Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.

388 5 TEM Applications of EELS
Spectrum imaging offers the possibility of extensive data manipulation after
spectrum acquisition. For example, it allows segmentation to be used for measuring
small concentrations of elements in particular organelles. Regions of similar com-
position can be recognized by examining the K-edges of major constituents (C, N,
O) and spectra from these regions can be summed to provide adequate statistics for
measuring average trace element concentrations. Leapman et al. (1993b)usedthis
technique to measure calcium concentrations (50–100 ppm) in mitochondria and
endoplasmic reticulum (see Fig. 5.67) with a precision of better than 20%. Further
discussion of the quantitative procedures involved is given in Aronova et al. (2009).
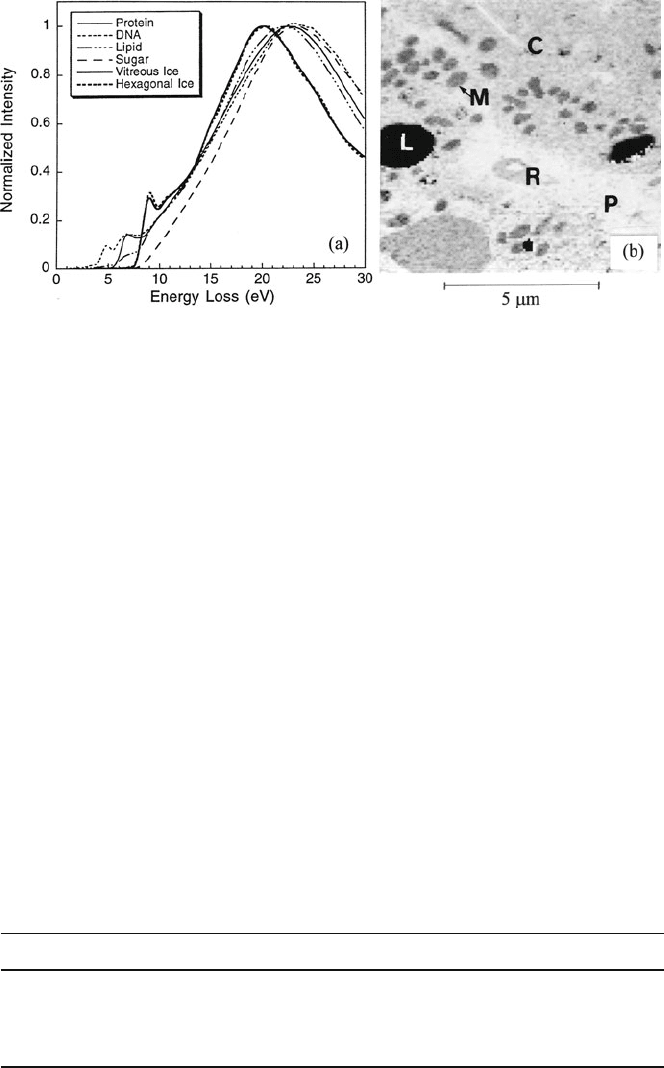
The low-loss spectra of biologically important substances exhibit a broad peak
around 23 eV, whereas ice shows a peak around 20 eV and a sharp rise around 9 eV,
probably due to excitation across the bandgap; see Fig. 5.68a. Sun et al. (1995)
exploited these differences in fine structure to measure the water content within
cells, with a precision of around 2% and a spatial resolution of 80 nm. Their pro-
cedure involved MLS fitting of spectrum image data (6–30 eV region) to standard
spectra from ice and protein. They also produced maps of water content, showing
pronounced differences between mitochondria, cytoplasm, red blood cells, plasma,
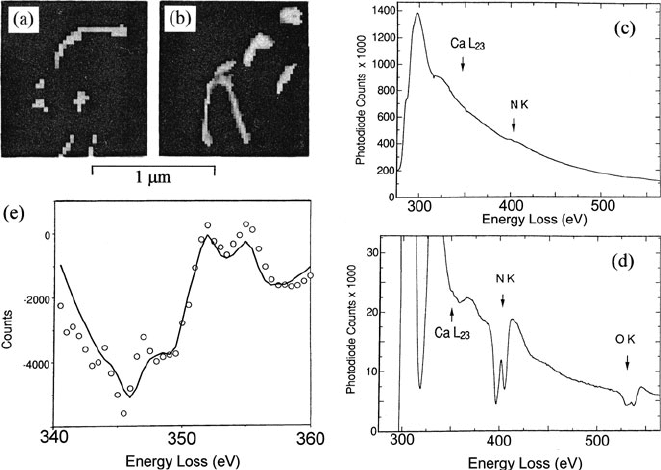
Fig. 5.67 (a, b) Regions of endoplasmic reticulum in mouse cerebellar cortex, segmented on
the basis of their nitrogen content. (c) Spectrum obtained by summing contributions from both
segmented regions. (d) First-difference spectrum, showing a weak Ca L-edge. (e)MLSfitofthe
Ca L-edge data points to a CaCl
2
reference spectrum. From Leapman et al. (1993b), copyright
Elsevier
5.7 Application to Specific Materials 389
Fig. 5.68 (a) Low-loss spectra of protein, DNA, lipid, sugar, and ice. (b) Water map of frozen
hydrated liver tissue: L = lipid droplets (zero water content), M = mitochondria (average content
57%), R = erythrocyte (65% water), P = plasma (91% water). From Sun et al. (1995), copyright
Wiley-Blackwell
and lipid components; see Fig. 5.68b. This same method has more recently been
