Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

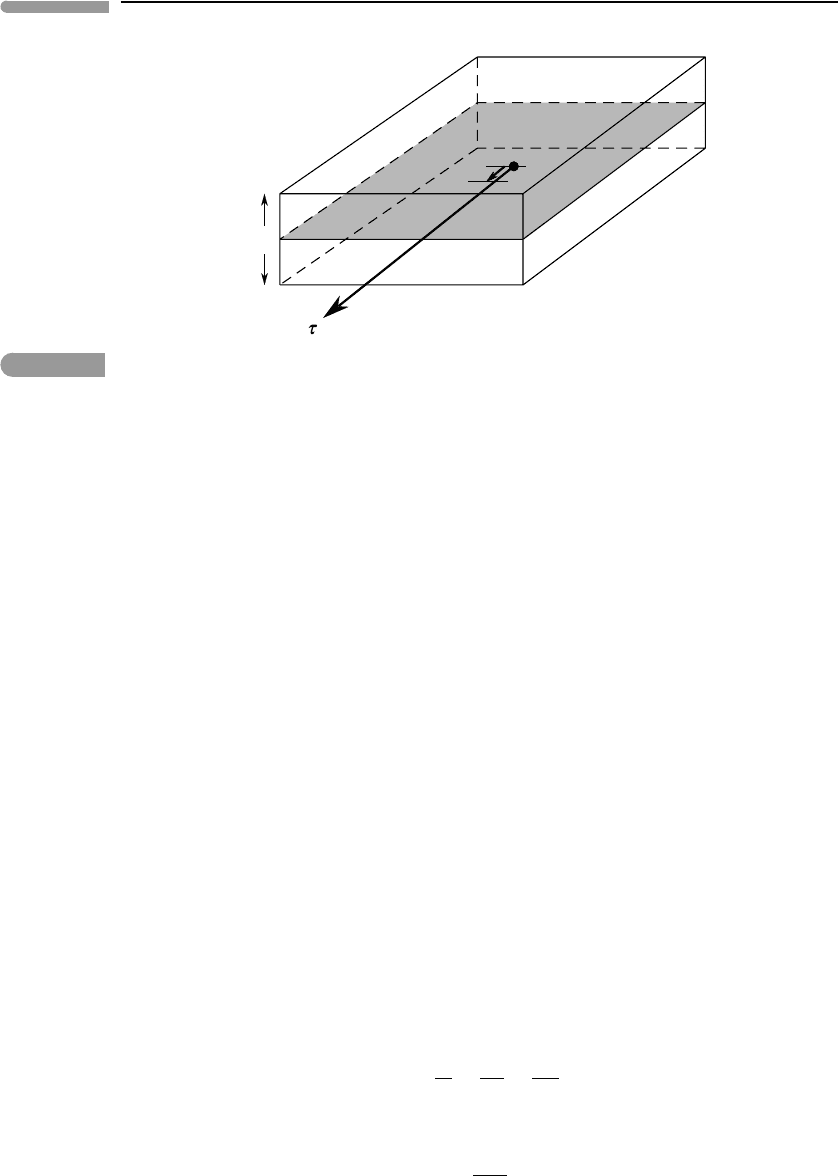
48 Energy in planetary processes
A
dX
z
A
Fig. 1.8
Frictional heating of a volume Az by dissipation of frictional work on a fault plane of area A (grey).
the frictional force on the system is dW . The work performed by the system against the
frictional force is thus −dW . A fault or shear zone moves when the force generated by the
shear stress component, τ , equals the frictional force. If the total surface area of the fault
plane is A then the frictional force is τ A. Calling the displacement along the fault dx, dW
is given by:
dW = τAdx, (1.78)
where, because frictional forces always act in the direction of displacement, I have been
sloppy and neglected the vector notation (see Box 1.1). Faulting can be treated as a constant
pressure process, because pressure inside a planet is determined by depth and the magnitude
of the instantaneous motion along a fault or shear zone is generally negligible compared to
depth. We thus write dE as follows:
dE = dH −PdV = C
P
dT −PdV (1.79)
so:
C
P
dT −PdV =−PdV −
(
−dW
)
. (1.80)
Substituting in (1.78):
C
P
dT = τAdx. (1.81)
Note that C
P
in this equation is the extensive variable, i.e. the total heat capacity of the
mass of heated rock. Let V =Az be the total volume of heated rock (Fig. 1.8), and N be the
number of mols of substance contained in this volume. If we symbolize the molar volume
of the substance with V and its molar heat capacity with C
P
, then:
N =
V
V
=
Az
V
=
C
P
C
P
. (1.82)
Substituting in (1.81) and rearranging we arriveat an equation in terms of intensive variables:
dT =
τV
zC
P
dx, (1.83)

49 1.13 Enthalpy associated with chemical reactions
from which we can also derive the rate of temperature increase, dT/dt (where t = time):
dT
dt
=
τV
zC
P
dx
dt
. (1.84)
The value of τ is determined either by the brittle shear strength for rocks (for a brittle fault)
or by the yield strength for a given ductile strain rate for a ductile shear zone; typical values
in both cases are of the order of 100 to a few 100 bar. Given that all three parameters: τ ,V
and C
P
vary within fairly restricted ranges for planetary materials, the rate of temperature
increase is controlled primarily by the width of the heated rock volume, z. Because the length
scale z is controlled by heat diffusion (Chapter 3) we can expect that it varies inversely with
the strain rate, which we can represent by dx/dt. We can thus expect a very strong dependency
of dT/dt on dx/dt, because as strain rate increases, z decreases and both effects combine to
make dT/dt larger. The ductile behavior of rocks is strongly temperature-dependent, with
viscosity decreasing exponentially with increasing temperature (see for instance Turcotte
& Schubert, 2002, Chapter 7). A positive feedback mechanism results, that focuses ductile
deformation in relatively narrow shear zones, in which most of the temperature increase,
and consequent lowering of the viscosity, take place.
