Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.


58 Energy in planetary processes
Choosing a surface perpendicular to one of the three axes that define the degrees of freedom
does not cause any loss of generality, because we can choose the orientation of the axes
(Fig. 1.11) any way we want, as long as they are mutually perpendicular. In other words,
pressure is isotropic.
If the molar volume of the gas is V then opposite walls are separated by a distance of
order V
1/3
and the time between consecutive collisions is (2V
1/3
)/u, because the particle
must move to the opposite wall and back. The rate of change of momentum of a particle
when it collides with a wall, i.e. the force exerted by a particle on a wall, is then given by:
2mu
2V
1/3
u
−1
=
mu
2
V
1/3
. (1.107)
The total pressure must equal the average force per particle, times the number of particles,
divided by the surface area of the wall. In order to account for the average force per particle
we must use the mean-square speed, u
2
, because we are averaging rates of change of
momentum, which scale as the square of speed (equation (1.107)). The surface area of the
wall is of order V
2/3
, so that the pressure is given by:
P = N
mu
2
V
1/3
1
V
2/3
=
Nmu
2
V
(1.108)
hence:
PV = Nmu
2
. (1.109)
Using the ideal gas EOS, PV = RT, we obtain:
T =
Nmu
2
R
=
mu
2
k
B
, (1.110)
where k
B
=R/N is the gas constant per molecule, known as Boltzmann’s constant. We can
also write equation (1.110) as:
1
2
k
B
T =
1
2
mu
2
. (1.111)
This equation relates the temperature of the gas to the average kinetic energy of the particles
along each degree of freedom. It allows us to calculate a characteristic value for molecular
speeds, the root-mean-square speed (RMS) of the molecules, c
2
1
2
=(3u
2
)
1
2
. Molecular
speeds were first calculated in this fashion by James Joule in the mid nineteenth century. The
root-mean-square speed is not the same as the most probable molecular speed mentioned in
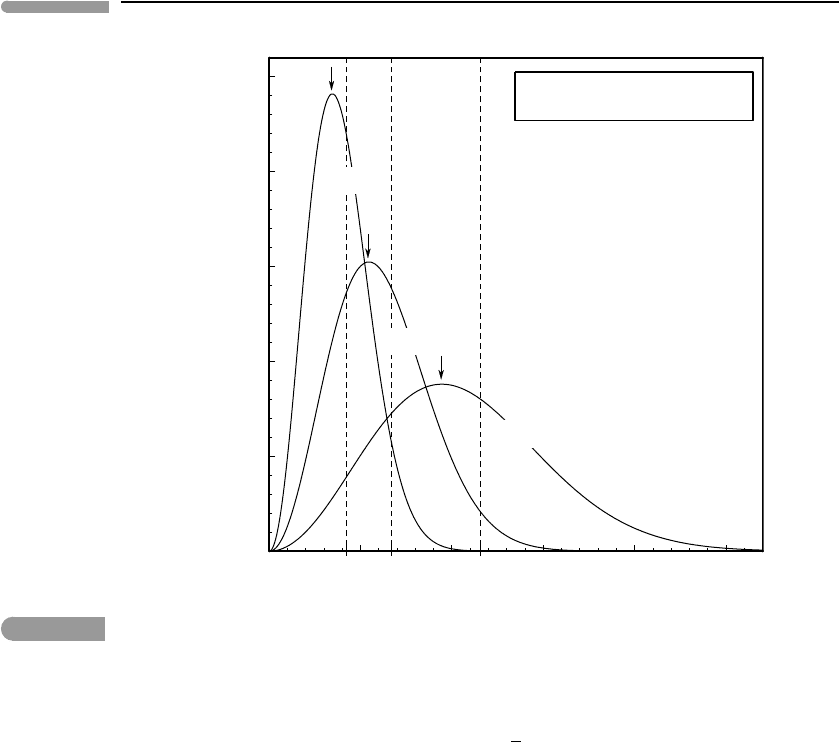
Section 1.4.2. The relationship between the two quantities is shown in Fig. 1.12. The RMS
molecular speed is always greater than the most probable speed, reflecting the long tail on
the high speed end of the Maxwell–Boltzmann distribution. This long tail, which implies
that there always are a significant number of molecules that are moving with speeds that
are much faster (by a factor of 2–3) than the most probable value, plays an important role
in the escape of light gas species from planetary atmospheres. This is especially the case
for escape of hydrogen from primordial planetary atmospheres, and will be discussed in
Chapter 13.
Equations (1.105) and (1.111) each relates a microscopic variable (E or T) to the kinetic
energyof the particles.We can substitute one equation into the other to arrive at an expression
59 1.14 Internal energy
0 500
1000
1500 2000
2500
0
0.001
0.002
Molecular speed (ms
–1
)
Probability density
Distribution of molecular speeds
and RMS speeds for N
2
200 K
500 K
1500 K
Fig. 1.12
Most probable speed (arrows) and RMS speed (dashed lines) for N
2
at 200, 500 and 1500 K.
for the molar internal energy of a monatomic ideal gas as a function of absolute temperature:
E
monatomic
=
3
2
RT . (1.112)
This equation shows that the internal energy of an ideal gas is a function of temperature
only, so that for any ideal gas (∂E/∂P )
T
= (∂E/∂V )
T
= 0. For real substances these
derivatives do not vanish. They vanish for ideal gases only because of our assumptions:
non-interacting, perfectly elastic particles with no volume. Also, since C
V
= (∂E/∂T )
V
,
we get C
V
=3/2 R for a monatomic ideal gas.
