals
where generation time is very short) to 13 million
years for dinoflagellates (which are largely asexual).
Speciation in the Fossil Record
It is generally accepted that most speciation events,
with the possible exception of those caused by hybrid-
ization and polyploidy (chromosomal duplication),
are too protracted to be detected in the Recent
world and too brief to be seen in the fossil record.
The lower limits of time that can be successively
sampled in the fossil record appear to be in the order
of 5000–10 000 years and such deposits are very rare,
restricted to lake deposits of relatively short duration
and small geographic range, and some deep-sea de-
posits. Nevertheless, there have been many studies
seeking to establish the precise pattern of speciation.
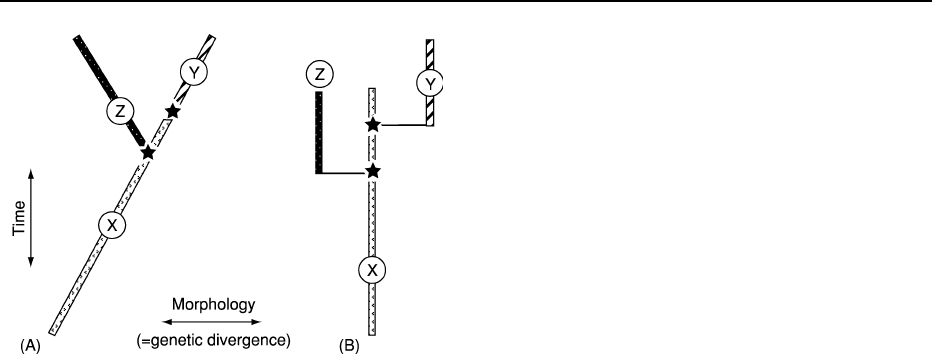
There are two polarized theories on the tempo and
pattern of speciation (Figure 2), punctuated equilibria
and phyletic gradualism. Both have support from the-
oretical and empirical studies in the modern world
(see legend to Figure 2) and it is likely that both modes
of speciation can happen.
Phyletic gradualism Phyletic gradualism holds that
morphological evolution is gradual and occurs inde-
pendently of speciation. Most of the studies support-
ing this mode of evolution involve organisms that
can be minutely sampled through continuous rock
sequences.
Figure 3 shows an example of one gastropod species
(see Fossil Invertebrates: Gastropods) changing or re-
placing another in an ancestor-descendent sequence
(gradual speciation by anagenesis) by a shift in the
modal shell form as measured by morphometric dis-
criminant analysis. In this example the gradual
change has been correlated with increasing depth
of water. The populations intermediate between
the two species were presumed to have lasted for
73 000–250 000 years. In this pattern there is no
BIOLOGICAL RADIATIONS AND SPECIATION 267
rapid shift in morphology and, in studies of this kind,
the species limits are usually recognized by comparing
the morphological variation over a period of time
with morphological variation of modern species and
dividing up the continuum accordingly.
In another study (Figure 4) involving radiolarians
recovered from Pacific Ocean deep-sea drilling cores,
an instance of speciation was detected occurring
during a period of about 500 000 years. In this case
both the ancestral and daughter species showed
marked deviation in many aspects of skeleton form.
Further, it is interesting to note that morphological
deviation, although slightly accelerated during the
speciation event, continued for some time after the
separation of morphotypes, suggesting strongly that
morphological evolution is decoupled from speci-
ation. In this instance both parental and daughter
species showed morphological evolution and it may
have been possible to suggest that a single ancestral
species gave rise to two daughter species. The author
of this study refrained from this because the ancestral
species, which still lives today, shows a high degree of
polymorphism and geographic variation, the scale
of which is comparable to the variation seen in the
lineage in this study.
It needs to be pointed out that many (but not all)
of the studies that support phyletic gradualism, report
information on continuously varying morphologies
such as lengths and shapes. These parameters are in-
herently gradualist in their measurement, unlike many
characters used in cladistic analysis such as presence/
absence or four toes versus five toes in which there can
be no intermediates.