As a numerical example, consider “slickensides”, that probably form as a result of instan-
taneous heating at a fault during brittle failure (i.e. earthquakes). For a slickenside width z
=5 mm, and fault motion during an earthquake x =1 m we obtain a maximum temperature
increase (for τ of a few hundred bars) T ≈ 2000 K. Fault motions during earthquakes
may last for only a few seconds, so that the heating rate may be of the order of hundreds of
degrees per second.
1.13 Enthalpy associated with chemical reactions
1.13.1 Enthalpy of reaction and enthalpy of formation
Phenomena such as condensation of solids in the solar nebula, generation and crystallization
of magmas, metamorphism, weathering, diagenesis, precipitation of carbonate minerals
from seawater and crystallization of evaporites, are chemical reactions. The essence of
a chemical reaction is that atoms of certain elements are transferred among phases, or
rearrange themselves into different molecules inside a homogeneous phase (e.g. chemical
reactions between gas species in a homogeneous atmosphere). Atomic bonds are broken
and form during chemical reactions, as a result of which there is a net surplus or deficit
of energy that is exchanged with the environment. The macroscopic manifestation of these
microscopic energy transactions is the enthalpy change associated with a chemical reaction.
This enthalpy is called the enthalpy of reaction and is denoted by
r
H. Enthalpy of reaction
is the amount of heat liberated or absorbed during a chemical reaction at constant pressure
and constant temperature. In Chapters 4 and 5 we will arrive at a precise and rigorous
definition of chemical equilibrium and we will see that enthalpy of reaction is an important
part of that definition and of the algebraic procedure by which we calculate the location of
chemical equilibria. Enthalpy of reaction behaves as latent heat: it is heat exchanged without
a change in temperature. You may think that this statement cannot possibly be correct, as
you are probably familiar with endothermic and exothermic reactions, during which the
temperature of the system decreases and increases, respectively. The answer to this puzzle

50 Energy in planetary processes
is that these temperature changes are caused by the fact that the rate of heat transfer is much
slower than the rate of chemical reaction. For example, during an exothermic reaction
enthalpy is liberated at a rate faster than the rate at which heat can be carried away from the
site where the reaction takes place. As a result, latent heat (enthalpy of reaction) is converted
to sensible heat (temperature increase).
We can write the algebraic definition of enthalpy of reaction by considering a generic
chemical reaction between reactant A and product B:
A →B. (1.85)
The enthalpy of reaction at a given T and P is the difference between the enthalpy of B and
the enthalpy of A at those conditions:
r
H = H
B
−H
A
. (1.86)
But what are the enthalpies of A and B? The First Law of Thermodynamics codifies the
law of energy conservation: it states that the total amount of energy is conserved, but says
nothing about the absolute magnitude of energy. A more formal statement of this fact is that
the First Law of Thermodynamics (equation (1.55)) is a differential equation and, as such,
it admits an infinite number of solutions that differ by an additive constant (the integration
constant). This is not a problem, because all we care about is the difference in enthalpy
between different states of a system. This is true in general: even though we did not state
it explicitly, in all examples that we have discussed thus far we have calculated differences
in enthalpy, internal energy or other types of energy, and not their absolute values. In the
case of a chemical reaction, we are not interested in the absolute magnitudes of H
A
or
H
B
, all we are interested in is
r
H . Because enthalpy is a state variable, the First Law of
Thermodynamics assures us that, as long as we define individual enthalpies relative to the
same reference level, the value of
r
H at any given T and P is unique and well defined.
We need to define some arbitrary reference level relative to which we will measure
enthalpies – the integration constant, if you wish. We did just this when we defined poten-
tial energy = 0 at infinity (Section 1.3.1), or when we specified that we were measuring
kinetic energy relative to a reference frame fixed to the Earth (Section 1.3.2). The universal
convention for chemical systems is to set the enthalpy of all pure chemical elements in
their stable configuration at 298.15 K and 1 bar equal to zero. The “stable configuration”
requirement is important. We cannot define rigorously what this means until after we have
defined chemical equilibrium (Chapters 4 and 5), but we can study examples that make the
meaning clear. The enthalpy of diatomic oxygen at 298.15 K and 1 bar is zero, because O
2
is the stable oxygen species at those conditions. In contrast, the enthalpies of atomic oxygen
(O) and of ozone (O
3
) at 298.15 K and 1 bar are not zero. For carbon, graphite has zero
enthalpy at 298.15 K and 1 bar. The enthalpy of pure elements in their stable configurations
at any other P–T combination is not zero either.