applied to mapping the water distribution in skin tissue (Yakovlev et al., 2010).
5.7.5 Radiation Damage and Hole Drilling
As discussed in Section 5.5.4, radiation damage provides a basic physical limit to the
spatial resolution of electron-beam analysis, so there is considerable interest in min-
imizing this damage. The basic damage mechanisms are summarized in Table 5.3,
together with some ways of reducing their effect (Egerton et al., 2004).
As discussed in Chapter 3, electrons undergo both elastic and inelastic scattering
in a TEM specimen. Elastic scattering below 100 mrad, used to form diffraction
patterns and bright-field images, involves negligible energy transfer (<0.1 eV) and
no damage to the specimen. However, electrons scattered through larger angles
can transfer several electron volts of energy and cause displacement (or knock-on)
damage if the incident energy exceeds some threshold value, as discussed in
Table 5.3 Mechanisms of radiation damage
Mechanism Possible antidotes
Knock-on displacement Reduce E
0
below threshold, surface coating
Electron-beam heating Reduce beam current, cool the specimen
Charging (SE production) Reduce beam current (possible threshold)
Radiolysis (ionization damage) Cool the specimen, surface coating
390 5 TEM Applications of EELS
Section 3.1.6. High-angle scattering is a rare event, so knock-on damage is impor-
tant only at high electron doses (>1000 C/cm
2
typically) and is noticeable only
in conducting materials (particularly metals), where the high density of free elec-
trons prevents damage by radiolysis. The consequences are permanent displacement
of atoms within a crystal or at grain boundaries (Bouchet and Colliex, 2003) and
removal of atoms from the specimen surface (electron-induced sputtering). In the
latter case, a thin carbon surface coating has been shown to be effective in protecting
the specimen for a limited period (Muller and Silcox, 1995b).
Inelastic scattering involves significant energy transfer to the specimen, on the
average several tens of electron volts per scattering event. Most of this energy ends
up as thermal vibration (heat) but some goes into secondary electron (SE) produc-
tion, giving rise to electrostatic charging of insulating specimens. In addition, the
electron transitions involved in inelastic scattering can result in ionization damage
(radiolysis).