Punctuated equilibrium Speciation following the
punctuated equilibrium model (Figure 2B) has been
claimed for many groups of organisms. This theory
suggests that species themselves exhibit morpho-
logical stasis and that speciation is, geologically spe-
aking, instantaneous and is accompanied by rapid
morphological shifts. In order to demonstrate this at
least three objections raised by advocates of phyletic
gradualism must be overcome. The first objection is
that what appear to be sudden changes only seem so
because either the sampling intervals are too wide or
the fossil record is lacking in the key years where
intermediate populations might have lived. There-
fore, it is necessary to demonstrate that the sampling
is dense. A second objection is that it is possible
that an isolated population, destined to become a
new species, was accumulating genetic and morpho-
logical distinctiveness in a gradualist manner, but that
the population was either so small that fossilization
potential was virtually zero, or that it was living
elsewhere. The sudden appearance is, therefore, ex-
plained not as any intrinsic property of speciation, but
by the new species population growing large enough
to leave a fossil record, or by immigration into the
area of study. The third objection is a taxonomic
argument. Many of the claims of punctuated equilib-
rium involve species recognized by qualitative charac-
ters where there are no intermediate states (see
above). If this is true then the sudden appearance of
morphologic changes must coincide with speciation
and must also remain static until the next ‘speciation’.
Despite the objections there are cases where punc-
tuated equilibrium can be demonstrated. Figure 5
illustrates the case of speciation in the bryozoan
genus Metrarhabdotus during the Neogene and Qua-
ternary of tropical America (see Fossil Invertebrates:
Bryozoans). The species were distinguished on both
continuously varying characters, as well as qualitative
differences. A stratophenetic tree was constructed.
There was particularly dense sampling of the fossil
record in the Dominican Republic between 8 and
4 million years ago, within which time there was the
successive origin of 12 species. Each of the species
appeared suddenly, accompanied by substantial
morphological differences. The species themselves ex-
hibited little or no change throughout their individual
Figure 2 Two theories of speciation modes in the fossil record.
(A) Phyletic gradualism suggests that speciation is a by-product
of changing ratios of gradual morphological variation (implied
gene frequency) within populations through time and that speci-
ation may happen through anagenesis (species X ! Y) as well as
cladogenesis (X ! Z). Phyletic gradualism agrees with Simp-
son’s evolutionary species concept (Figure 1B), coupled with
Fisher’s notion of ‘directed’ selection of gene frequencies within
populations. (B) Punctuated equilibrium claims that individual
species remain morphologically constant throughout time and
that all or most of the morphological (and implied genetic)
change is associated with cladogenesis: the speciation event.
This theory agrees with Mayr’s biological species concept
(Figure 1A) coupled with Sewell Wright’s idea of stabilizing
selection. In these diagrams the species are denoted by letters
and different shadings and the speciation events by stars.
268 BIOLOGICAL RADIATIONS AND SPECIATION
existence and this is a key feature of the punctuated
equilibrium model. Although the possibility of immi-
gration cannot be completely dismissed this is un-
likely because the basal species of this lineage are
morphologically very similar to species occurring
earlier in the same area.
Therefore, it appears as though there is evidence for
both patterns of speciation in the fossil record.
Indeed, there may be instances where both kinds can
be recognized within the same sequence, affecting
closely related organisms. More commonly, species
within the same lineage may show gradualism at
some times and punctuated patterns at others (but
here there is always the objection of differential sam-
pling). Rather than trying to prove that all speciation
complies with one or the other model it may be more
productive to try to determine when and why one or
the other is predominant.
Radiations
Radiations are episodes of increased rates of speci-
ation relative to extinctions resulting in rapid net
increases in diversity. They may be accompanied by
evolution of more diverse body forms than in normal
periods when extinction and origination rates are
approximately equal. Radiations are nearly always
explained in terms of adaptation, hence ‘adaptive ra-
diations’, the latter being defined by Dolph Schluter as
the evolution of ecological and morphological diver-
sity within a rapidly speciating lineage. There are diffi-
culties with defining both ecological and phenotypic
diversity and it is not always easy to describe precisely
the adaptation and even more difficult to isolate the
cause, but the following factors have been suggested as
triggers for radiations (adaptive or otherwise):
1. Abiotic causes – such as changing levels in O
2
/CO
2
concentrations or significant changes in ambient
temperature, changes in ocean current circulation,
fragmentation of landmasses and consequent
changes in both the length and ecological differen-
tiation of coastlines, and changes in latitudinal
distributions.