We define the enthalpy of formation of a substance (compound or element) at the reference
pressure and temperature as the value of
r
H for the reaction that forms the substance from
the elements (in their stable configurations) at 298.15K and 1 bar. In the thermodynamics
literature there are, unfortunately, different symbols in use for this quantity. In this book we
will symbolize the enthalpy of formation at the reference pressure (1 bar) and temperature
(298.15 K) by
f
H
0
1,298
. This notation is not in widespread use. Enthalpy of formation at
298.15 K and 1 bar is one of the values that are listed in tables of thermodynamic properties
of substances, and is commonly symbolized
f
H
0
. The convenience of adding the actual

51 1.13 Enthalpy associated with chemical reactions
values of the reference pressure and temperature, as I do here, will become clear in due
course, as will the meaning of the
0
superscript which appears in both notations.
We will not discuss in this book how enthalpies of formation are measured, as excellent
discussions are available in classical textbooks on chemical and geochemical thermody-
namics (see, for example, Anderson, 2005, Chapter 5), but we will look at some examples
to make the concept clear. The enthalpy of formation,
f
H
0
1,298
, of liquid H
2
O is equal to
r
H for the reaction:
H
2
+
1
2
O
2
⇒H
2
O
liquid
. (1.87)
at 298.15 K and 1 bar. Because this is a constant pressure process,
f
H
0
1,298
is simply the
heat liberated when 1 mol of liquid water forms from a stoichiometric mixture of hydrogen
and oxygen gas at 298.15 K and 1 bar (see equation (1.62)). Similarly,
f
H
0
1,298
of diamond
is the heat exchanged by the following polymorphic transformation at 298.15 K and 1 bar,
per mol of carbon:
C
graphite
⇒C
diamond
. (1.88)
The enthalpy of formation of graphite is zero. Whether or not the formation reaction can
actually take place at 298.15 K and 1 bar, or whether or not the substance in question is
stable at those conditions, is not important. For example, we can define
f
H
0
1,298
for H
2
O
gas even though H
2
O gas is not stable at 298.15 K and 1 bar – although of course, the
enthalpy of formation of H
2
O gas at the reference conditions 298.15 K and 1 bar is different
from that of liquid H
2
O at the same conditions. Defining and evaluating
f
H
0
1,298
for a
substance that is not stable at 298.15 K and 1 bar is no more a problem than the fact that it
is not possible to locate a body at infinity, yet infinity provides a convenient reference level
for potential energy.
The quantity
f
H
0
1,298
is sometimes called the standard state enthalpy of formation.
Unfortunately, the phrase “standard state” applied to thermodynamic variables (including
enthalpy) can also have another, quite different, meaning. We will come across this alternate
meaning in Chapter 5 and we will see that the intended meaning is commonly (but not
always) evident from the context. I will make the meaning explicit whenever there is a
possibility of confusion (for an in-depth look at this and other terminology issues, see
Anderson, 2005). More trivially, why choose the reference conditions at a strange value
such as 298.15 K? Because this temperature corresponds to 25
◦
C, which historically has
been considered “standard” room temperature (arbitrarily so, and uncomfortably warm in
the view of this writer). I usually abbreviate 298.15 as 298.
1.13.2 Enthalpy of reaction as a function of temperature
I will use this example to describe the procedure that is used to calculate
r
H at any
arbitrary temperature and 1 bar. These calculation procedures will be used in later chapters
to calculate chemical equilibrium.