Note what we have done here: from a simple hypothesis about the microscopic nature of
an ideal gas and the principle of equipartition of energy we have derived a mathematical
expression for the relationship between two macroscopic variables. The validity of our
assumptions can then be tested by comparing predicted internal energies of ideal gases
with measurements for real gases at conditions such that their behavior approaches that of
ideal gases. We will do this in the next section, using C
V
rather than E, because C
V
can be
obtained from direct experimental measurements whereas E cannot.
We could have derived these same results from statistical mechanics, with one crucial
difference. In our derivation using only kinetic theory we had to use the ideal gas EOS
(in equation (1.110)), which arises from macroscopic observations. Statistical mechanics
allows us to do away with this last piece of macroscopic physics – we can derive both
the expression for internal energy (equation (1.112)) and the ideal gas EOS solely from
the (microscopic) partition function. The importance of this is that the same principles and
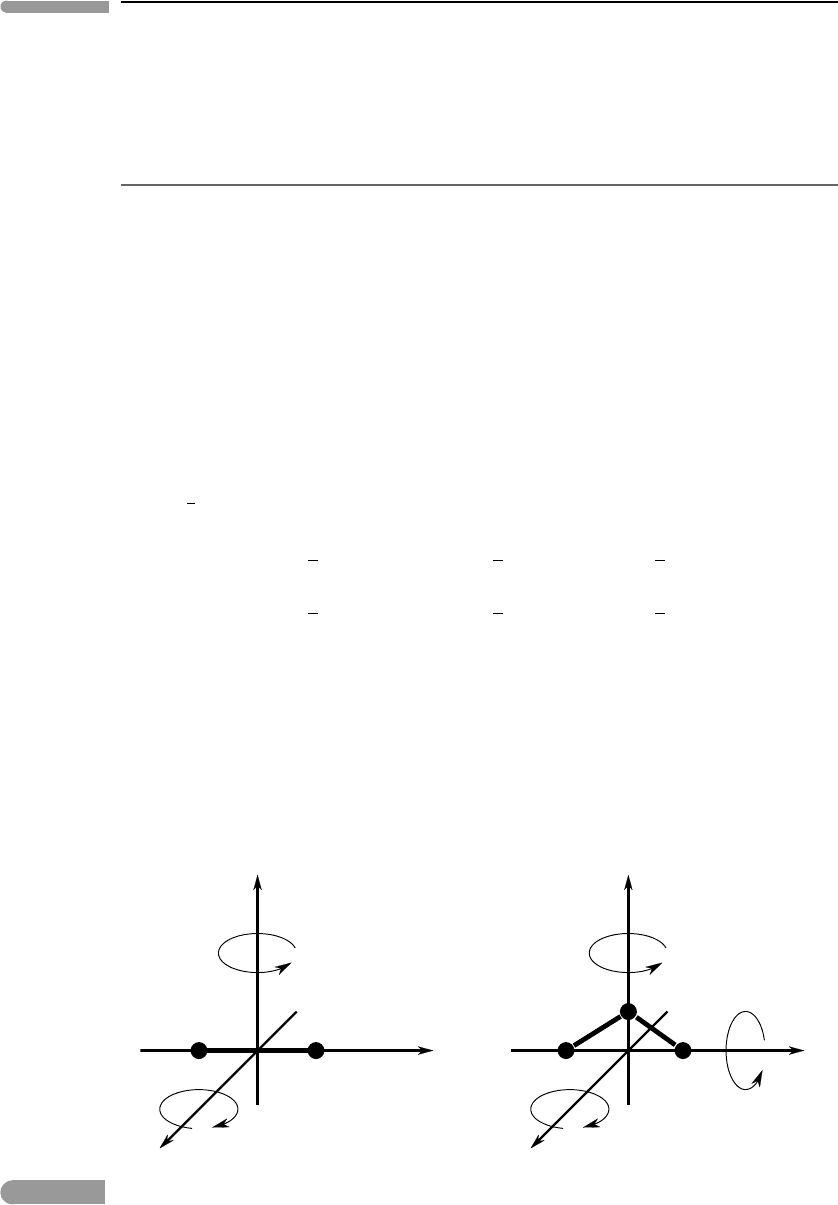
60 Energy in planetary processes
formalism of statistical mechanics that would allow us to do that for an ideal gas can be
used to derive equations of state for materials of planetary interest at very high pressures
and temperatures, that may be experimentally inaccessible.