The average energy E deposited in a specimen, per incident electron, can be
evaluated from its energy-loss spectrum:
E=
EJ(E)dE
#
J(E)dE (5.36)
where the integration is over the entire spectrum including the zero-loss peak
(Egerton, 1982b). Because Eq. (5.36) includes plural scattering contributions to
J(E), E increases more than linearly with specimen thickness. Assuming that
only a small fraction of the energy transfer goes into SE production and radiolysis,
the temperature rise in the beam can be estimated by equating the rates of energy
deposition and heat loss, giving
IE(t/λ
i
) = 4πκt(T − T
0
)/[0.58 +2ln(2R
0
/d)] +(π/2)d
2
εσ(T
4
−T
4
0
) (5.37)
where I is the beam current (in A), E is in eV, and λ
i
is the total inelastic mean
free path. Radial heat conduction is assumed, κ being the thermal conductivity of
the specimen, R
0
the distance between the beam and a thermal sink (e.g., grid bars,
assumed to be at the ambient temperature T
0
); d is the electron beam diameter.
Heat loss by radiation from the upper and lower surfaces of the specimen is
represented by the last term in Eq. (5.37), ε being the emissivity of the specimen and
σ Stefan’s constant. However, in almost all cases this term is negligible compared to
the conduction term (Reimer and Kohl, 2008). The temperature rise T = T −T
0
is
then approximately independent of specimen thickness and depends mainly on the
beam current (not current density) and only logarithmically on the beam diameter:
for I = 5nA,T increases from 0.5
◦
to 1.5
◦
Casd decreases from 1 μmto0.5nm
(Egerton et al., 2004).
The temperature rise is usually negligible for small probes, whereas for beam cur-
rents of many nanoamperes, it can be tens or hundreds of degrees (Reimer and Kohl,
2008). In beam-sensitive specimens such as polymers, the result can be melting or
warping of the specimen, especially when accompanied by electrostatic charging.
5.7 Application to Specific Materials 391
Reducing the beam current is helpful, even if it results in a longer exposure time
to acquire the data. Because radiolysis occurs more rapidly at higher temperature,
beam heating may account for the higher radiation sensitivity of polymer films at
higher dose rate (current density) observed by Payne and Beamson (1993).
Radiolysis occurs because the electron excitation is not necessarily a reversible
process: when an atom or molecule returns to its ground state, the chemical bonds
with neighboring atoms may reconfigure, resulting in a permanent structural change.
In crystalline specimens, structural disorder is seen as a disappearance of lattice
fringes and a gradual fading of the spot diffraction pattern (Glaeser, 1975; Zeitler,
1982). The disruption of chemical bonding can be seen more directly as a disappear-
ance of the fine structure in an optical-absorption and energy-loss spectra (Reimer,
1975; Isaacson, 1977). Radiolysis may also result in the removal of atoms from the
irradiated area, known as mass loss. This process is of concern in elemental analysis
by EELS or EDX spectroscopy because some elements are removed more rapidly
than others, resulting in a change in chemical composition.
5.7.5.1 Damage Measurements on Organic Specimens
Although radiation damage is detrimental to electron-beam measurements, EELS
has proven useful for examining the sensitivities of different types of specimen, the
damage mechanisms involved, and ways of reducing the damage. Core-loss spec-
troscopy has been used to monitor the loss of particular elements from organic
specimens, while low-loss or core-loss fine structure has been used as a measure
of structural order.
The dose required for a single measurement is reduced if the spectrum is col-
lected from as large an area of specimen as possible. In TEM image mode, this
implies a low magnification and a large spectrometer entrance aperture. If a spec-
trum is recorded at a time t after the start of irradiation, the accumulated dose is
D = It/A, where I is the beam current and A the cross-sectional area of the beam
at the specimen. The remaining amount ( N atoms/area) of a particular element is
calculated from its ionization edge, making use of Eq. (4.65). If log(N) is then plot-
ted against D, the initial slope of the data gives the characteristic or critical dose
D
c
(the dose that would cause N to fall to 1/e of its initial value, if the kinetics
remained strictly exponential). The value of D
c
is an inverse measure of the radiation
sensitivity of the specimen.
Measurements on organic materials have shown that mass loss depends on the
accumulated dose and not on the dose rate (i.e., D
c
is independent of current den-
sity). Table 5.4 lists D
c
for selected organic compounds exposed to 100-keV incident
electrons. Values for other incident energies can be estimated by assuming D
c
to be
proportional to the effective incident energy: T = m
0
v
2
/2 (Isaacson, 1977). Not
surprisingly, D
c
is low for compounds containing unstable groups such as nitrates.