Consider the problem of condensation of solids in the solar nebula. During the forma-
tive period of planetary systems the chemical elements are progressively extracted from
a gas phase by formation of solid phases. Which phases and elements condense depends
on temperature. Refractory phases such as perovskite (CaTiO
3
) and corundum (Al
2
O
3
)
condense at temperatures of the order of 1600 K, whereas very volatile phases (generically
52 Energy in planetary processes
called “ices”) in which the chief constituents are C, O, N and H condense at much lower
temperatures, 300 K or less. Water ice is one of the most abundant of planetary “ices”. If
condensation takes place at pressures lower than the pressure of the triple point of H
2
O
(∼0.006 bar), then the solid phase forms directly by reaction of chemical species in the gas
phase, because the liquid phase is not stable at those conditions (Chapter 6). Suppose that
we want to know the enthalpy change (heat released) during condensation of H
2
O ice from
gaseous H
2
and O
2
at 273 K and 10
−4
bar. The strategy to calculate the enthalpy change
for this reaction is to break up the problem into two parts. First, we calculate the enthalpy
of reaction at the temperature of interest (273 K in this case) and the reference pressure
of 1 bar. Let us call this enthalpy of reaction
r
H
1,T
. Then, we calculate how enthalpy of
reaction varies as a function of pressure at a constant temperature of 273 K, integrate this
function from 1 bar to the pressure of interest (in this case, 10
−4
bar) and add the result to
r
H at 273 K and 1 bar. Symbolically:
r
H
P ,T
=
r
H
1,T
+
P
1
∂
(
r
H
)
∂P
T
dP , (1.89)
where, for the example that we are considering, T = 273 K and P = 10
−4
bar. Here we
will look only at the first part of the problem, i.e. calculation of the enthalpy of reaction at
273 K and 1 bar:
r
H
1,273
. The pressure integral (second term on the right-hand side of
equation (1.100)) will be discussed in Chapters 5, 8 and 9, because it relies on concepts that
we have not discussed yet. Here we will focus on the fact that, even though equilibrium
condensation of the solid from the gas does not occur at 1 bar (because liquid is stable at
that pressure), calculation of the enthalpy change at 1 bar is always possible.
Relative to
f
H
0
1,298
of liquid H
2
O (equation (1.87)), there are two differences in
r
H for the reaction that forms H
2
O ice from H
2
and O
2
gas at 273 K: the temperature is
different and the H
2
O phase is different. In order to visualize how to proceed it is best to
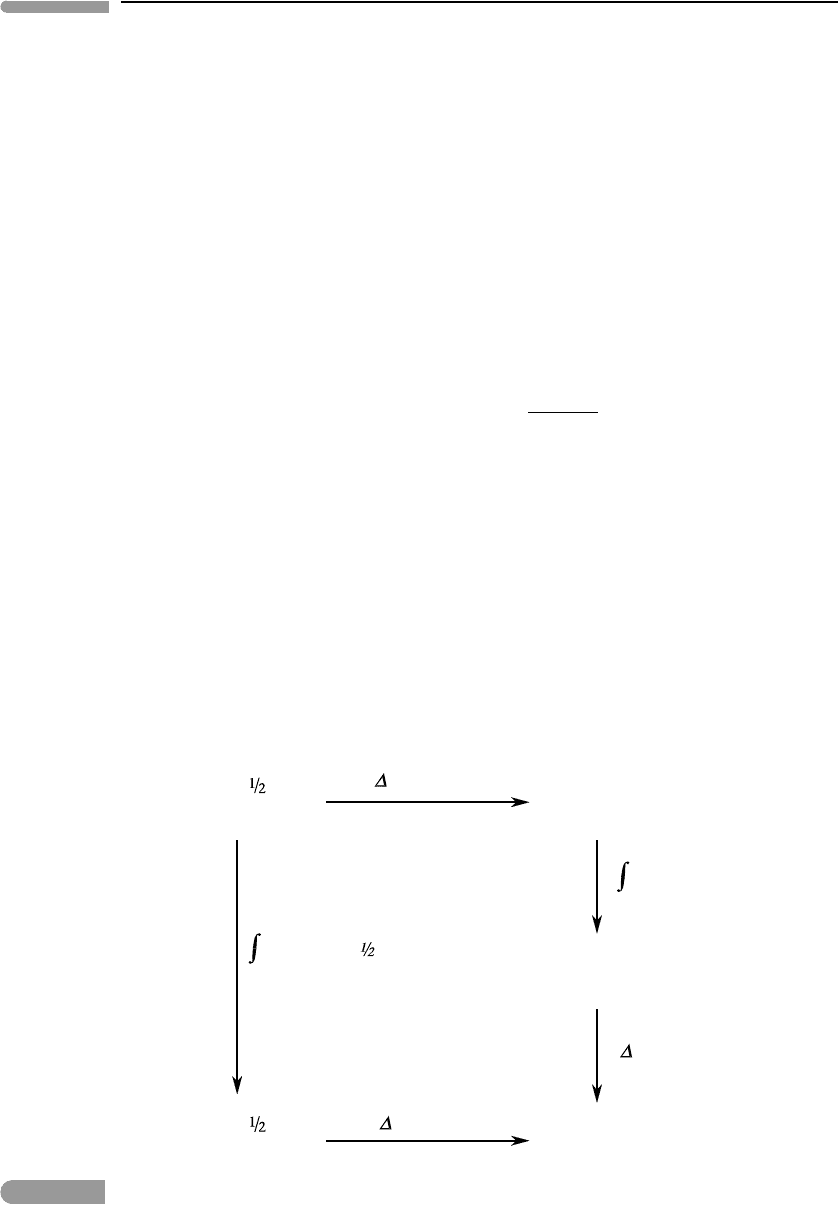
draw a diagram (Fig. 1.9). Because enthalpy is a state variable its value is independent of
H
2
+ O
2
1 bar, 298.15 K
H
2
O (liquid)
1 bar, 298.15 K
H
2
O (liquid)
1bar,273K
H
2
O (ice)
1 bar, 273 K
H
2
+ O
2
1 bar, 273 K
f
H
0
1, 298
r
H
1, 273
H
freezing
298
273
C
P, H
2
O
liquid
d
T
298
273
[ C
P,H
2
+ C
P,O
2
] dT
Fig. 1.9
Enthalpy of condensation of H
2
O ice from the elements at 273 K and 1 bar.