1.14.2 Degrees of freedom and heat capacities of polyatomic gases
The internal energy function given by equation (1.112) is appropriate for a monatomic ideal
gas, but it must be modified for diatomic and polyatomic gases. This is so because, if the
molecule contains more than one atom, then additional degrees of freedom are possible,
and by the principle of equipartition of energy each of these additional degrees of freedom
carries an additional amount of energy. For example (Fig. 1.13), a diatomic molecule has
non-negligible moment of inertia relative to two orthogonal axes perpendicular to the atomic
bond, and a polyatomic molecule (unless the atoms are arranged in a straight line) has non-
negligible moment of inertia relative to three orthogonal axes. This means that diatomic and
polyatomic molecules have kinetic energy of rotation in addition to that of translation, and
each of the rotation axes adds a degree of freedom. As molecules collide with one another
kinetic energy is exchanged between translational and rotational degrees of freedom, and
by the principle of equipartition of energy all degrees of freedom carry the same amount of
energy:
1
2
k
B
T . The corresponding internal energies are, thus:
E
diatomic
=
3
2
RT (translational) +
2
2
RT (rotational) =
5
2
RT
E
polyatomic
=
3
2
RT (translational) +
3
2
RT (rotational) =
6
2
RT .
(1.113)
The constant volume heat capacities of ideal gases are, therefore: 3/2R,5/2R and 3R, for
monatomic, diatomic and non-linear polyatomic ideal gases, respectively. Heat capacities
can be determined directly by experiment and are a test of the predictions of the microscopic
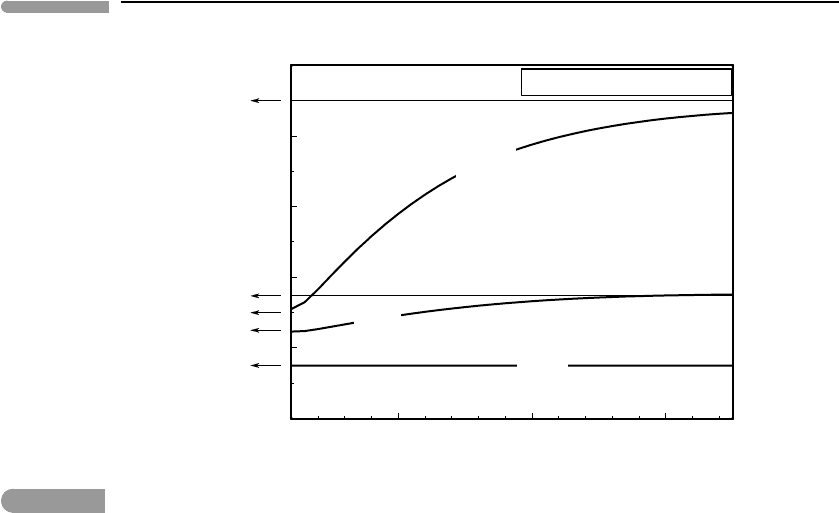
description of ideal gases. Figure 1.14 shows measured constant volumes heat capacities
at low pressure as a function of temperature, for a monatomic gas (neon), a diatomic gas
(nitrogen) and a polyatomic gas with a three-dimensional molecule (ammonia). The low
pressure constraint is what makes the behavior of the gases approach that of ideal gases,
x
z
y
x
z
y
Fig. 1.13
Rotational degrees of freedom for a linear molecule and a non-linear molecule.
61 1.14 Internal energy
1000 2000 3000
0
2
4
6
8
10
T (K)
C
V
/R
NH
3
N
2
Ne
3/2
5/2
6/2
7/2
9
Constant volume heat capacities
Fig. 1.14
Measured constant volume heat capacities for a monatomic gas (Ne), a diatomic gas (N
2
) and a polyatomic gas with
non-linear molecules (NH
3
). Data from NIST Chemistry WebBook. The values expected from kinetic theory are 3/2 R,
7/2 R and 9 R, respectively. Heat capacity is plotted as the non-dimensional variable C
V
/R.
by keeping them far from their condensation conditions. The agreement of the measured
values with those predicted by kinetic theory is essentially perfect for neon. For nitrogen
the agreement is good at temperatures up to approximately room temperature (∼300 K) but
then C
V
increases smoothly and reaches a plateau of approximately 7/2R. In the case of
ammonia there is good agreement at low temperature (200–300 K), and then, as for nitrogen,
C
V
increase and reaches a plateau, in this case close to 9R.
Do these discrepancies mean that the premises that we used to calculate C
V
for gases
other than monatomic gas are incorrect? Well, no, what it means in this case is that they are
incomplete. In other words, gases that contain more than one atom in their molecules have
other degrees of freedom in addition to those corresponding to translation and rotation. These
are called vibrational degrees of freedom, and they arise from the fact that atomic bonds are
not rigid, but can stretch, contract and bend as an elastic material (e.g. a spring), and behave
like harmonic oscillators. These oscillations carry energy and thus represent additional
degrees of freedom. The principle of equipartition of energy applies to vibrational degrees
of freedom too, but there are two important differences with translational and rotational
degrees of freedom, which we can see by examining Fig. 1.14 in some detail.