Aromatic compounds are generally more stable than aliphatic ones, and it has been
proposed that damage to aromatics requires K-shell ionization (Howie et al., 1985).
Replacement of hydrogen by halogen atoms (as in chlorinated phthalocyanine) fur-
ther reduces the radiation sensitivity, due to the increased steric hindrance (cage
392 5 TEM Applications of EELS
Table 5.4 Characteristic dose for removal of specified elements from organic compounds by 100-
keV electrons (Egerton, 1982b; Ciliax et al., 1993)
Material Element removed D
c
(C/cm
2
) 300 K D
c
(C/cm
2
) 100 K
Nitrocellulose (collodion) C
N
O
0.07
0.002
0.007
0.4
0.3
0.6
Poly(methyl methacrylate)
(PMMA)
C
O
0.6
0.07
1
0.6
Copper phthalocyanine N 0.8
Cl
15
Cu-phthalocyanine Cl 4 >10
Perfluorotetracosane F 0.2 0.2
Amidinotetrafluorostilbene F 0.8 >10
4
effect) from surrounding atoms. Fluorine attached directly to an aromatic ring can
be remarkably stable, especially at low temperatures (Ciliax et al., 1993).
As seen in Table 5.4, cooling an organic specimen to 100 K reduces the rate
of mass loss, sometimes by a large factor. Cryogenic operation may not change
the number of broken bonds but it prevents atoms from leaving the irradiated area
by reducing their diffusion rate. EELS measurements confirm that gaseous atoms
leave the irradiated area when the s pecimen returns to room temperature (Egerton,
1980c). Lamvik et al. (1989) found that mass loss in collodion is further reduced at
a temperature of 10 K.
An alternative way of reducing mass loss is to coat the specimen on both sides
withathin(≈10 nm) film of carbon or a metal. Perhaps because each surface film
acts as diffusion barrier, mass loss is reduced by a factor of typically 2–6 (Egerton
et al., 1987). Carbon contamination films produced in the electron beam are believed
to have a similar protective effect. According to Fryer and Holland (1984), encapsu-
lation also helps to preserve crystallinity, perhaps by aiding recombination processes
or acting as an electron source.
Radiation effects can also be monitored from the low-loss spectrum, with a
lower dose required for measurement. From the plasmon peak intensity, Egerton and
Rossouw (1976) measured the rate of hydrocarbon contamination as a function of
specimen temperature and found that it became negative (indicating etching by oxy-
gen or water vapor) below −50
◦
C. By measuring a shift in the main plasmon energy
toward that of amorphous carbon, Ditchfield et al. (1973) found that polyethylene
loses a significant fraction of its hydrogen at doses as low as 10
−3
C/cm
2
. In addi-
tion, they observed the creation of double bonds (indicating cross-linking) from
the appearance of a π-excitation peak around 6 eV; in the case of polystyrene, an
initially visible π peak decreased upon irradiation, showing that double bonds were
being broken. Fine structure below 10 eV in the spectrum of nucleic acid bases grad-
ually disappears during the irradiation (Isaacson, 1972a). Doses that cause these
bonding changes are usually intermediate between those needed that destroy the
diffraction pattern (of a crystalline specimen) and the larger values associated with
mass loss (Isaacson, 1977).
5.7 Application to Specific Materials 393
Core-loss fine structure provides another indication of bonding, with the advan-
tage that different ionization edges can be recorded to determine the atomic site
at which damage occurs. In the case of Ge-O-phthalocyanine, Kurata et al. (1992)
found a decrease in the π
∗
threshold peak to be more rapid at the nitrogen edge than
at the carbon edge, suggesting chemical reaction between N and adjacent H atoms
released during irradiation. Conversely, the emergence of a π
∗
peak at carbon and/or
nitrogen edges has been observed during electron irradiation of fluorinated com-
pounds, providing evidence for the formation of double bonds and aromatization of
ring structures (Ciliax et al., 1993).