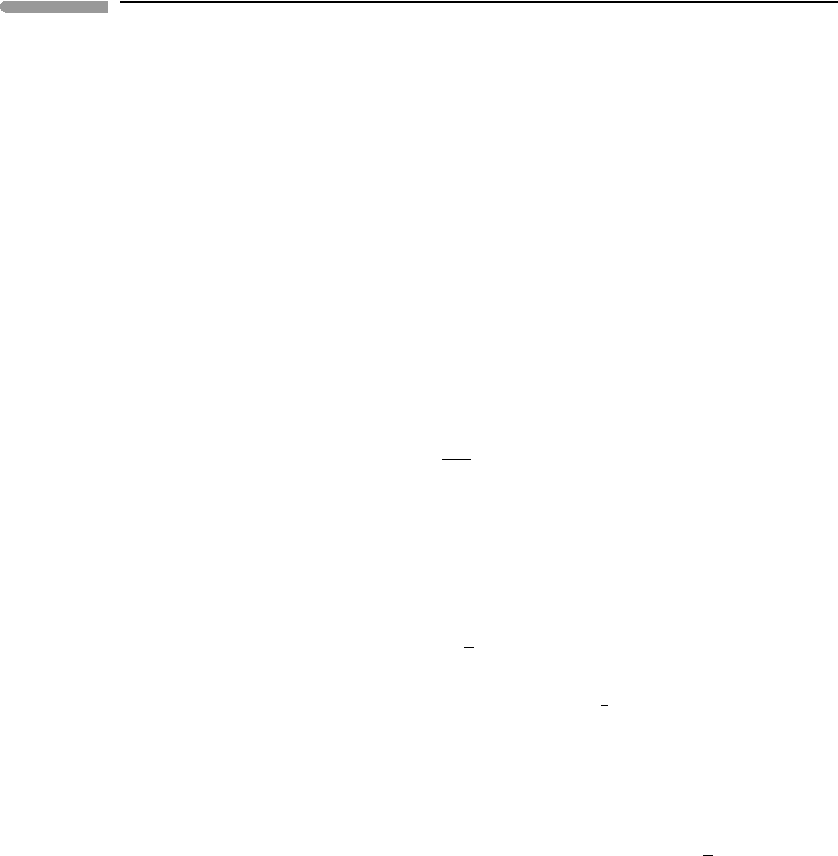
53 1.13 Enthalpy associated with chemical reactions
the path that we use to calculate it, it only depends on the state of the system that we are
considering. In our case the state of interest is H
2
O ice at 273 K and 1 bar. The diagram
(Fig. 1.9) shows that, starting from an initial state consisting of hydrogen and oxygen gas
at 298 K and 1 bar (the reference conditions at which the enthalpies of the elements are
zero), there are two paths by which we can arrive at the desired final state. One way is to
form liquid H
2
O at 298 K and 1 bar, then cool the liquid to 273 K, then freeze the liquid at
273 K to ice at 273 K. The total enthalpy change along this path is:
H
1
=
f
H
0
1,298
+
273
298
C
P,liquidH
2
O
dT +H
freezing
, (1.90)
where
f
H
0
1,298
is the enthalpy of formation of liquid H
2
O (equation (1.87)), H
freezing
=
−H
melting
is the heat given off when water freezes to ice at 273 K and 1 bar, and the change
of enthalpy associated with an isobaric change in temperature, i.e. the middle term in the
right-hand side of equation (1.90), is given in general by:
H
(
T
1
)
=H
(
T
0
)
+
T
1
T
0
∂H
∂T
P
dT = H
(
T
0
)
+
T
1
T
0
C
P
dT . (1.91)
Another way to arrive at ice at 273 K and 1 bar is to cool the gas mixture to 273 K and
then form ice directly by reaction between the gases at this temperature. This is the process
that takes place in the solar nebula, and the one that we want to calculate the enthalpy of
reaction for:
r
H
1,273
. The enthalpy change along this path (Fig. 1.9) is:
H
2
=
273
298
C
P ,H
2
dT +
1
2
273
298
C
P ,O
2
dT +
r
H
1,273
. (1.92)
The heat capacity integral for O
2
is preceded by a factor of
1
2
, which is the stoichiometric
coefficient for O
2
in the balanced chemical reaction – recall that C
p
is the molar heat
capacity. Stoichiometric coefficients are always present, but they are 1 for H
2
and H
2
O.
Now, because enthalpy is a state variable, it must be H
1
=H
2
, and we see immediately
that:
r
H
1,273
=
f
H
0
1,298
+H
freezing
+
273
298
C
P,liquid H
2
O
−C
P ,H
2
−
1
2
C
P ,O
2
dT .