2. Biotic factors – such as the evolution of a particu-
lar character or characters that may change
aspects of life history leading to rapid speciation
and/or ecological differentiation.
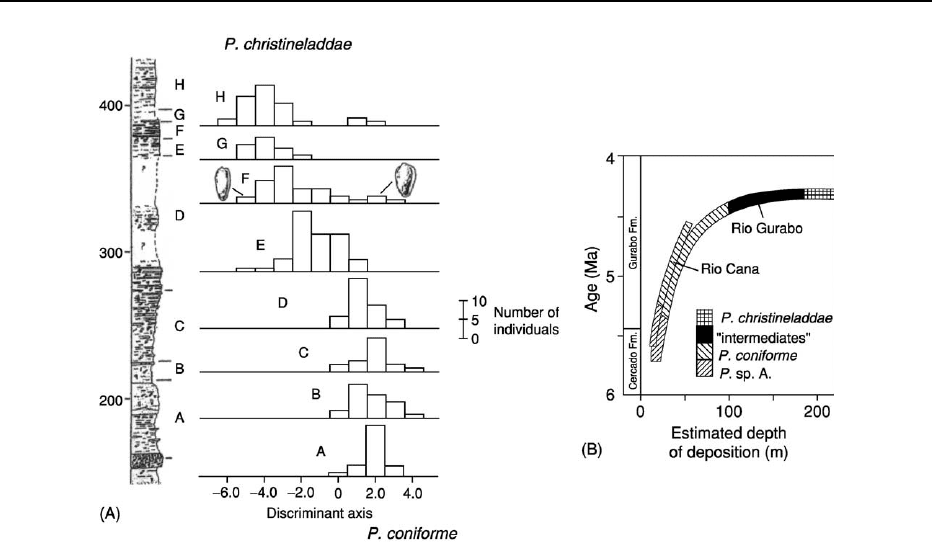
Figure 3 Speciation through anagenesis. In this study a species of marginellid gastropod – (Prunum coniforme) – living in the Mio-
Pliocene of the Dominican Republic was seen to show strong directional selection in shell morphology towards a new species –
(
Prunum christineladdae). (A) Histogram of individuals on the discriminant axis that best distinguishes the two species (endpoints of
sampling) collected from successively higher stratigraphic levels within the section at Rio Gurabo, Dominican Republic (section
thickness marked in meters at left). Notice that at level F there appears to be a mixed population which appears to have occupied
0.6–2.5% of the entire range of the ancestral species – (
P. coniforme). (B) Over a longer time scale the species are distributed in
sediments inferred to have been deposited in increasingly deeper water. Part of the history at Rio Gurabo is repeated at neighbouring
Rio Cana. Reproduced from Nehm RH and Geary DH (1994) A gradual morphologic transition during a rapid speciation event
in marginellid gastropods (Neogene; Dominican Republic).
Journal of Paleontology 68: 787–795.
BIOLOGICAL RADIATIONS AND SPECIATION 269
3. Interactions between organisms – such as the ex-
tinction of one kind allowing ecological and
phenotypic diversification in another. Alterna-
tively it may be true that the morphological and
diversification of one group allows similar phe-
nomena in another (e.g., insects and plants). For
example, in fishes, it has long been noted that the
evolution of many durophagous (mollusc crush-
ing) lineages coincides with the Mesozoic diversifi-
cation of bivalve and gastropod molluscs. Many of
these ‘associations’ are anecdotal and, while they
may well be true, they need firm experimental
evidence for their justification.
It is rarely possible to isolate a single cause and in
all probability complex interactions between two
or more factors are ultimately responsible for radi-
ations. Therefore, the study of these evolutionary
phenomena is both frustrating and challenging,
demanding lengthy and detailed data collection.