First, vibrational degrees of freedom in gases are inactive at low temperature and begin
to carry energy only as temperature rises. In other words, gas molecules must have a
minimum amount of kinetic energy before collisions are able to transfer some of this energy
to vibrations of the atomic bonds (if you suspect a connection with quantum mechanics here
you are right). The situation is different for solids and liquids, in which vibrational modes
are important at relatively low temperatures, simply because translational and rotational
degrees of freedom do not exist in them (although liquids have additional complexities).
Second, in the diatomic gas there is only one atomic bond, and thus there can only be
one vibrational degree of freedom, which corresponds to stretching of the atomic bond.

62 Energy in planetary processes
Heat capacity for diatomic nitrogen levels off at a value of approximately 7/2R, suggesting
that the vibrational degree of freedom carries twice as much energy as the translational
and rotational ones, i.e. k
B
T. This is indeed the case, and the explanation is that harmonic
oscillators (such as an atomic bond that stretches and contracts, or a pendulum, which is
mathematically equivalent) carry both kinetic and potential energy, and equipartition of
energy causes each of these energy modes to have an energy content of
1
2
k
B
T . In the case
of ammonia the difference between the measured high-temperature heat capacity (9R) and
the heat capacity predicted from translation and rotation only (3R) indicates that there are
six vibrational degrees of freedom, which include stretching of the N–H bonds, changes in
the angles between N–H bonds, and bending relative to a plane containing all four atoms.
The general rule for gases is that molecules have a total of 3n degrees of freedom, where
n is the number of atoms in the molecule. Of these, 3 degrees of freedom are always
translational, and either 2 or 3 are rotational, depending on whether the molecule is linear
or not (Fig. 1.13). Each of these degrees of freedom carries an energy equal to
1
2
k
B
T . The
remaining 3n −5 (linear molecule) or 3n − 6 (non-linear molecule) degrees of freedom
are vibrational and each carries an energy of k
B
T. Translational and rotational degrees of
freedom are always active, whereas vibrational degrees of freedom only become active (or
“excited”) above a certain temperature.
Constant volume heat capacities for ideal gases follow immediately from the generaliza-
tions given in the previous paragraph. In most planetary sciences applications, however, we
are interested in C
P
rather than C
V ,
and these are also easily derived. From the definition
of enthalpy we have:
C
P
=
∂H
∂T
P
=
∂E
∂T
P
+P
∂V
∂T
P
. (1.114)
Using identity (1.3.12)inBox1.3 and the fact that, for an ideal gas, (∂E/∂V )
T
=0, we see
that:
∂E
∂T
P
=
∂E
∂T
V
+
∂E
∂V
T
∂V
∂T
P
=C
V
(1.115)
and from the ideal gas EOS:
P
∂V
∂T
P
=P
R
P
=R (1.116)
so, for an ideal gas:
C
P
=C
V
+R. (1.117)
The extra amount of energy, R per Kelvin per mol, is a macroscopic contribution from
expansion work and does not reflect a change in the microscopic state of the system.
For gases that cannot be assumed to behave ideally equation (1.117) is not valid, but the
appropriate equation can always be derived from the equation of state, starting from equation
(1.114) which is true in general. Thus, with some important additional considerations, the
physical concepts developed in this section are the basis for understanding and quantifying
the behavior of real fluids at high pressures and temperatures, something that we will do in
Chapter 9.
63 1.14 Internal energy
1.14.3 Heat capacities of solids
In a crystalline solid there are only vibrational degrees of freedom. The atoms have fixed
positions in the crystalline lattice. They vibrate about their equilibrium positions, but they
can neither translate not rotate. Each atom can vibrate in three independent directions. We
could thus expect the heat capacity of crystalline solids to be approximately 3R per gram
atom, reflecting the three vibrational degrees of freedom of each atom, and the fact that
each vibrational degree of freedom carries an energy of k
B
T per atom. The molar heat
capacity of a substance that contains n atoms in its molecule should thus be 3nR. This has
been empirically known to be the case since the nineteenth century. It is known as Dulong
and Petit’s law, but it works only for temperatures comparable to or greater than room
temperature.