5.7.5.2 Damage Measurements on Inorganic Materials
TEM-EELS has also been used to investigate electron-beam damage to inorganic
materials. Hydrides are among the most radiation sensitive: a dose of 0.1 C/cm
2
con-
verts NaH to metallic sodium inside the electron microscope (Herley et al., 1987), as
seen from the emergence of crystalline needles whose plasmon-loss spectrum con-
tains sharp peaks. Metal halides are also rather beam sensitive: an efficient excitonic
mechanism results in the creation of halogen vacancies (F-centers) and interstitials
(H-centers), which may diffuse to the surface, resulting in the ejection of halogen
atoms (Hobbs, 1984). Halogen loss apparently depends on the dose rate as well
as the accumulated dose (Egerton, 1980f). Other radiolytic processes in inorganic
materials include inner-shell ionization followed by an interatomic Auger decay
(Knotek, 1984).
Thomas (1982, 1984)usedK-shell spectroscopy to monitor the effect of a field-
emission STEM probe on compounds such as Cr
3
C
2
,TiC
0.94
,Cr
2
N, and Fe
2
O
3
.The
dose required for the removal of 50% of the nonmetallic element was in the range
10
5
–10
6
C/cm
2
and did not change when specimens were cooled to 143 K, sug-
gesting a knock-on or sputtering process as the damage mechanism. Hole drilling in
silicon nitride was judged to be due to sputtering because the process occurred only
above a threshold incident energy (120 keV) and because the specimen thickness
decreased linearly (rather than exponentially) with time (Howitt et al., 2008).
A sputtering rate can be estimated from the displacement cross section σ
d
of the appropriate element, calculated as a Rutherford or Mott cross section
(Section 3.1.6), but only if the surface-displacement energy E
d
is known (Oen,
1973; Bradley, 1988). The latter is usually taken as the sublimation energy E
s
,but
E
d
= (5/3)E
s
appears to give better agreement with experimental data for metals
(Egerton et al., 2010). The sputtering rate in monolayers per second is then (J/e)σ
d
where J is the incident electron current density (e.g., A/cm
2
) and e is the electron
charge.
Because sputtering and bulk displacement processes are absent below some
threshold incident energy, considerable interest has been devoted to designing TEM-
EELS systems that work at lower accelerating voltages, while still achieving good
spatial and energy resolution. For example, a TEM fitted with a delta-type aberra-
tion corrector has achieved atomic resolution and 0.3-eV energy resolution at 30 kV
(Sasaki et al., 2010).
394 5 TEM Applications of EELS
5.7.5.3 Electron-Beam Lithography and Hole Drilling
High-brightness electron sources and aberration-corrected lenses allow the pro-
duction of nanometer-scale electron probes with very high current densities (>10
6
A/cm
2
) that can be quickly damaging to a TEM specimen. The implications for
nanolithography have also been explored, with high-density information storage
as one of the stated applications, and EELS has played an important part in these
investigations.
Muray et al. (1985) used a 100-kV field-emission STEM (vacuum of 10
−9
torr
in the specimen chamber) with a s erial-recording spectrometer to investigate beam
damage in vacuum-evaporated films of metal halides. After a dose of 1 C/cm
2
,a
50-nm film of NaCl is largely converted to sodium, as shown by the appearance
of sharp surface (3.8 eV) and volume (5.7 eV) plasmon peaks. A dose of 100
C/cm
2
removes the sodium, creating a 2-nm-diameter hole. Similar behavior was
observed for LiF but hole formation required only 10
−2
C/cm
2
. In the case of MgF
2
,
magnesium was formed (bulk plasmon peak at 10.2 eV) for D ≈ 1C/cm
2
but not
removed by prolonged irradiation. In CaF
2
, bubbles of molecular fluorine have been
detected from the appearance of a sharp peak at 682 eV (K-edge threshold) but only
with fine-grained films evaporated onto a low-temperature substrate (Zanetti et al.,
1994).