Factors which may distort our view of radiations
include the following:
1. Imperfections of the fossil record. Large hiatuses or
differential preservation will inevitably distort our
perception of radiations. For example, the sudden
appearance and apparent radiation of many cat-
fishes (most of which are freshwater) in the Early
Tertiary must be judged against the knowledge that
there are very few freshwater fish-bearing deposits
in the underlying Upper Cretaceous.
2. Appearance of many diverse animals and plants
associated with Lagersta
¨
tten deposits. Such deposits
dramatically increase the numbers and morpho-
logical diversity of species that may, in reality,
have had a long unrecorded history.
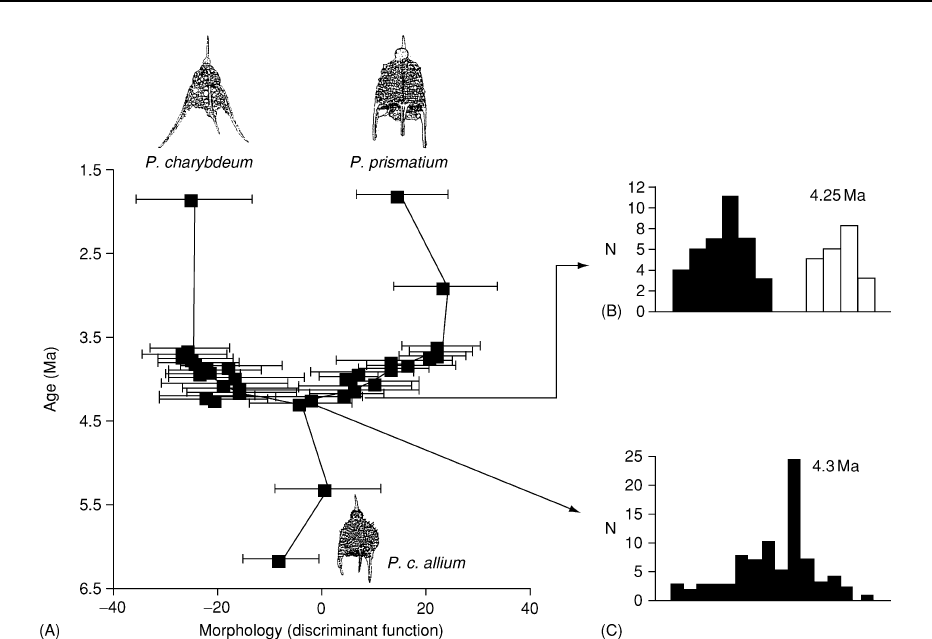
Figure 4 Speciation and the gradualist model. The present day radiolarian species Pterocanium charybdeum extends well back into
the Miocene where a subspecies (
P. c. allium is recognized). At about 4 million years ago a new species, P. prismatium arose and
subsequently went extinct about 1.8 million years ago. To compile this diagram samples representing populations at 50 000 year
intervals were taken from piston cores during the key 4.5–3.5 million year interval (much more widely spaced outside of this time
band). The radiolarian tests were measured for 32 morphological characters such as length, breadth, angles and outlines and
subjected to a multivariate discriminant analysis. The x-axis of the diagram shows the value of the discriminant function that best
separates the species. The boxes are the population means 1 standard error. The histograms on the right show discriminate scores
at the point of the intermediate population (lower diagram) and the sampling level 50 000 years later (upper diagram), showing clear
separation at this time; these two documenting the speciation event. Notice that both lineages continue to diverge morphologically in a
gradualist manner long after the speciation event. Reproduced from Lazarus DB (2001) Speciation and morphological evolution. In:
Briggs DE and Crowther PR (eds.)
Palaeobiology II, pp. 133–137. Oxford: Blackwell Scientific.
270 BIOLOGICAL RADIATIONS AND SPECIATION
3. Taxonomic artifact. Many diversity studies are
based on estimates of numbers of genera or fam-
ilies. Dependent on how those taxa are recognized
in successive stratigraphic levels, the expansion (or
decrease) in diversity may be exaggerated or dis-
torted. For example, it has been pointed out that
the apparent sudden increase in new trilobite fam-
ilies in the earliest Ordovician may be an artifact
of taxonomy of Cambrian trilobites, since many
phylogenetic lines can now be drawn across the
boundary linking families that were previously
thought to be quite distinct.