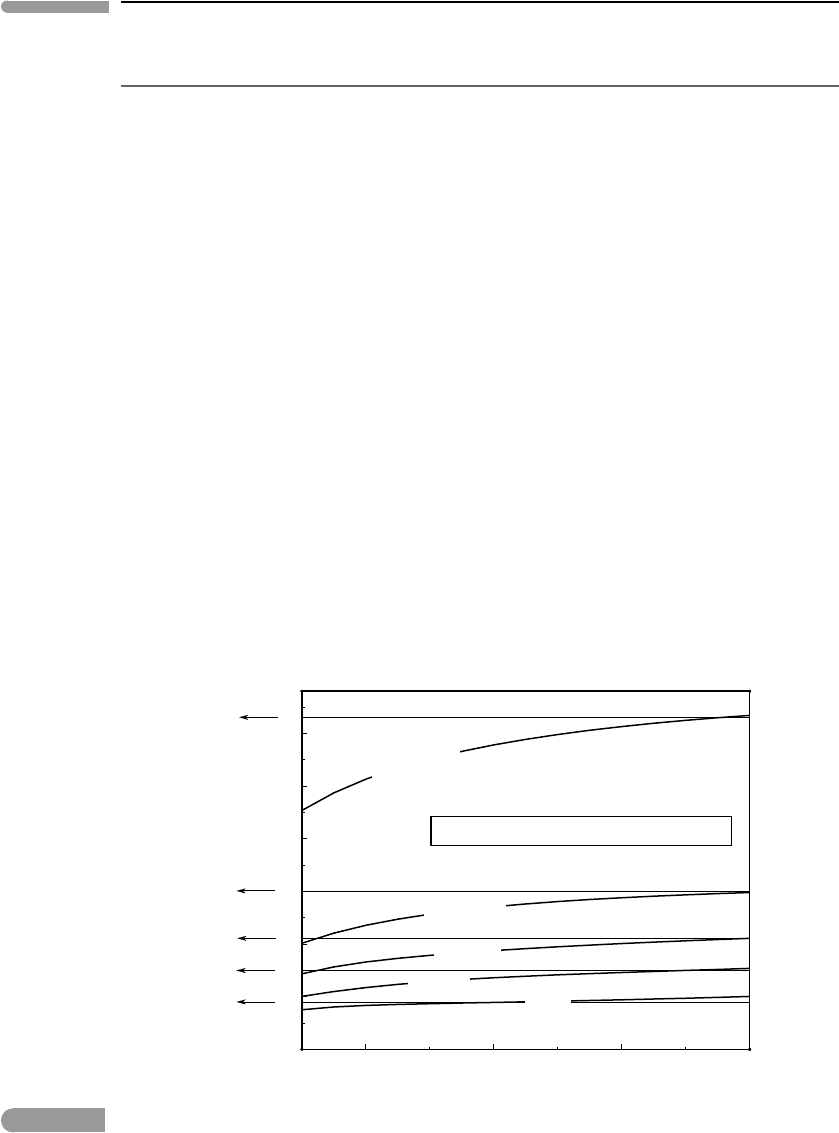
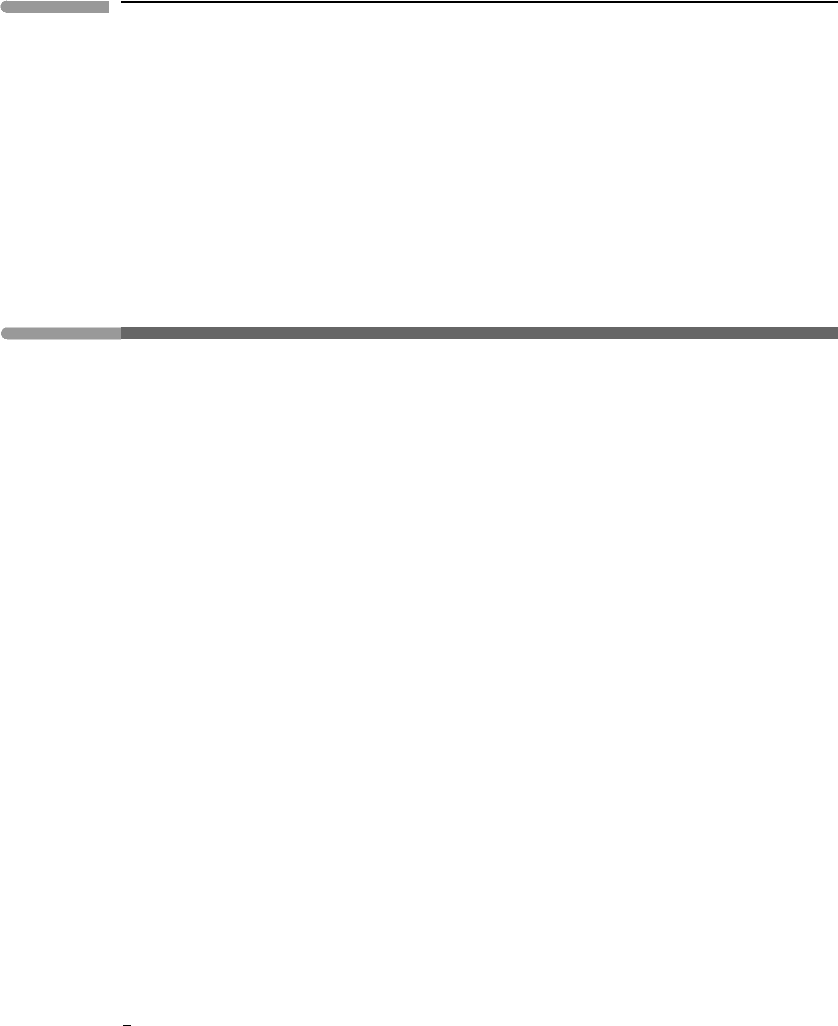
Figure 1.15 shows constant pressure heat capacities for a few minerals (C
P
and C
V
for
solids differ by a very small amount, because of the incompressibility of solids, see Chapter
8). They all approach Dulong and Petit’s behavior at high temperature (∼10
3
K), but heat
capacities fall off fairly steeply with decreasing temperature. The data confirm the general
microscopic picture (atoms in a solid have vibrational degrees of freedom only) but also
show that some fundamental physics is missing from the picture. The missing ingredient is
quantum mechanics, and although the details are beyond the scope of this book a qualitative
understanding of the behavior depicted in Fig. 1.15 will become important in subsequent
discussions. Atomic vibrations in a crystal are quantized, meaning that only some definite
energy levels are allowed. At low temperature the allowed energy levels are few and far
apart, and degenerate to a single energy level as absolute zero is approached. The number
of allowed energy levels increases with temperature, and as this happens so does the heat
400 600 800 100
0
0
10
20
30
40
50
60
T (K)
C
P
/R
FeS
2
MgSiO
3
Mg
2
SiO
4
CaMgSi
2
O
6
Ca
5
(PO
4
)
3
F
21 x 3
10 x 3
7x3
5x3
3x3
Constant pressure heat capacity of solids
Fig. 1.15
Constant pressure heat capacities for pyrite, enstatite, forsterite, diopside and fluorapatite. Horizontal lines show
Dulong and Petit’s approximation, labeled next to the arrows: the number of atoms in the formula times the number
of vibrational degrees of freedom per atom (=3). Measured heat capacity data from Robie and Hemingway (1995).