(1.93)
We are also interested in
r
H for reactions in which no pure elements take part. For
example, the following reaction among spinel (MgAl
2
O
4
), enstatite (Mg
2
Si
2
O
6
), forsterite
(Mg
2
SiO
4
) and pyrope (Mg
3
Al
2
Si
3
O
12
) is an end-member model for the transition between
spinel lherzolites and garnet lherzolites in the mantles of the Earth and other terrestrial
planets:
MgAl
2
O
4
+2Mg
2
Si
2
O
6
⇒Mg
2
SiO
4
+Mg
3
Al
2
Si
3
O
12
. (1.94)
Starting from the elements, we can reach the assemblage forsterite + pyrope at an arbitrary
temperature T (and P = 1 bar) by either (1) forming forsterite + pyrope at 298 K and then
heating this assemblage to T, or (2) forming spinel + 2 enstatite at 298 K, heating this
assemblage to T, and then reacting it to form forsterite + pyrope (Fig. 1.10). The enthalpy
54 Energy in planetary processes
5Mg+2Al
+4Si+8O
2
1 bar, 298.15 K
Fo + Prp
1 bar, 298.15 K
1 bar, T
(
f
H
0
1, 298
)
Fo
+(
f
H
0
1, 298
)
Prp
r
H
1, T
Fo + Prp
298
T
[C
P, Fo
+C
P,Prp
] d
T
Sp + 2 En
1 bar, 298.15 K
Sp + 2 En
1bar,T
298
T
[C
P, Sp
+2C
P,En
] dT
(
f
H
0
1, 298
)
Sp
+2(
f
H
0
1, 298
)
En
Fig. 1.10
Enthalpy of the reaction spinel + 4 estatite →forsterite + pyrope, at T and 1 bar.
change along the first path is:
H
1
=
f
H
0
1,298
prp
+
f
H
0
1,298
fo
+
T
298
C
P,prp
dT +
T
298
C
P, fo
dT (1.95)
and along the second path (note the explicit stoichiometric coefficient of enstatite):
H
2
=
f
H
0
1,298
sp
+2
f
H
0
1,298
en
+
T
298
C
P,sp
dT +2
T
298
C
P ,en
dT +
r
H
1,T
.
(1.96)
As enthalpy is a state variable, H
1
=H
2
, so:
r
H =
f
H
0
1,298
prp
+
f
H
0
1,298
fo
+
T
298
C
P,prp
dT +
T
298
C
P, fo
dT
−
f
H
0
1,298
sp
+2
f
H
0
1,298
en
+
T
298
C
P,sp
dT +2
T
298
C
P,en
dT
.
(1.97)
Let us now introduce some additional notation that will simplify this equation. The sum of
the four enthalpies of formation is simply the enthalpy of reaction at 298 K and 1 bar. Let
us call this sum
r
H
0
1,298
:
r
H
0
1,298
=
f
H
0
1,298
prp
+
f
H
0
1,298
fo
−
f
H
0
1,298
sp
+2
f
H
0
1,298
en
.
(1.98)
Because all the integrals are evaluated over the same temperature interval we can also
collect all the heat capacity functions (assuming that they are all described by the same
function) in a single function, which we call
r
C
P
:
r
C
P
=C
P,prp
+C
P,fo
−
C
P,sp
+2C
P,en
. (1.99)
55 1.14 Internal energy
Finally, we call the enthalpy of reaction at the temperature and pressure of interest
r
H
P,T
.
In this case P = 1 bar, so our equation for
r
H
1,T
becomes:
r
H
1,T
=
r
H
0
1,298
+
T
298
r
C
P
dT . (1.100)
This equation is completely general. It applies to any chemical reaction, as long as there are
no phase transitions along any of the paths. If phase transitions occur then their enthalpies are
simply added separately, as was done with H
freezing
in equation (1.93), which is otherwise
identical to (1.100). These calculations will very quickly become second nature and you will
be able to do away with the diagrams. When in doubt, however, sketching diagrams such
as Figs. 1.9 and 1.10 will always point you to the correct result. Simple Maple procedures
to carry out the numerical calculations are described in Software Box 1.1.
Calculating the effect of pressure on
r
H is less straightforward than calculating theeffect
of temperature. This is so partly because the equation for (∂H /∂P )
T
is not a simple function
of other thermodynamic variables or material properties (compare to (∂H /∂T )
P
=C
P
). The
partial derivative (∂H /∂P )
T
is a function of molar volume, so that integating H =H(P)
requires an EOS. These are different for gases and condensed phases, and we defer their
discussion to later chapters.
1.14 Internal energy and the relationship between macroscopic
thermodynamics and the microscopic world
The internal energy of a system is the sum of energy contributions from translation, rotation
and vibration of molecules, their electronic configurations, their nuclear configurations,
and their electrostatic interactions (i.e. chemical bonding). If we do not consider nuclear
reactions, including radioactive decay, then the nuclear contribution to E stays constant.