A finely focused electron beam can also create nanometer-scale holes in metal-
lic oxides. Sometimes hole drilling is seen only above a threshold current density,
typically of the order of 1000 A/cm
2
(Salisbury et al., 1984). The existence of this
threshold may indicate that a radial electric field around the beam axis (positive
potential at the center because of the high secondary electron yield of insulators)
must be established, of sufficient strength to remove cations in a Coulomb-explosion
process (Humphreys et al., 1990; Cazaux, 1995). The fact that the current den-
sity threshold for alumina was reduced by cooling the sample of 85 K (enabling
hole drilling to be performed with a tungsten filament electron source) suggests
inward diffusion of metal, less effective at low temperatures (Devenish et al.,
1989).
Berger et al. (1987) used their field-emission STEM to create holes in amor-
phous alumina. During drilling, the low-loss spectrum exhibited a peak at about
9 eV (Fig. 5.69a), the oxygen K-edge spectrum developed a sharp threshold reso-
nance (Fig. 5.69b), and the O/Al ratio, measured from areas under the O K- and Al
L-edges, increased from 1.5 to 7 or more, all consistent with the creation of a bubble
containing molecular oxygen. The bubble burst after an average time of 40 s, leav-
ing 5-nm-diameter hole. Hole drilling in sodium β-alumina proceeded somewhat
differently. A sharp peak appeared, with a shoulder at 9 eV and maximum at 15 eV
(Fig. 5.69c), suggesting surface and bulk modes in small Al spheres. At the same
time, the Al L-edge shifted 2 eV lower in energy and became more rounded in shape
(Fig. 5.69d), consistent with the formation of aluminum metal from an insulating
oxide. The oxygen K-edge gradually weakened, the O/Al ratio decreasing from 1.5
to 0.6 typically. Berger et al. (1987) suggested that oxygen is lost from both surfaces,
forming surface indentations that grow inward, leaving behind Al particles coating
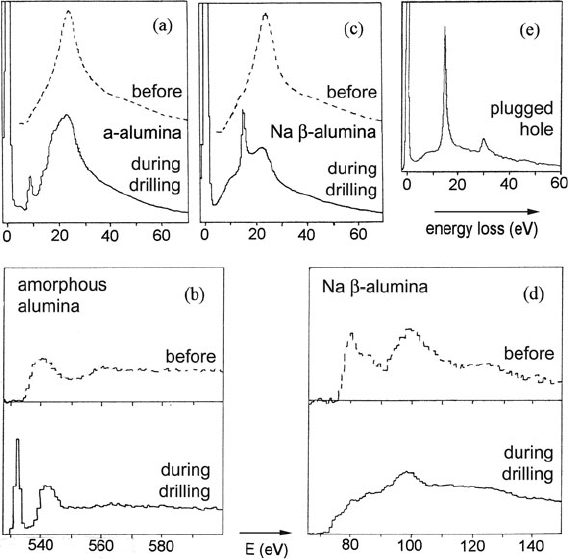
5.7 Application to Specific Materials 395
Fig. 5.69 (a) Low-loss and (b) oxygen K-loss spectra of amorphous alumina, before and during
hole drilling. (c) Low-loss and (d)AlL-loss spectra of Na β-Al
2
O
3
before and during drilling.
(e) Low-loss spectrum of a hole plugged with aluminum. From Berger et al. (1987), copyright
Taylor and Francis Ltd
the inside walls. Although in most cases a hole is formed after 30 s, the zero-loss
intensity remained well below the incident beam current, indicating scattering from
Al within the incident beam. Occasionally, the hole became filled with a plug of
continuous aluminum, as evidenced by bulk plasmon peaks in the low-loss spec-
trum (Fig. 5.69e). This metallization is similar to the normal irradiation behavior of
halides such as MgF
2
.
Hole drilling has been demonstrated in many other oxides, with doses mainly in
the range 10
4
–10
6
C/cm
2
(Hollenbeck and Buchanan, 1990). In the case of crys-
talline MgO, square holes are formed from growth of an indentation on the electron
exit surface (Turner et al., 1990). Hole formation in metallic films such as aluminum
(Bullough, 1997) and metallic alloys (Muller and Silcox, 1995b) can be attributed
to electron-induced sputtering.