Heat capacity plotted as the non-dimensional variable C
P
/R.
64 Energy in planetary processes
capacity. Above a certain temperature the quantum energy levels become so close together
that the classical continuous approximation – Dulong and Petit’s law – becomes valid. This
temperature is characteristic for each substance and is known as the Debye temperature,
θ
D
. The Debye temperature for most minerals is of the order of several hundred to ∼10
3
K.
Heat capacity becomes a weak function of temperature above θ
D
, as shown by Fig. 1.15.
Interestingly, Dulong and Petit’s approximation also works for some liquids. For example,
the heat capacity of water is ∼75JK
−1
mol
−1
≈ 9R, corresponding to 3R for each of the
three atoms in the H
2
O molecule.
1.15 An overview of the properties of matter and equations of state
Equations of state (EOS) are an essentialcomponent in the study of the physics and chemistry
of planetary bodies. We will discuss different types of EOS in considerable detail in Chapters
8 and 9. Here we define some concepts about the possible states of matter in planetary
environments. This will allow us to better define the P –V –T ranges over which different
types of EOS are applicable. Solids and liquids are condensed phases, whereas gases are
non-condensed phases. Both liquids and gases are fluids. Gases are non-condensed fluids,
which means that they expand indefinitely as pressure decreases, whereas we call liquids
condensed fluids because, as solids, they do not behave in this manner. These contrasting
macroscopic behaviors arise from differences between condensed and non-condensed fluids
in the relative magnitudes of the intermolecular potentials (or forces), compared to thermal
energy. A vapor is a gas in equilibrium with its liquid, and a melt is a liquid in equilibrium
with its solid. For all substances it is an empirical observation that, as temperature increases,
the material properties of liquid and vapor at equilibrium approach each other, until the two
phases become indistinguishable at a temperature called the critical temperature. Above
the critical temperature a single fluid phase is stable, called a supercritical fluid.
Here we describe the behavior of matter in terms of temperature and density (i.e. the
inverse of volume). Density is a better choice than pressure for this exercise because we
can relate it directly to a description of the material at the atomic scale, something which is
not generally the case with pressure. The arguments and conclusions are summarized in a
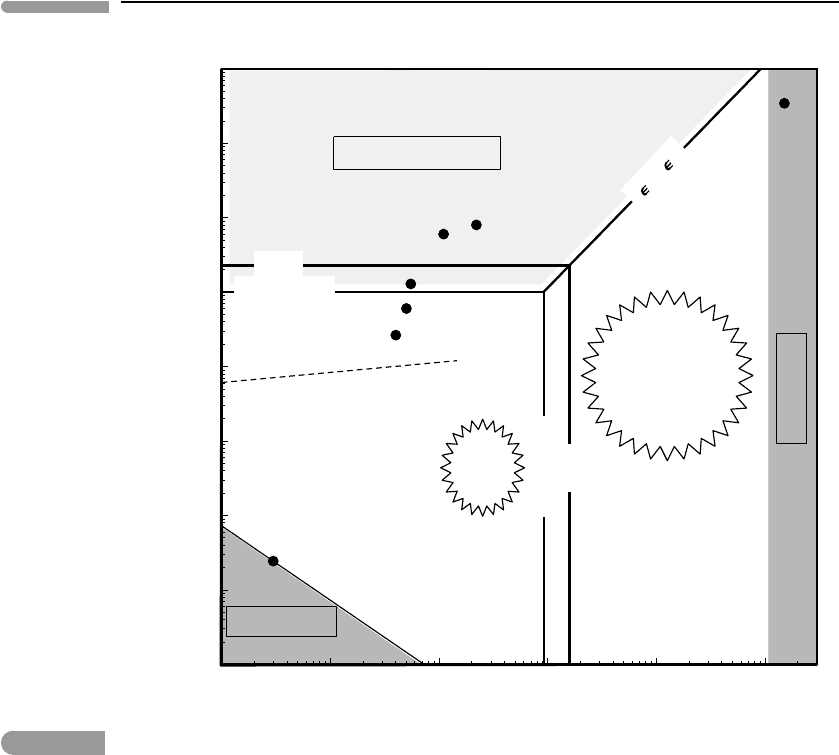
density–temperature diagram, Fig. 1.16, which is rather busy and will take some explaining.