If we also exclude chemical reactions and excitation of electronic shells by high-energy
electromagnetic radiation then the electronic and electrostatic contributions to E also stay
constant. With these restrictions, changes in E arise from changes in the translational, rota-
tional and vibrational energies of the molecules, in response to changes in the macroscopic
variables temperature and pressure. We seek a mathematical relationship between internal
energy and temperature as a first step towards understanding some of the ways in which the
macroscopic language of thermodynamics reflects processes that take place at the micro-
scopic scale. A simple derivation which relies on classical deterministic physics, called the
kinetic theory of gases, has been known since the mid nineteenth century. The kinetic theory
of gases is only applicable to the simplest of systems: a gas made up of non-interacting
particles, i.e. an ideal gas. For all other systems, the link between phenomenological ther-
modynamics and microscopic processes can only be constructed on the basis of statistical
mechanics and must include considerations of quantum mechanics as well. This is beyond
the scope of this book.
1.14.1 Internal energy of a monatomic ideal gas
Consider a gas made up of non-interacting point-like particles, i.e. a monatomic ideal gas.
In such a substance, the only possible kind of energy that can exist at the microscopic level
56 Energy in planetary processes
is the kinetic energy of motion, or translation, of the particles. The internal energy of such
a gas must therefore be equal to the sum of the translational kinetic energies of all of the
particles that make up the gas. In this case the link between the macroscopic state variable
E and the microscopic repository of energy admits no other possible interpretation, because
of the restrictions that we have imposed on the nature of the substance: non-interacting,
point-like particles.
Not all particles have the same kinetic energy. As we saw in Section 1.4.2, molecular
speeds follow a statistical distribution known as the Maxwell–Boltzmann distribution (Fig.
1.4). A consequence of this distribution of molecular speeds is that if a gas is at equilibrium
at a given pressure and temperature, then the average kinetic energy of the particles, U
k
,
is a well defined and unique value. This is the microscopic reason why E is a state variable.
The molar internal energy is then given by:
E =NU
k
=N
1
2
mc
2
, (1.101)
where N is Avogadro’s number, m is the particle mass and c
2
is the mean-square particle
speed. This is not the square of the mean, but the mean of the squares. The reason for this
is that we are averaging kinetic energy, which scales as the square of the speed.
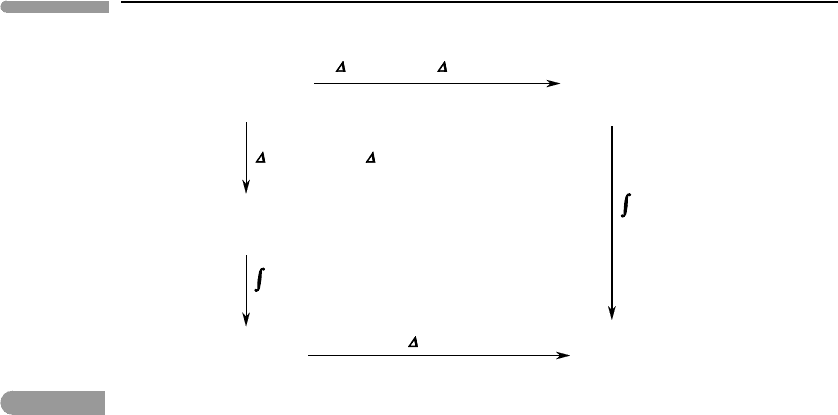
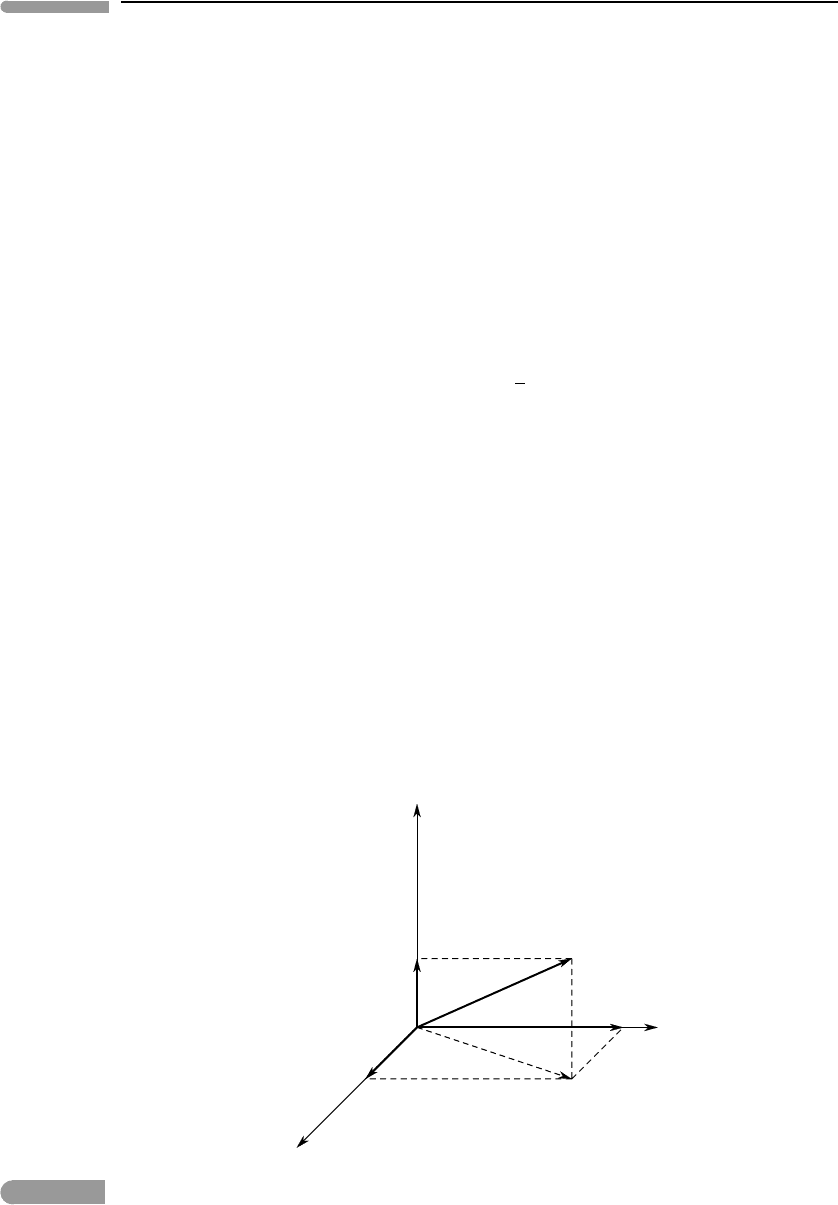
The velocity of each particle can be written in terms of three independent components:
c
x
, c
y
, c
z,
along three orthogonal directions (Fig. 1.11) such that:
c
2
=c
2
x
+c
2
y
+c
2
z
. (1.102)
Now, by symmetry, it must be:
c
2
x
=c
2
y
=c
2
z
=u
2
(1.103)
so that:
c
2
=3u
2
. (1.104)
x
z
y
c
c
x
c
z
c
y
Fig. 1.11
Translational degrees of freedom of a particle.

57 1.14 Internal energy
This result also arises from Maxwell’s principle of equipartition of energy. What the prin-
ciple states is that, in a collection of particles, energy is distributed evenly among all of the