High-angle elastic scattering, which can lead to the displacement of atoms within
a crystal or sputtering from the surface of a specimen, is discussed in Section 3.1.6.
Displacement occurs only for an incident electron energy above threshold value,

396 5 TEM Applications of EELS
Table 5.5 Sublimation energy E
sub
and threshold energy E
0
th
for electron-induced sputtering of
elemental solids. The sublimation energy of carbon may be as low as 5 eV in an organic compound
or as high as 11 eV in diamond
Element symbol Atomic wt. AE
sub
(eV)
E
0
th
(KeV)
for E
d
= E
sub
E
0
th
(KeV)
for E
d
= (5/3) E
sub
Li 6.94 1.66 5.2 8.7
C 12.0 ≈8 ≈42 ≈68
Al 27.0 3.42 40 65
Si 28.1 4.63 56 91
Ti 47.9 4.86 97 154
V 50.9 5.31 111 175
Cr 52.0 4.10 89 142
Mn 53.9 2.93 68 109
Fe 55.9 4.29 100 158
Co 58.9 4.47 109 171
Ni 58.7 4.52 109 172
Cu 63.6 3.49 93 147
Zn 65.4 1.35 39 63
Ge 72.6 3.86 115 181
Sr 87.6 1.72 65 104
Zr 91.2 6.26 215 328
Nb 92.9 7.50 254 385
Mo 95.9 6.83 242 366
Ag 107.9 2.95 129 202
Ta 180.9 8.12 461 673
W 183.9 8.92 501 728
Pt 195.1 5.85 379 560
Au 197.0 3.80 270 407
From Egerton et al. (2010), copyright Elsevier
which in the case of electron-induced sputtering of elements can be calculated
from the sublimation energy E
sub
. As seen in Table 5.5, these threshold energies
are mostly below 200 keV, even if the surface-displacement energy E
d
is taken as
(5/3)E
sub
(Egerton et al., 2010).
The sputtering rate can be estimated as J
e
σ
d
/e monolayers/s, where J
e
is the
current density in A cm
–2
and σ
d
is a surface-displacement cross section in cm
2
.For
a field-emission source and aberration-corrected probe, J
e
can exceed 10
6
A/cm
2
,
giving sputtering rates of many nanometers per second. The predicted sputtering
rate is typically a few times lower if cross sections are based on a planar escape
potential (Section 3.1.6).
Alternatively, low-loss EELS can be used to measure the decrease in thickness
of a specimen with time, or core-loss measurements can reveal the loss of a specific
element. For example, Fig. 5.70 shows that aluminum is sputtered predominantly
from the bottom surface of a C/Al bilayer film; with carbon on the bottom surface,
loss of Al K-signal is delayed until the carbon is removed by sputtering. Because
sputtering is a slow process, a relatively high beam current is helpful for accurate
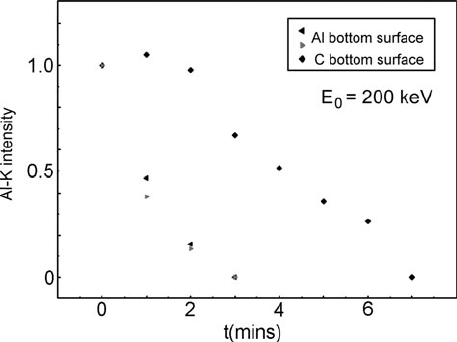
5.7 Application to Specific Materials 397
Fig. 5.70 Decrease in Al K-loss signal with irradiation time for a C/Al bilayer film, before and
after inversion in the TEM. Measurements were made in a TEM with a LaB
6
thermionic electron
source. Reproduced from (Egerton et al., 2006b), with permission from Cambridge University
Press
measurement of the sputtering rate, favoring the use of a thermionic (W-filament or
LaB
6
) electron source. A field-emission source can deliver a higher current density,
but with lower probe diameter and current. If the probe diameter is comparable to
or less than the depth of the crater formed by sputtering, some sputtered material is
re-deposited on the sides of the hole, reducing the measured thinning rate.