The physical inspiration for this discussion comes from Shalom et al.(2002), who present
detailed mathematical arguments; I have added the planetary applications. A review of
recent developments in the high density region of the diagram is given by Drake (2010).
The horizontal coordinate in the diagram is temperature in Kelvin. Let the thermal energy of
microscopic particles (molecules, atoms, ions, etc.) be ε
T
. From our discussion in Section
1.14 we conclude that ε
T
is of order k
B
T, which we symbolize with the ∼ symbol, i.e.
ε
T .
∼ k
B
T. The justification for this statement is that particles carry an energy equal to
1
2
k
B
T per degree of freedom, and the number of degrees of freedom is a small number, of
order 1–10.
At sufficiently high temperature atoms become ionized as a result of interatomic col-
lisions. This happens when their thermal energy is of the same order as their ionization
energy, i.e., the energy required to detach an electron from an atom. By equating the ion-
ization energy of an element to k
B
T we can estimate the temperature at which thermal
ionization takes place. Note, however, that because energies of individual atoms are not
all the same but follow a statistical distribution (e.g., Fig. 1.12), ionization actually takes
65 1.15 An overview of the properties of matter
10
2
10
3
10
4
10
5
10
6
10
7
10
32
10
31
10
30
10
29
10
28
10
27
10
26
10
25
10
24
Temperature (K)
Particle density (m
–3
)
Earth atmosphere
Earth CMB
Earth deep core
Uranus & Neptune
deep interior
Saturn deep interior
Jupiter deep interior
Solar core
Fe, Si, Mg, C
Fe, Si, Mg, C
H, O
H, O
F
=
T
electron degeneracy
Ideal gas EOS
Ideal gas at 1 bar
non-condensed fluids
condensed fluids and solids
Ideal gas EOS
Non-ideal gases
Here
there
be
dragons...
...and
here
too
Fig. 1.16
Density–temperature diagram of matter. Dark gray regions correspond to ideal gas behavior. Pressure ionization
occurs in the light gray region. The lines labeled “H, O” and “Fe, Si, Mg, C” represent approximate ionization conditions
caused by temperature and pressure. CMB =core–mantle boundary.
place over a temperature range. The vertical lines labeled “H, O” and “Fe, Si, Mg, C” show
characteristic ionization temperatures for these most important of planet-forming elements.
Many other abundant elements plot within this range, the only important exception being
helium, which has an ionization energy almost twice that of hydrogen. Thermal ionization
occurs at temperatures of order 10
5
K, which are not attained in any planet in the Solar
System, but may be possible inside large extrasolar planets and brown dwarfs (failed stars).
Most of the solar interior is of course ionized.
Density is shown on the vertical axis as particle density, i.e. number of particles per unit
volume. This is simply Avogadro’s number divided by molar volume, and has units of m
−3
.
Using these units we can compare the degree of packing independently of particle mass.
The figure shows some reference values. An ideal gas at 1 bar and 298 K (e.g., the terrestrial
atmosphere) has a particle density of ∼2.4 ×10
25
m
−3
. The variation of this number with
temperature at a constant pressure of 1 bar, i.e. the ideal gas law, is shown in the diagram
with a negatively sloping line. The ideal gas EOS reproduces P –V –T relations to a high
degree of accuracy for conditions below this line (P<1 bar) and perhaps over a narrow

66 Energy in planetary processes
interval above the line. With increasing particle density the behavior of real gases diverges
from the predictions of the ideal gas EOS, owing to the existence of interatomic potentials,
i.e. attractive and repulsive forces. Up to particle densities of ∼10
27
–10
28
m
−3
, substances
behave as non-condensed fluids (i.e., gases) even if their densities are higher than what we
typically associate with a gas.
Condensation occurs at particle densities of ∼10
28
m
−3
, with overlapping ranges for
liquids and solids. Condensed matter is orders of magnitude less compressible than gases,
a fact that is expressed in Fig. 1.16 by the (apparent) proximity of a wide range of con-
densed planetary materials. The examples shown are: Mg
1.8
Fe
0.2
SiO
4
at the conditions of
the Earth’s core–mantle boundary (Chapter 8), an H
2
O–CH
4
–NH
3
mixture at conditions
thought to represent the deep interiors of Uranus and Neptune, Fe in the Earth’s inner core,
and H–He mixtures in Jupiter’s and Saturn’s deep interiors. These examples are representa-
tive of the upper density–temperature bounds likely to be encountered in the Solar System,
with the exception of the solar interior, which is also shown for comparison.