degrees of freedom of the particles. By degrees of freedom we mean, in general, variables
that can vary independently of one another. We will come across different ways in which
this terminology is used, and the actual meaning will in every case be explained and then
become obvious from the context. When studying the behavior of particles in a micro-
scopic system, by degrees of freedom we mean independent ways in which particles can
carry energy. If particles only have translational kinetic energy, then they have three degrees
of freedom, corresponding to the three perpendicular directions in which they can move.
The principle of equipartition of energy then says that the average kinetic energy along each
of the three directions must be the same, for if particles are moving more slowly in one of
the three directions then collisions will eventually increase their speed in that direction at
the expense of the speed (=kinetic energy) in the other directions. This intuitive explana-
tion makes sense, but it does not prove the validity of the principle. Equipartition of energy
remains a principle, i.e. a statement whose truth is assumed a priori, and that is accepted
because it leads to results that agree with observations. The same is true, of course, of the
laws of thermodynamics, the principle of conservation of momentum, and all other physical
laws and principles.
Because of the principle of equipartition of energy, we can use equation (1.104) in order
to re-write equation (1.101) as follows:
E =
3
2
Nmu
2
. (1.105)
This equation says that the average kinetic energy of the particles for each degree of freedom
is
1
2
mu
2
, and the internal energy, which equals the total kinetic energy of the particles,
equals this energy times three (one for each degree of freedom) times the total number of
particles.
We know that internal energy is a function of temperature and we want to find out what
this function is on the basis of the microscopic description of the system. It is a remarkable
fact that this is in principle always possible, no matter how complicated the system may be.
The formal method for doing this relies on finding a fundamental function that is defined
in statistical mechanics and is called the partition function (see, for example, the textbooks
by Hill, 1986, or Glazer & Wark, 2001).
All thermodynamic state variables can be derived from a substance’s partition function.
The problem is that it is seldom easy to find what the partition function for a given substance
is. For the simplest of cases, ideal gases, it is possible to derive the value of the state functions
both from the partition function and from the much simpler formalism of kinetic theory, as
we do here. This is not true in general, though.
We begin by working out an expression for the pressure of an ideal gas, which must arise
from collisions of the particles against the walls of the container. Pressure is force per unit
area, and we recall from elementary physics (Newton’s second law of motion) that force
equals the rate of change of momentum. We assume that the particles that make up the ideal
gas are perfectly elastic. Therefore, when a particle collides with a surface perpendicular to
any one of the three independent directions of translation it reverses direction but does not
lose speed, so that its momentum changes by:
mu −
(
−mu
)
=2mu. (1.106)