Despite these problems there are good examples of
association of cause with radiations.
Environmental Shift
One general pattern that has been identified after
extensive collection of both taxic and geological
data is that many invertebrate clades originated in
nearshore marine environments and subsequently
expanded in species numbers to occupy deeper
water, at the same time becoming morphologically
more diverse. Figure 6 shows one example of the
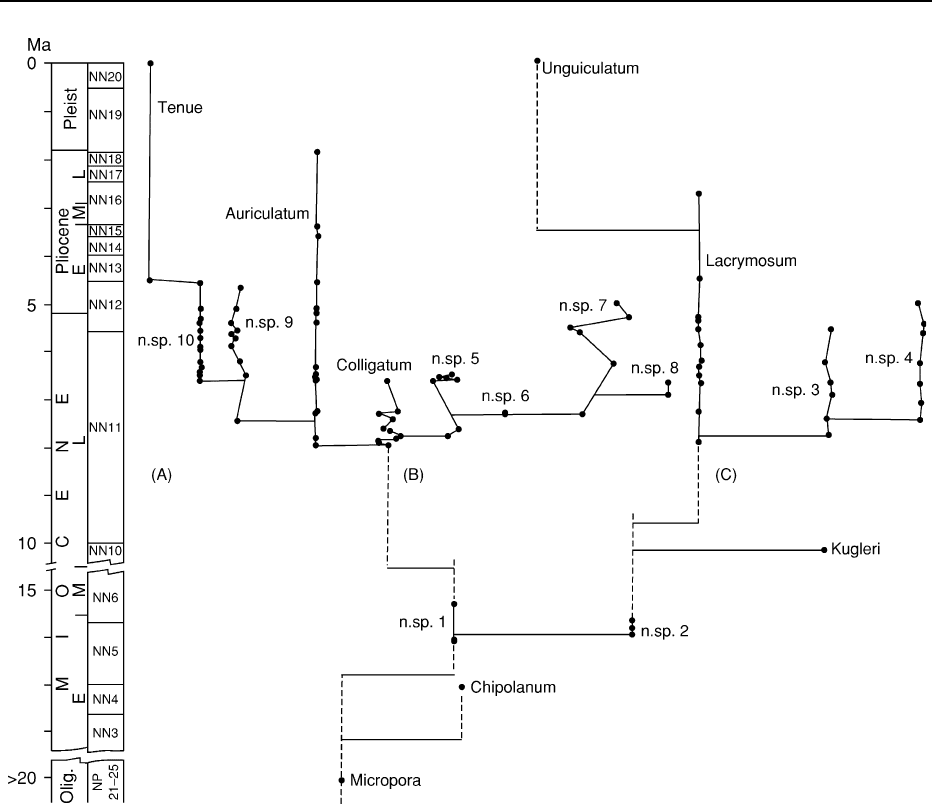
Figure 5 Punctuated equilibrium in Neogene bryozoans. The cheilostome genus Metrarhabdotos is widely distributed in Miocene and
Pliocene deposits of the Caribbean. Sections in the Dominican Republic are particularly rich and uninterrupted, such that is possible to
sample extensively. This study used 46 measurements, counts or codings of colony morphology of the species (some were left un-
named). These were subjected to multivariate analysis and the stratophenetic tree shown here was produced. The dots are sampling
points. The x-axis is a measure of the morphologic distance. Notice the sudden shift in morphological variation at points of
cladogenesis. The within species variability is very small compared with the between species differences as predicted by the
punctuated equilibrium model, as is also the prediction that the ancestral species continues to live alongside the descendant. The
age, epoch and nannoplankton zones are shown on the y-axis. Reproduced from Cheetham AH (1986) Tempo of evolution in a Neogene
bryozoan: rates of morphological change within and across species boundaries.
Paleobiology 12: 190–202.
BIOLOGICAL RADIATIONS AND SPECIATION 271