At particle densities somewhat above 10
29
m
−3
it is necessary to consider quantum
effects. We define the quantum energy, ε
q
, for a particle of mass m and momentum p
as ε
q
= p
2
/m.If particles are packed with a particle density n, then each particle occupies
a volume n
−1
, which means that there is an uncertainty in the position of the particle of
order n
−1/3
.According to the uncertainty principle the relationship between p and n is given
(approximately) by:
pn
−1/3
≈h, (1.118)
where h is Planck’s constant. We then get for the quantum energy:
ε
q
≈
h
2
n
2/3
2m
. (1.119)
For an electron with mass m
e
, accounting for the fact that each volume element can be
occupied by at most two electrons with opposite spins, and allowing for spherical symmetry,
the quantum energy becomes the Fermi energy, ε
F
, given by:
ε
F
=
h
2
n
2/3
8m
e
3
π
2/3
. (1.120)
If the Fermi energy exceeds the ionization energy and ε
F
>ε
T
then the material undergoes
pressure ionization. This takes place even if the thermal energy of the material is lower than
its ionization energy, because bound electrons are excluded by the uncertainty principle from
occupying the same volume element. The resulting condition is termed electron degeneracy.
The horizontal lines labeled “H, O” and “Fe, Si, Mg, C” show the approximate densities
at which electron degeneracy can be expected to occur in planetary materials. Much of
Jupiter’s and Saturn’s interiors are likely to consist of pressure-ionized hydrogen, and some
degree of pressure ionization may also occur in the Earth’s deeper core. Most of the core, as
well as the Earth’s mantle, and the entire volume of the ice giants (Uranus and Neptune) are,
in contrast, composed of non-degenerate matter. Given that pressures in the other terrestrial
planets are lower than in the Earth’s interior this conclusion also applies to them. EOS for
degenerate matter are needed in order to model the interiors of the giant planets.
In contrast to “cold” dense matter, “hot” dense matter (ε
T
>ε
F
) is not degenerate. This
includes the entire solar interior. At temperatures of order 10
7
K interatomic potentials are
negligible compared to thermal energy. Under such conditions matter consists of a highly

67 Exercises for Chapter 1
ionized plasma that is accurately described as a monatomic ideal gas (more on this in Chapter
2). We are left, as the figure shows, with a wide range of intermediate temperatures and
densities in which the behavior of matter is poorly understood and for which equations of
state are very complex, if they exist at all. Fortunately, these regions are of little interest to
planetary scientists. Focusing now on the range of conditions for non-degenerate matter in
planetary interiors (T < 10
4
K, n < 10
29
m
−3
), which is what we will mostly be concerned
with, we can identify three regions that require distinct equations of state. At very low
densities the ideal gas EOS is all we need. At the other end, a class of EOS that will be
introduced in Chapter 8 is generally appropriate for the entire range of densities of non-
degenerate condensed matter, both solids and liquids. This leaves the intermediate density
range, roughly between 10
26
and 10
28
m
−3
, occupied by non-ideal gases. Equations of state
for non-ideal gases are discussed in Chapter 9.
Exercises for Chapter 1
1.1 A mass m
i
of ice at temperature T
i
is added to a mass m
w
of water at temperature T
w
inside a perfectly isolated container. Let the heat capacities of ice and water be C
p,i
and C
p,w
, and the enthalpy of fusion of ice be
f
H > 0. Derive a set of criteria to
decide what the final equilibrium state of the system is, i.e. pure ice, pure water or
ice + water.
1.2 Calculate the gravitational potential energy stored in the Earth’s largest active volcano
(Mauna Loa) and in the Solar System’s largest volcano (Olympus Mons in Mars).
Assume that Mauna Loa is a cone with a basal radius (on the floor of the Pacific
Ocean) of 50 km, and an elevation of 10 km relative to the ocean floor, and Olympus
Mons a cone with a radius of 600 km and an elevation of 27 km. The gravitational
accelerations of Earth and Mars are 9.8 and 3.7 m s
−2
. Assume that Mauna Loa is
2 ×10
5
years old. Calculate the average power expended in building up Mauna Loa.
Assuming that this rate of storage of potential energy is characteristic of large basaltic
shield volcanoes, estimate how long the building of Olympus Mons may have taken.
How does this compare with the building time of Olympus Mons if you assume the
same mass supply rate as in Mauna Loa? Discuss the relative merits of extrapolating
terrestrial mass supply rate or energy supply rate to Mars. Compare the rate of storage
of potential energy in Mauna Loa with the power output of the Hawaiian hot spot,
∼10
11
Js
−1
. Comment on your results.
1.3 What is the potential energy stored in a rod-shaped asteroid, 100 × 50 × 50 km, with
density = 3000 kg m
−3
? The stable shape of the asteroid is a sphere with the same
volume.
1.4 Derive the formula for kinetic energy (equation (1.12)) by considering conservation
of energy of a free-falling body in a uniform gravitational field.
1.5 Consider the asteroid–Earth collision (Worked Example 1.2). What is the relationship
between m and M that results in the greatest dissipation of kinetic energy? You can
do this formally, by maximizing the function in equation (1.15), or intuitively, from
symmetry considerations.
1.6 What is the maximum amount of power that can in principle be harvested from wind
with a velocity v, by a wind turbine with diameter d ? The actual power output of the
turbine must be less than this, why?
